Abstract
Cat jaw-closing muscles are a distinct muscle allotype characterized by the expression of masticatory-specific myofibrillar proteins. Transplantation studies showed that expression of masticatory myosin heavy chain (m-MyHC) is promoted by fast motor nerves, but suppressed by slow motor nerves. We investigated whether masticatory myosin-binding protein-C (m-MBP-C) and masticatory tropomyosin (m-Tm) are similarly regulated. Temporalis muscle strips were transplanted into limb muscle beds to allow innervation by fast or slow muscle nerve during regeneration. Regenerated muscles were examined postoperatively up to 168 days by peroxidase IHC using monoclonal antibodies to m-MyHC, m-MBP-C, and m-Tm. Regenerates in both muscle beds expressed fetal and slow MyHCs, m-MyHC, m-MBP-C, and m-Tm during the first 4 weeks. Longer-term regenerates innervated by fast nerve suppressed fetal and slow MyHCs, retaining m-MyHC, m-MBP-C, and m-Tm, whereas fibers innervated by slow nerve suppressed fetal MyHCs and the three masticatory-specific proteins, induced slow MyHC, and showed immunohistochemical characteristics of jaw-slow fibers. We concluded that expression of m-MBP-C and m-Tm is coregulated by m-MyHC and that neural impulses to limb slow muscle are capable of suppressing masticatory-specific proteins and to channel gene expression along the jaw-slow phenotype unique to jaw-closing muscle. (J Histochem Cytochem 58:989–1004, 2010)
Keywords: masticatory muscle, fiber types, muscle allotype, muscle regeneration, neural influence, cat, myosin heavy chain, myosin-binding protein-C, tropomyosin
Mammalian jaw and limb muscles perform different types of functions and have evolved different fiber types to match functional demands. Limb fibers may be classified into several phenotypes: slow (type I) and up to three subtypes of fast (type II) fibers, 2a, 2x, and 2b, in the order of increasing speed, which is due principally to the specific myosin isoform they express (Bottinelli et al. 1991). Slow fibers express slow (or β-cardiac) myosin heavy chain (MyHC), whereas the three fast fiber types express 2A, 2X, and 2B MyHC, respectively (Bar and Pette 1988; Hoh 1992). Fast and slow muscles also express different isoforms of limb-specific myosin-binding protein-C (MBP-C) (Dhoot et al. 1985), tropomyosin (Tm), and other myofibrillar proteins (Hoh 1992).
Cat jaw-closing muscles have two types of fibers with different mechanical properties (Hoh et al. 2007): masticatory fibers that express a jaw-specific myosin comprising masticatory myosin heavy chains (m-MyHCs) and masticatory light chains and jaw-slow fibers that express jaw-slow myosin comprising slow MyHCs associated with masticatory light chains (Sciote et al. 1995; Hoh et al. 2007). Recent work has shown that masticatory light chain 1 is identical to the embryonic/atrial light chain 1 (Reiser et al. 2009,2010). Masticatory fibers also express masticatory-specific isoforms of Tm (m-Tm) (Rowlerson et al. 1983) and myosin-binding protein-C (m-MBP-C), which are found in thin and thick filaments, respectively, whereas jaw-slow fibers express different isoforms of these proteins (Kang et al. 2010). Masticatory fibers are associated with a unique set of physiological properties: high Ca sensitivity (Kato et al. 1985), high force per unit cross-sectional area (Saeki et al. 1987; Toniolo et al. 2008), high tension cost (Saeki et al. 1987), moderate cross-bridge cycling rate (Hoh et al. 2007), and moderate speed of shortening (Toniolo et al. 2008). These characteristics ensure rapid development of high isometric force important for a carnivorous lifestyle.
Both limb and jaw muscle fibers show physiological plasticity. Myosin composition, speed of contraction, fatigue characteristics, and other properties of fast and slow limb muscle fibers are controlled by the nerves to these muscles. When the nerves to fast and slow limb muscles are surgically crossed, the characteristics of the reinnervated muscles are reversed (Hoh 1975; Hoh et al. 1980; Pette and Vrbova 1985). When cat masticatory muscle is transplanted into a fast limb muscle bed to allow the regenerating muscle to be innervated by its nerve, the regenerated muscle expressed m-MyHC but not limb MyHCs (Hoh and Hughes 1988). However, when a masticatory muscle regenerate is innervated by the slow motor nerve, m-MyHC is initially expressed, but in the long term is replaced by slow MyHC (Hoh and Hughes 1988).
The fact that regenerating cat jaw muscle innervated by a limb muscle nerve re-expresses m-MyHC suggests that jaw muscle cells are intrinsically different from limb muscles. This capacity of regenerating cat jaw muscle to express m-MyHC is independent of innervation (Hoh and Hughes 1991b) and the basal lamina of jaw muscle (Hoh and Hughes 1991a). Myotubes derived from satellite cells of cat masticatory fibers express not only m-MyHC but also m-Tm and m-MBP-C (Kang et al. 2010) in contrast to limb muscle satellite cells, which express limb fast and slow MyHCs in culture (LaFramboise et al. 2003) but not masticatory myofibrillar proteins (Kang et al. 2010). These studies suggest that masticatory muscles belong to a distinct muscle allotype whose satellite cells are preprogrammed to express masticatory myofibrillar proteins during myogenesis.
As the earlier study (Hoh and Hughes 1988) on neural regulation of regenerating cat masticatory muscle used only m-MyHC as the marker for the masticatory phenotype, we investigate here whether the other known masticatory-specific proteins, m-MBP-C and m-Tm, are similarly regulated. We use IHC to study the expression of m-MyHC, m-MBP-C, and m-Tm in cat temporalis muscle regenerating under the influence of fast and slow limb muscle nerves. We show that fast muscle nerve promotes the coexpression of these masticatory myofibrillar proteins, whereas the nerve to slow muscle represses them, channeling gene expression along the jaw-slow phenotype.
Materials and Methods
Muscle Transplantation
Transplantation experiments were carried out on 16 kittens (body weight around 1 kg; ∼6 weeks old) as described previously (Hoh and Hughes 1988,1991b). Briefly, in half of the kittens, the fast extensor digitorum longus (EDL) muscle was completely removed from its bed and a strip of temporalis muscle (170–270 mg) was transplanted in its place, overlying the stump of the host muscle nerve. As a control, EDL muscle in the contralateral leg of the same kitten was removed and reimplanted into its own bed. In the other kittens, a temporalis transplant was similarly made into the soleus muscle bed. Regenerated muscles were harvested for study at 9, 14, 28, 56, 63, 91, 112, and 168 days after operation.
The experiments were done with the approval of the Animal Ethics Review Committees of the University of Sydney and comply with the current laws governing animal experimentation in Australia.
