Abstract
Objective
This study explored the relations between sleep, fatigue and health-related quality of life in a sample of individuals with chronic insomnia.
Methods
A total of 160 adults meeting diagnostic criteria for chronic insomnia underwent three nights of polysomnography (PSG) and completed sleep diaries and questionnaires assessing daytime functioning including fatigue and health-related quality of life.
Results
A cluster analysis was conducted based on PSG-defined sleep disturbances and fatigue severity. A four-cluster solution (R2 = 0.68) was found, classifying individuals as having either (a) both severe sleep disturbance and severe fatigue (n = 15); (b) severe sleep disturbance but milder fatigue (n = 15); (c) milder sleep disturbance but severe fatigue (n = 68) or (d) both milder sleep disturbance and milder fatigue (n = 61). Health-related quality of life was lower in both clusters with severe fatigue compared to those with milder fatigue, and was further decreased when severe sleep disturbances were present. Relations between several indicators of fatigue and health-related quality of life were then examined using factor analysis in order to identify different domains of impairment. A three-factor structure was selected, suggesting that daytime symptoms can be classified as relating to fatigue, physical health, or mental health. These different subtypes of daytime impairment were predicted by distinct sets of variables.
Conclusion
More severe fatigue is not necessarily related to poorer PSG-defined sleep but appears associated with greater impairment in health-related quality of life. Fatigue and health-related quality of life appear to be distinct but interrelated constructs.
Keywords: insomnia, sleep disturbances, fatigue, health-related quality of life, daytime impairment
Introduction
Insomnia has been shown to affect between 6% and 9.5% of adults in the general population (1, 2). Although the presenting complaint of individuals with insomnia is usually focused on their inability to get adequate sleep, the manifestations of insomnia generally extend beyond nighttime sleep to alter the overall sense of well-being and impair the ability to carry on daytime activities. While they are often assumed to be rather mild in severity (3), daytime symptoms are