Immunohistochemistry
Regenerated muscles were frozen in isopentane cooled in liquid nitrogen, cut in a cryostat, and analyzed by peroxidase IHC as described previously (Hoh et al. 1988a). Primary antibodies used were a polyclonal antibody (STE) raised in sheep against fetal (embryonic and neonatal) MyHCs (Hoh et al. 1988b) and the following monoclonal antibodies (MAbs): anti-m-MyHC 2F4 (Kang et al. 1994) (from Developmental Studies Hybridoma Bank, University of Iowa, Iowa), anti-m-Tm 1H2 and anti-m-BMP-C 3F10 (Kang et al. 2010), anti-slow MyHC NOQ-7-5-4D and anti-fast MyHC NOQ-7-5-2B (Hoh et al. 1988b), anti-tropomyosin CH1 (Lin et al. 1985), and MAb 1A10, which is specific for jaw-slow myosin (Hoh et al. 1991). Horseradish peroxidase (HRP)-labeled rabbit anti-mouse or anti-sheep immunoglobulin antibody (Dako; Glostrup, Denmark) was used as the secondary antibody.
SDS-PAGE and Western Blotting
Crude myosin was extracted from cryostat sections of temporalis and EDL regenerates in 30 μl of extraction buffer (0.1 M sodium pyrophosphate, 5 mM EGTA, and 5 mM dithiothreitol), centrifuged, then diluted in sample buffer (0.625 M Tris, 10% glycerol, 2% SDS, 5% β-mercaptoethanol, 0.0013% (w/v) bromophenol blue), and boiled for 4 min. SDS-PAGE and Western blots were carried out in 8% gels as described before (Lucas and Hoh 1997). Primary antibodies used for Western blotting were MAb 3F10 against m-MBP-C and MAb 2F4 against m-MyHC, but MAb 1H2 against m-Tm was not used because it did not react with denatured antigen in Western blots. The secondary antibody used was HRP-conjugated rabbit anti-mouse immunoglobulin antibody.
Results
IHC of Temporalis and EDL Regenerates Innervated by the EDL Nerve
Immunohistochemical analysis of semiserial sections of temporalis transplants into the EDL bed at 9 days shows that the core of the tissue is dominated by necrotic masticatory fibers that stain with masticatory-specific MAbs but not with anti-fetal or anti-slow MyHC antibodies (Figure 1). Although myotubes expressing fetal MyHCs are seen scattered throughout the section, regeneration is at a more advanced stage at the periphery, as indicated by the replacement of necrotic tissue with bundles of myotubes that express fetal MyHCs. At higher power, most of these myotubes are seen to also express masticatory-specific proteins; some express slow MyHC and others express slow and masticatory-specific proteins (data not shown). The 14-day regenerate shows widespread staining for fetal MyHCs, but there is now a marked increase in the proportion of fibers staining for slow MyHC and masticatory-specific proteins, especially at the periphery of the regenerate (data not shown).
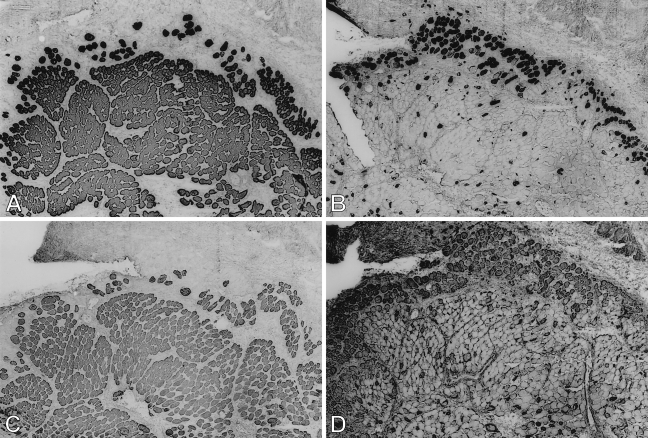
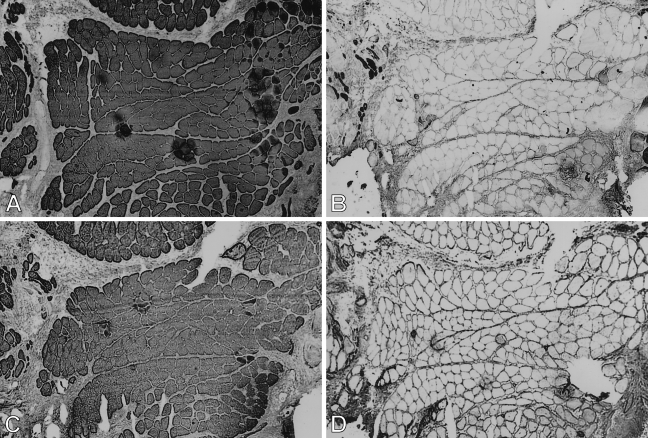
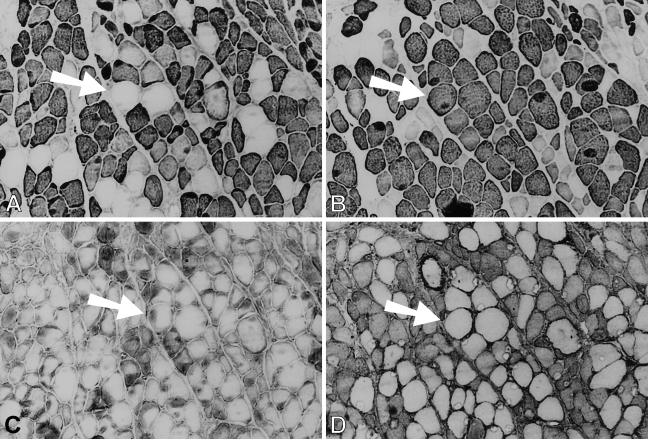
Figure 1.
Low-power view of semiserial sections of temporalis muscle transplanted into the extensor digitorum longus (EDL) bed studied 9 days postoperatively, stained by immunoperoxidase with anti-masticatory myosin heavy chain monoclonal antibody (anti-m-MyHC MAb) 2F4 (A), anti-slow MyHC MAb NOQ-7-5-4D (B), anti-masticatory myosin-binding protein-C (anti-m-MBP-C) MAb 3F10 (C), anti-fetal MyHC antibody STE (D), and anti-masticatory-tropomyosin (anti-m-Tm) MAb 1H2 (E). Bar = 100 μm.
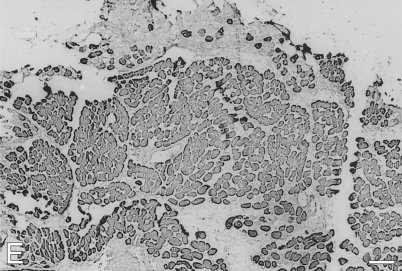
By 28 days postoperatively (Figure 2), cellular debris from the original transplant have been almost completely replaced by mature-looking fibers and smaller myotubes. All fibers, large and small, stain moderately for fetal MyHCs (Figure 2D), and many fibers, especially the larger ones, now stain strongly for m-MyHC (Figure 2A), m-MBP-C (Figure 2C), and m-Tm (Figure 2E); the more mature of these fibers (example arrowed) have lost staining for slow MyHC (Figure 2B). Expression of slow MyHC is now mostly confined to small myotubes and medium-sized fibers.
Figure 2.
Immunoperoxidase staining of semiserial sections of temporalis muscle transplanted into the fast EDL bed examined 28 days postoperatively. Antibodies used were anti-m-MyHC (A), anti-slow MyHC (B), anti-m-MBP-C (C), anti-fetal MyHC (D), and anti-m-Tm (E). The arrow points to the same, relatively large diameter fiber in each panel that no longer expresses slow MyHC. Bar = 100 μm.
In the 56-day-old regenerate, fibers are relatively uniform in size and the vast majority of them stain for m-MyHC, m-Tm, and m-MBP-C (data not shown). A small subpopulation of fibers staining for m-MyHC and m-Tm does not stain for m-MBP-C. Slow MyHC is still expressed in some fibers. Fetal MyHCs are still expressed in a few scattered fibers.
At 63 days after operation (Figure 3), the vast majority of fibers are large and strongly express masticatory-specific myofibrillar proteins (Figures 3A, 3C, and 3E). Fetal MyHCs are no longer expressed (Figure 3D), whereas slow MyHC is expressed in a group of small fibers at the periphery of the regenerate (Figure 3B). By 112 days (Figure 4), all fibers uniformly express jaw-specific myofibrillar proteins (Figures 4A, 4C, and 4E) and no fibers react with anti-fetal MyHC antibody (Figure 4D), but a few scattered fibers still stained weakly for slow MyHC (Figure 4B). At no stage during the regeneration of the temporalis muscle reinnervated by the EDL nerve does any muscle fiber stain significantly for fast MyHC (data not shown).
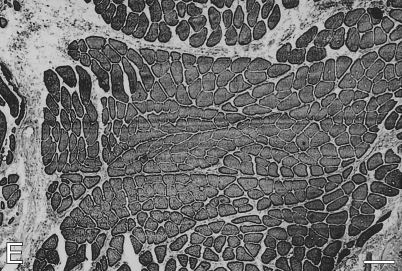
Figure 3.
Low-power view of immunoperoxidase staining of semiserial sections of temporalis muscle transplanted into the EDL bed, studied 63 days postoperatively. Antibodies used were anti-m-MyHC (A), anti-slow MyHC (B), anti-m-MBP-C (C), anti-fetal MyHC (D), and anti-m-Tm (E). Bar = 100 μm.
Figure 4.
Immunoperoxidase staining of semiserial sections of temporalis muscle transplanted into the fast EDL bed studied 112 days postoperatively. Antibodies used were anti-m-MyHC (A), anti-slow MyHC (B), anti-m-MBP-C (C), anti-fetal MyHC (D), and anti-m-Tm (E). Bar = 50 μm.
EDL muscles regenerating in their own beds follow a time course of cellular changes similar to that described for temporalis regenerates. In the early EDL regenerates, fetal, slow, and fast MyHCs are expressed, whereas masticatory-specific proteins are not expressed (data not shown). At 56 days, most fibers stain for both slow and fast MyHCs and staining for fetal MyHCs persists. By 112 days after operation, virtually all fibers stain for fast MyHC, but some fibers still express slow MyHC as well. At no stage do the EDL regenerates in their own beds express masticatory-specific proteins.
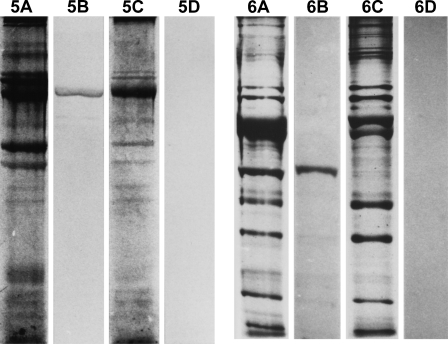
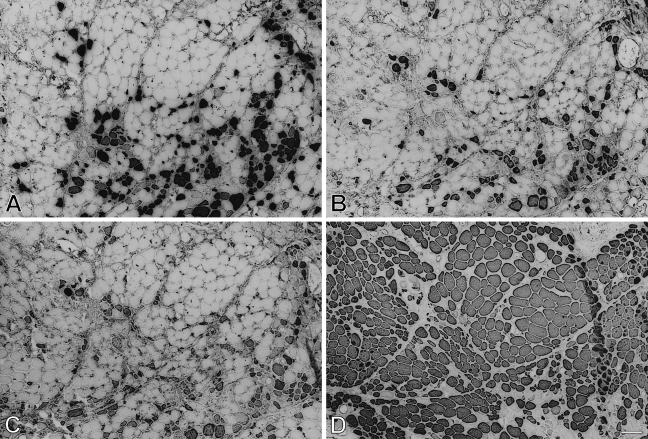
Myofibrillar protein extracts from 112-day temporalis and EDL regenerates innervated by the EDL nerve are analyzed by SDS-PAGE and Western blotting using MAbs against m-MyHC (Figure 5) and m-MBP-C (Figure 6). The results show that only the temporalis regenerate expresses m-MyHC and m-MBP-C, confirming the above immunohistochemical results.
Figure 5.
Analysis of myofibrillar proteins from 112-day regenerates of temporalis (A,B) and EDL (C,D) muscles innervated by the EDL nerve. A and C are protein-stained SDS-PAGE gels; B and D are Western blots using anti-m-MyHC.
Figure 6.
Analysis of myofibrillar proteins from 112-day regenerates of temporalis (A,B) and EDL (C,D) muscles innervated by the EDL nerve. A and C are protein-stained SDS-PAGE gels; B and D are Western blots using anti-m-MBP-C.
IHC of Regenerated Temporalis Muscle Innervated by the Soleus Nerve
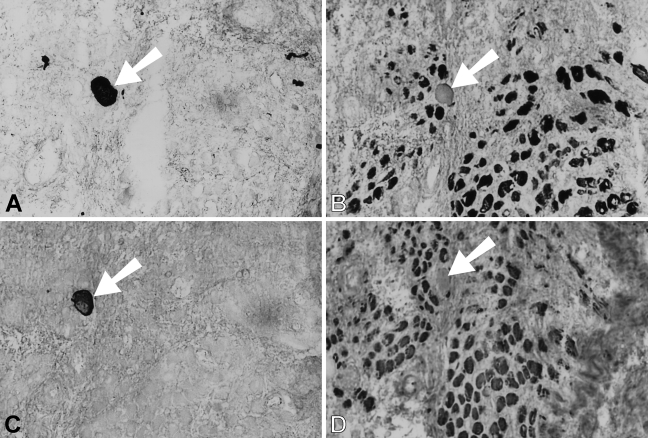
During the first month after surgery, temporalis regenerates in the soleus bed are rather similar to those in the EDL bed in the pattern of myofibrillar protein gene expression. Figure 7 shows a high-power view at the periphery of a 9-day regenerate where necrotic tissue has largely been cleared. Bundles of small myotubes are seen to react with anti-slow and anti-fetal antibodies (Figures 7B and 7D, respectively), but not with any of the masticatory-specific MAbs (Figures 7A, 7C, and 7E). However, an island of necrotic tissue (Figure 7, arrowed), which is negative for fetal and slow MyHCs, stains with masticatory-specific MAbs. At 14 days, myotubes are uniformly stained with anti-fetal and anti-slow antibodies and some myotubes also weakly express masticatory-specific myofibrillar proteins (data not shown).
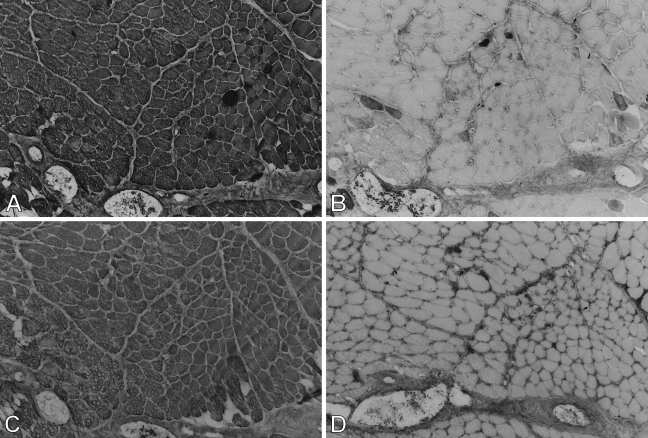
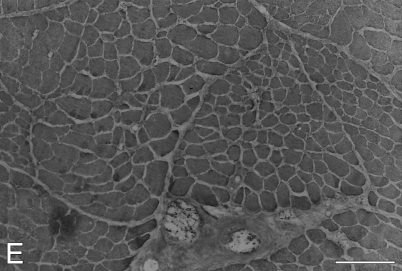
Figure 7.
High-power views of immunoperoxidase-stained semiserial sections of regenerating temporalis muscle 9 days after transplantation into the soleus muscle bed. Antibodies used were anti-m-MyHC (A), anti-slow MyHC (B), anti-m-MBP-C (C), anti-fetal MyHC (D), and anti-m-Tm (E). The arrow points to an island of necrotic tissue in each panel. Bar = 50 μm.
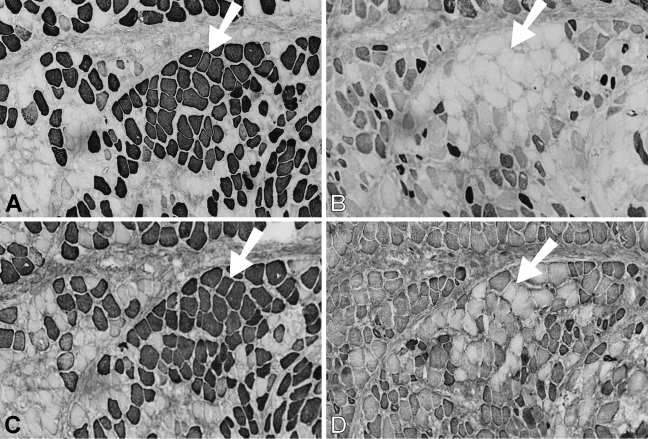
Figure 8 presents a 28-day temporalis regenerate in the soleus bed. Relative to 14-day regenerates, there is a significant increase in fiber size, intensity of staining, and proportion of fibers staining for m-MyHC, m-MBP-C, and m-Tm (Figures 8A, 8C, and 8E, respectively). A subpopulation of these masticatory protein expressing fibers with larger diameters (arrowed) has lost staining for slow MyHC (Figure 8B) and some of these fibers have even lost staining for fetal MyHCs (Figure 8D) and resemble mature masticatory fibers. Most fibers still stain moderately for fetal MyHC, whereas staining for slow MyHC is now weak and mostly confined to small myotubes and medium-sized fibers. These features are very similar to those of regenerates in the EDL bed at the same stage (Figure 2).
Figure 8.
Immunoperoxidase staining of semiserial sections of regenerating temporalis muscle 28 days after transplantation into the soleus muscle bed. Antibodies used were anti-m-MyHC (A), anti-slow MyHC (B), anti-m-MBP-C (C), anti-fetal MyHC (D), and anti-m-Tm (E). Arrow in each panel points to a group of large fibers that no longer expresses slow and fetal MyHCs. Bar = 50 μm.
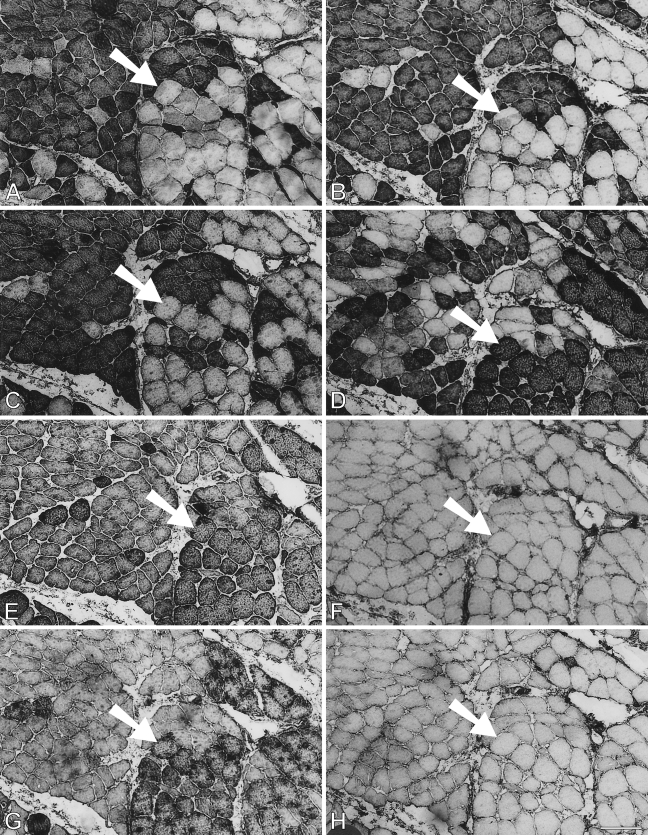
At 63 days (Figure 9), a dramatic change in antibody staining pattern is seen with respect to both the corresponding regenerate in the EDL bed and the 28-day regenerate in the soleus bed. In contrast to the drastic loss of slow MyHC staining in the EDL bed (Figure 3B), the larger fibers in the soleus bed now acquired strong staining for slow MyHC (Figure 9B), whereas the less mature smaller fibers are weakly stained. This correlation of large fiber size with staining intensity for slow MyHC is the reverse of that seen in the 28-day regenerate in the soleus bed, suggesting that these large fibers have changed from slow MyHC inhibition to slow MyHC induction. Staining for m-MyHC (Figure 9A), particularly for m-MBP-C, m-Tm, and fetal MyHCs (Figures 9C, 9E, and 9D, respectively), has become weak, and the largest fibers (examples arrowed), which stain strongly for slow MyHC, are not stained for these proteins. These large fibers have, therefore, completely transformed into slow fibers at this stage, whereas the smaller, less mature fibers still stain weakly for fetal MyHCs and masticatory-specific proteins.
Figure 9.
Immunoperoxidase staining of semiserial sections of regenerating temporalis muscle 63 days after transplantation into the soleus muscle bed. Antibodies used were anti-m-MyHC (A), anti-slow MyHC (B), anti-m-MBP-C (C), anti-fetal MyHC (D), and anti-m-Tm (E). Arrow in each panel points to a pair of large fibers that have completely transformed into slow fibers. Bar = 50 μm.
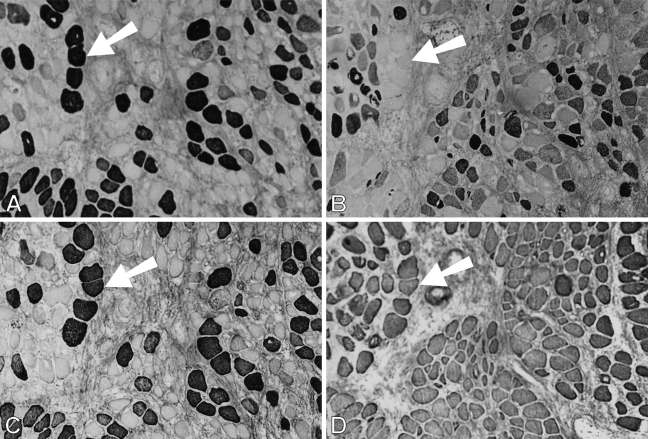
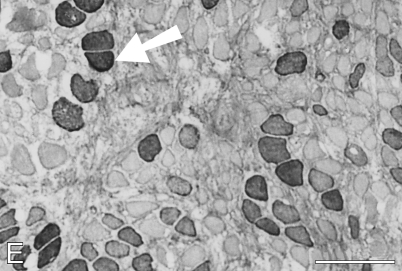
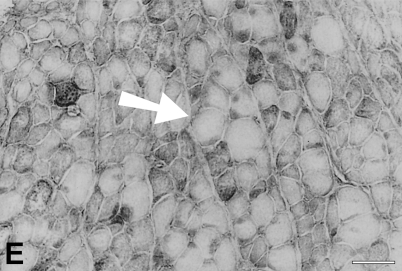
At 91 days, staining for fetal MyHCs is no longer seen. The majority of fibers stain for slow MyHC, but many fibers still stain for masticatory-specific proteins. Figure 10 shows semiserial sections of a selected region with slow and masticatory fibers stained with the five antibodies used hitherto plus three additional MAbs to probe other phenotypic characteristics of the regenerated fibers. Many fibers in this region (mostly located in the left half of each photomicrograph) show costaining with m-MyHC, m-MBP-C, and m-Tm (Figures 10A–10C, respectively), some of which are negative for slow MyHC (Figure 10D). Anti-slow MyHC MAb stains a group of fibers, as shown in the lower right corner of the photomicrograph (Figure 10D), that do not stain for masticatory-specific proteins and thus have completely transformed into slow fibers (one example indicated by arrow). Most of the fibers that stain for slow MyHC elsewhere overlap in staining for masticatory-specific proteins, indicating that they are incompletely transformed into slow fibers. The completely transformed slow fibers are better demarcated by MAbs 1A10 (Figure 10E) and CH1 (Figure 10G). The pattern of staining for these MAbs is complementary to that for masticatory-specific myofibrillar proteins, whereas fibers coexpressing slow MyHC and masticatory-specific myofibrillar proteins are not stained. MAb 1A10 is known to react with fetal and fast MyHCs, but is specific for jaw-slow and not for limb-slow fibers (Hoh et al. 1991). The slow fibers stained by MAb 1A10 are stained neither by anti-fast MyHC MAb (Figure 10F) nor by anti-fetal MyHC antibody (Figure 10H), indicating that MAb 1A10 is responding to jaw-slow myosin (see details in Discussion). MAb CH1 is specific for α-Tm and β-Tm in limb muscle fibers (Hoh et al. 1989). Staining of completely transformed slow fibers suggests that their m-Tm has been replaced by these isoforms.
Figure 10.
Immunoperoxidase staining of semiserial sections of regenerating temporalis muscle 91 days after transplantation into the soleus muscle bed. Antibodies used were anti-m-Tm (A), anti-m-MBP-C (B), anti-m-MyHC (C), anti-slow MyHC (D), MAb 1A10 (E), anti-fast MyHC (F), anti-limb Tm CH1 (G), and anti-fetal MyHC (H). The arrow in each panel points to a jaw-slow fiber. Bar = 100 μm.
In the 168-day regenerate, fibers costaining for m-MyHC, m-Tm, and m-MBP-C (Figures 11A–11C respectively) are still present, but are confined to a small subpopulation that varied widely in fiber size. As with the 63-day regenerate, staining for m-MyHC is more intense and more extensive than for m-Tm and m-MBP-C. Most fibers stain only with anti-slow MyHC MAb (Figure 11D).
Figure 11.
Immunoperoxidase staining of semiserial sections of temporalis muscle regenerated in the soleus bed 168 days postoperatively. Antibodies used were anti-m-MyHC (A), anti-m-Tm (B), anti-m-MBP-C (C), and anti-slow MyHC (D). Bar = 100 μm.
Discussion
Expression of Myofibrillar Proteins in Early Temporalis Muscle Regenerates: Influence of Muscle Allotype
Immunohistochemical analyses of the regenerates of temporalis transplants during the first 4 weeks revealed that the pattern of myofibrillar protein gene expression was similar in the EDL and soleus muscle beds. In both beds, regenerating fibers strongly expressed fetal MyHCs and coexpressed slow MyHC and/or masticatory-specific proteins. These changes were observed in 9-day regenerates in the EDL bed, but in the soleus bed, expression of masticatory proteins did not appear until day 14. By day 28, the immunohistochemical profile of the regenerates in the two muscle beds became very similar. Regenerative myogenesis apparently occurred at a somewhat slower rate in the soleus bed, but because the observations were based on single animals at each time point, the early difference in myogenic progression between the beds is of uncertain statistical significance. However, local factors may have contributed to this difference. The regenerate in the soleus bed was more susceptible to disturbance by movement due to the overlying gastrocnemius muscle, whereas that in the EDL bed was comfortably accommodated within a muscle compartment. This difference might have adversely affected the early stages of revascularization of the regenerate in the soleus bed.
The present work complements earlier works on jaw regenerates (Hoh and Hughes 1988,1991a,b) by showing that early jaw regenerates express not only fetal, slow, and masticatory MyHCs but also two other masticatory-specific myofibrillar proteins, m-MBP-C and m-Tm. This pattern of gene expression in vivo was identical to that of cultured myotubes derived from temporalis satellite cells (Kang et al. 2010). In contrast, limb fast muscle reimplanted in its own beds coexpressed fetal, fast, and slow MyHCs during the first 4 weeks of regeneration, which is also the pattern of MyHC expression of myotubes in culture derived from satellite cells of fast or slow limb muscles (LaFramboise et al. 2003). Limb muscle regenerates did not express masticatory-specific myofibrillar proteins. Differences in MyHC and MBP-C gene expression between masticatory and limb muscle regenerates were confirmed by SDS-PAGE and Western blotting. These results reflect the nature of the masticatory and limb muscle allotypes: the satellite cells of the former are preprogrammed to express masticatory myofibrillar proteins during myogenesis (Kang et al. 2010), whereas those of the latter are preprogrammed to express limb-specific isoforms (LaFramboise et al. 2003).
By 28 days, myotubes in both muscle beds began to show signs of maturity by increasing in diameter and in intensity of expression of masticatory-specific myofibrillar proteins, whereas expression of slow and fetal MyHCs decreased or disappeared. Similar features were reported in both denervated and innervated regenerates at 27 days (Hoh and Hughes 1991b), showing that these signs of maturity emerged independent of innervation, suggesting that the changing pattern of myofibrillar gene expression is intrinsic to the myogenic program of masticatory muscle allotype. However, myotube maturity as indicated by fetal and slow MyHC suppression was not observed in vitro (Kang et al. 2010), reflecting the more favorable conditions present in vivo, such as the presence of a blood supply and humoral factors. Important among humoral factors that could influence muscle maturity during regeneration are the thyroid hormones.
Thyroid hormones are essential for normal muscle development among vertebrates (Hulbert 2000). There is a surge of thyroid hormone levels during the postnatal development of mammalian muscles that is correlated with the differentiation of muscle fiber types. Thyroid hormone accelerates the withdrawal of fetal MyHCs and their replacement by fast MyHCs in developing fast muscles (Adams et al. 1999). It does so indirectly by binding to thyroid response elements to induce the expression of the myogenic factor MyoD, which directly regulates myofibrillar gene expression. MyoD dimerizes with ubiquitous E-proteins to bind with specific nucleotide sequences CANNTG called E-boxes found in the regulatory regions of many muscle-specific protein genes, including those for 2B (Wheeler et al. 1999), 2X, and 2A MyHCs (Allen et al. 2001) and fast myosin light chain 1/3 (Rao et al. 1996). The postnatal surge in thyroid hormone is involved in the development of masticatory muscles in the rat (Maeda et al. 1981) and presumably in the developmental and regenerative myogenesis of masticatory muscle in other mammalian species. It seems likely that thyroid hormones played a large part in the maturation of temporalis regenerates and the withdrawal of fetal and slow MyHC expression in both muscle beds around 28 days.
In common with limb muscle, myogenic cells of the masticatory allotype use the MyoD family of regulatory proteins to induce myogenesis (Hacker and Guthrie 1998; Noden et al. 1999). However, during early embryonic development, mesodermal cells giving rise to jaw and limb muscle precursor cells use different signaling cascades to lead them into the myogenic pathway (reviewed in Kang et al. 2010). The role of thyroid hormones in jaw muscle development and the manner whereby differences in upstream signaling cascades for myoblast specification lead to different programs of myofibrillar protein gene expression during the subsequent myogenic progression are interesting questions for future research.
Jaw muscles of carnivorous lower vertebrates express m-MyHC, a phylogenetically ancient MyHC (Qin et al. 2002). This phenotype is also found in eutherian and marsupial carnivores and in members of many other mammalian orders (Hoh 2002; Hoh et al. 2006; Reiser et al. 2010), including some rodents (Reiser et al. 2009). In adapting to the diverse types of food they consume, some mammals have replaced m-MyHC with one or more MyHCs normally expressed in developing muscle, adult fast and slow limb muscle, or in the heart (α-cardiac MyHC) (Rowlerson et al. 1981; Hoh 2002; Hoh et al. 2006). It is likely that in those species where m-MyHC has been replaced by another isoform, the myogenic cells from their jaw muscles would also be preprogrammed to express the MyHC and other myofibrillar proteins found in their jaw-closing muscle fibers.
Limb muscles reimplanted in their own beds coexpressed fetal, fast, and slow MyHCs during the first 4 weeks of regeneration. This pattern of gene expression is similar to that of myotubes in culture derived from satellite cells of fast or slow limb muscles (LaFramboise et al. 2003). They did not express masticatory-specific myofibrillar proteins. Differences in MyHC and MBP-C gene expression between masticatory and limb muscle regenerates were confirmed by SDS-PAGE and Western blotting and were consistent with their different muscle allotypes.
Expression of Myofibrillar Proteins in Late Temporalis Muscle Regenerates: Influence of Innervation
The gene expression pattern of temporalis muscle transplants innervated by fast and slow nerves diverged dramatically beyond 28 days. Regenerates innervated by the EDL nerve seamlessly continued the trend of increasing masticatory-specific protein expression and inhibition of fetal and slow MyHC expression initiated by the myogenic influence. By 63 days and beyond, fetal and slow MyHC expression was virtually completely suppressed and all regenerated fibers eventually ended up strongly coexpressing the three masticatory-specific myofibrillar proteins. These results complement earlier work on temporalis regenerates innervated by the nerve to fast muscle (Hoh and Hughes 1988) by showing that, in the long term, these regenerates not only expressed m-MyHC but also expressed the other two currently known masticatory-specific myofibrillar proteins, m-Tm and m-MBP-C. It is likely that other, currently uncharacterized masticatory fiber-specific proteins were also expressed in these regenerates.
In contrast, temporalis regenerates innervated by the slow muscle nerve followed a very different course. After strongly expressing masticatory myofibrillar proteins and suppressing fetal and slow MyHCs at 28 days due to the myogenic influence, these regenerating fibers dramatically reversed these changes by switching on slow MyHC expression and suppressing masticatory-specific myofibrillar proteins while continuing to mature by inhibiting fetal MyHCs. At 63 days, the largest fibers had completely transformed into jaw-slow fibers, whereas smaller fibers still express masticatory-specific proteins and fetal MyHCs. This mixture of transformed and partially transformed fibers generated the complex pattern of staining seen in Figure 9. The suppression of slow MyHC around 28 days was missed in the earlier study (Hoh and Hughes 1988) due to a gap in the data. In the long term, both the present and the earlier study showed that the vast majority of fibers innervated by the slow motor nerve expressed slow MyHC, whereas only a small population of small fibers continued to express m-MyHC. We have shown here that all three masticatory myofibrillar proteins were repressed in temporalis regenerates innervated by the slow muscle nerve, with m-Tm and m-MBP-C apparently being more easily repressed than m-MyHC, suggesting that signaling pathways for their inhibition may not be identical.
The present work also complements the earlier work by showing that the regenerated jaw-slow fibers can be distinguished from limb slow fibers immunohistochemically by their reaction with MAbs 1A10 and CH1. MAb 1A10 does not bind limb slow MyHC, but binds jaw-slow fibers and limb fast and fetal MyHCs (Hoh et al. 1991). Because the cDNA of slow MyHC expressed in cat jaw-slow fibers appears to be the same as that in limb slow muscle (Hoh et al. 2007), the unique affinity of jaw-slow fibers for MAb 1A10 has been attributed to the jaw-slow myosin, in which slow MyHCs are associated with masticatory light chains (Hoh et al. 2007). The transformed jaw-slow fibers in regenerates innervated by the soleus nerve bound MAb 1A10 in the absence of fetal or fast MyHCs, suggesting that MAb 1A10 was binding to the jaw-slow myosin. The transformed jaw-slow fibers also no longer expressed m-Tm, as shown by their failure to bind MAb 1H2. Instead, they bound MAb CH1, which is known to bind limb α-Tm and β-Tm isoforms (Hoh et al. 1989), suggesting that one or both of these isoforms have replaced m-Tm. The presence of β-Tm, presumably from jaw-slow fibers, has been identified by SDS-PAGE of myofibrillar proteins from cat masseter muscle that has masticatory and jaw-slow fibers (Kang et al. 2010). Thus, the present work shows that the transformed slow fibers have unique phenotypic characteristics of normal cat jaw-slow fibers rather than limb slow fibers.
The present findings indicate that motor nerves to limb muscles are competent in regulating muscle fibers of the jaw allotype, the fast and slow nerves channeling these fibers to express the masticatory and jaw-slow fiber phenotypes, respectively. The influence of the soleus nerve started to emerge only after 28 days; this delay may be attributed to the preceding regenerative myogenesis and reinnervation. Although regenerates were likely to have been partially innervated by 28 days, the new neuromuscular junctions may not be able to faithfully transmit the tonic neural signals (see later) needed to transform emerging masticatory fibers into jaw-slow fibers. These two neurally inducible phenotypic options are unique to masticatory muscle allotype. Somehow, the signaling cascades used to specify myogenic cells of masticatory muscle have primed them to respond in these two specific ways to neural signals.
Limb muscle nerves are known to carry a variety of impulse patterns that are thought to regulate different patterns of muscle gene expression (Hennig and Lømo 1985). Nerves to slow muscle fibers carry tonic, low frequency impulses, with high impulse counts per day, whereas those to fast fibers are characterized by phasic, high frequency impulses, with low-to-moderate impulse counts per day. Stimulating a fast muscle with chronic, low frequency impulses, either by cross-innervating it with the nerve to a slow muscle or directly via an implanted stimulator, causes fast fibers to transform into slow ones (Pette and Vrbova 1985). It is currently thought that fast-to-slow transformation of limb muscle results from the sustained rise of intracellular Ca2+ activating the Ca–calmodulin-dependent phosphatase, calcineurin. Calcineurin then dephosphorylates the nuclear factor NFAT, which enters the nucleus to induce the expression of slow muscle genes (Chin et al. 1998; McCullagh et al. 2004). Recent work on limb muscles has shown that the same pathway is involved in the suppression of a fast myofibrillar gene in response to chronic, low-frequency stimulation (Rana et al. 2008).
We propose that the calcineurin/NFAT signaling pathway is also involved in both the induction of jaw-slow myofibrillar genes and the suppression of m-MyHC, m-Tm, m-MBP-C, and other masticatory-specific myofibrillar proteins mediated by the tonic, low frequency impulses carried by the soleus nerve. However, the observed transient expression of slow MyHC in early masticatory muscle regenerates in both fast and slow muscle beds is most probably due to some other mechanism, as slow MyHC expression during embryonic development has been shown to be independent of calcineurin (Oh et al. 2005).
It is currently not known how the nerve to fast limb muscle promotes the coexpression of masticatory myofibrillar proteins. The phasic, high frequency impulses they carry may activate a specific gene regulatory pathway that leads to masticatory-specific gene expression. Unlike mechanisms regulating limb muscles, which have to cater for slow and three fast fiber types, each requiring a distinct regulatory pathway (Allen et al. 2001), the mechanism regulating cat jaw-closing muscle has only two alternative end points: masticatory fibers or jaw-slow fibers. This allows the formulation of a simple alternative hypothesis for the regulation of masticatory fibers: expression of masticatory myofibrillar genes is the default state during myogenesis; the absence of sustained low-frequency stimulation allows the myogenic factors to sustain the expression of masticatory myofibrillar protein isoforms. The sustained expression of m-MyHC in uninnervated temporalis muscle regenerates (Hoh and Hughes 1991b) supports this hypothesis, as do expressions of masticatory myofibrillar proteins in temporalis satellite cell cultures (Kang et al. 2010) and in early temporalis regenerates in the soleus bed before the functional maturity of the soleus nerve.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia and the Australian Research Council.
We thank Dr. A.W. Everett for MAb 1A10, Dr. R.B. Fitzsimons for MAbs NOQ-7-5-4D and NOQ-7-5-2B, R. Bestak for technical assistance, and E. Hoh for help with figures.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Adams GR, McCue SA, Zeng M, Baldwin KM (1999) Time course of myosin heavy chain transitions in neonatal rats: importance of innervation and thyroid state. Am J Physiol 276:R954–961 [DOI] [PubMed] [Google Scholar]
- Allen DL, Sartorius CA, Sycuro LK, Leinwand LA (2001) Different pathways regulate expression of the skeletal myosin heavy chain genes. J Biol Chem 276:43524–43533 [DOI] [PubMed] [Google Scholar]
- Bar A, Pette D (1988) Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett 235:153–155 [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Schiaffino S, Reggiani C (1991) Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol 437:655–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, et al. (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12:2499–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhoot GK, Hales MC, Grail BM, Perry SV (1985) The isoforms of C protein and their distribution in mammalian skeletal muscle. J Muscle Res Cell Motil 6:487–505 [DOI] [PubMed] [Google Scholar]
- Hacker A, Guthrie S (1998) A distinct developmental programme for the cranial paraxial mesoderm in the chick embryo. Development 125:3461–3472 [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T (1985) Firing patterns of motor units in normal rats. Nature 314:164–166 [DOI] [PubMed] [Google Scholar]
- Hoh JFY (1975) Neural regulation of mammalian fast and slow muscle myosins: an electrophoretic analysis. Biochemistry 14:742–747 [DOI] [PubMed] [Google Scholar]
- Hoh JFY (1992) Muscle fiber types and function. Curr Opin Rheumatol 4:801–808 [PubMed] [Google Scholar]
- Hoh JFY (2002) ‘Superfast’ or masticatory myosin and the evolution of jaw-closing muscles of vertebrates. J Exp Biol 205:2203–2210 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Hughes S (1988) Myogenic and neurogenic regulation of myosin gene expression in cat jaw-closing muscles regenerating in fast and slow limb muscle beds. J Muscle Res Cell Motil 9:59–72 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Hughes S (1991a) Basal lamina and superfast myosin expression in regenerating cat jaw muscle. Muscle Nerve 14:398–406 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Hughes S (1991b) Expression of superfast myosin in aneural regenerates of cat jaw muscle. Muscle Nerve 14:316–325 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Hughes S, Chow C, Hale PT, Fitzsimons RB (1988a) Immunocytochemical and electrophoretic analyses of changes in myosin gene expression in cat posterior temporalis muscle during postnatal development. J Muscle Res Cell Motil 9:48–58 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Hughes S, Hale PT, Fitzsimons RB (1988b) Immunocytochemical and electrophoretic analyses of changes in myosin gene expression in cat limb fast and slow muscles during postnatal development. J Muscle Res Cell Motil 9:30–47 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Hughes S, Walker ML, Kang LHD, Everett AW (1991) Slow myosin heavy chains in cat jaw and limb muscles are phenotypically distinct: expression of jaw-specific slow myosin phenotype in regenerated and chronically stimulated jaw muscles. Basic Appl Myol 1:285–294 [Google Scholar]
- Hoh JFY, Kang LHD, Sieber LG, Lim JH, Zhong WW (2006) Myosin isoforms and fibre types in jaw-closing muscles of Australian marsupials. J Comp Physiol B 176:685–695 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Kwan BTS, Dunlop C, Kim BH (1980) Effects of nerve cross-union and cordotomy on myosin isoenzymes in fast-twitch and slow-twitch muscles of the rat. In Pette D, ed. Plasticity of Muscle. Berlin, Walter de Gruyter, 339–352
- Hoh JFY, Li ZB, Qin H, Hsu MK, Rossmanith GH (2007) Cross-bridge kinetics of fast and slow fibres of cat jaw and limb muscles: correlations with myosin subunit composition. J Muscle Res Cell Motil 28:329–341 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Walker ML, Lin JJC (1989) A unique isoform of skeletal tropomyosin in cat jaw-closing muscle and its developmental expression. Proc Aust Physiol Pharmacol Soc 20:192P [Google Scholar]
- Hulbert AJ (2000) Thyroid hormones and their effects: a new perspective. Biol Rev Camb Philos Soc 75:519–631 [DOI] [PubMed] [Google Scholar]
- Kang LHD, Hughes S, Pettigrew JD, Hoh JFY (1994) Jaw-specific myosin heavy chain gene expression in sheep, dog, monkey, flying fox and microbat jaw-closing muscles. Basic Appl Myol 4:381–392 [Google Scholar]
- Kang LHD, Rughani A, Walker ML, Bestak R, Hoh JF (2010) Expression of masticatory-specific isoforms of myosin heavy chain, myosin-binding protein-C, and tropomyosin in muscle fibers and satellite cell cultures of cat masticatory muscle. J Histochem Cytochem 58:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato C, Saeki Y, Yanagisawa K (1985) Ca2+ sensitivities and transient tension responses to step-length stretches in feline mechanically-stripped single-fibre jaw-muscle preparations. Arch Oral Biol 30:429–432 [DOI] [PubMed] [Google Scholar]
- LaFramboise WA, Guthrie RD, Scalise D, Elborne V, Bombach KL, Armanious CS, Magovern JA (2003) Effect of muscle origin and phenotype on satellite cell muscle-specific gene expression. J Mol Cell Cardiol 35:1307–1318 [DOI] [PubMed] [Google Scholar]
- Lin JJ, Chou CS, Lin JL (1985) Monoclonal antibodies against chicken tropomyosin isoforms: production, characterization, and application. Hybridoma 4:223–241 [DOI] [PubMed] [Google Scholar]
- Lucas CA, Hoh JFY (1997) Extraocular fast myosin heavy chain expression in the levator palpebrae and retractor bulbi muscles. Invest Opthalmol Vis Sci 38:2817–2825 [PubMed] [Google Scholar]
- Maeda N, Hanai H, Kumegawa M (1981) Postnatal development of masticatory organs in rats. II. Effects of hormones on the postnatal development of the M. masseter superficialis. Anat Anz 149:425–436 [PubMed] [Google Scholar]
- McCullagh KJA, Calabria E, Pallafacchina G, Ciciliot S, Serrano AL, Argentini C, Kalhovde JM, et al. (2004) NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc Natl Acad Sci USA 101:10590–10595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM, Marcucio R, Borycki AG, Emerson CP Jr (1999) Differentiation of avian craniofacial muscles. I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Dev Dyn 216:96–112 [DOI] [PubMed] [Google Scholar]
- Oh M, Rybkin II, Copeland V, Czubryt MP, Shelton JM, Van Rooij E, Richardson JA, et al. (2005) Calcineurin is necessary for the maintenance but not embryonic development of slow muscle fibers. Mol Cell Biol 25:6629–6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D, Vrbova G (1985) Invited review: neural control of phenotypic expression in mammalian muscle fibres. Muscle Nerve 8:676–689 [DOI] [PubMed] [Google Scholar]
- Qin H, Hsu MK, Morris BJ, Hoh JFY (2002) A distinct subclass of mammalian striated myosins: structure and molecular evolution of “superfast” or masticatory myosin heavy chain. J Mol Evol 55:544–552 [DOI] [PubMed] [Google Scholar]
- Rana ZA, Gundersen K, Buonanno A (2008) Activity-dependent repression of muscle genes by NFAT. Proc Natl Acad Sci U S A 105:5921–5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Donoghue MJ, Merlie JP, Sanes JR (1996) Distinct regulatory elements control muscle-specific, fiber-type-selective, and axially graded expression of a myosin light-chain gene in transgenic mice. Mol Cell Biol 16:3909–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser PJ, Bicer S, Chen Q, Zhu L, Quan N (2009) Masticatory (‘superfast’) myosin heavy chain and embryonic/atrial myosin light chain 1 in rodent jaw-closing muscles. J Exp Biol 212:2511–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser PJ, Bicer S, Patel R, An Y, Chen Q, Quan N (2010) The myosin light chain 1 isoform associated with masticatory myosin heavy chain in mammals and reptiles is embryonic/atrial MLC1. J Exp Biol 213:1633–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlerson A, Heizmann CW, Jenny E (1983) Type-specific proteins of single IIM fibres from cat muscle. Biochem Biophys Res Commun 113:519–525 [DOI] [PubMed] [Google Scholar]
- Rowlerson A, Pope B, Murray J, Whalen RG, Weeds AG (1981) A novel myosin present in cat jaw-closing muscles. J Muscle Res Cell Motil 2:415–438 [Google Scholar]
- Saeki Y, Kato C, Satomi M, Yanagisawa K (1987) ATPase activity and tension development in mechanically-skinned feline jaw muscle. Arch Oral Biol 32:207–210 [DOI] [PubMed] [Google Scholar]
- Sciote JJ, Rowlerson AM, Carlson DS (1995) Myosin expression in the jaw-closing muscles of the domestic cat and American opossum. Arch Oral Biol 40:405–413 [DOI] [PubMed] [Google Scholar]
- Toniolo L, Cancellara P, Maccatrozzo L, Patruno M, Mascarello F, Reggiani C (2008) Masticatory myosin unveiled: first determination of contractile parameters of muscle fibers from carnivore jaw muscles. Am J Physiol Cell Physiol 295:C1535–1542 [DOI] [PubMed] [Google Scholar]
- Wheeler MT, Snyder EC, Patterson MN, Swoap SJ (1999) An E-box within the MHC IIB gene is bound by MyoD and is required for gene expression in fast muscle. Am J Physiol 276:C1069–1078 [DOI] [PubMed] [Google Scholar]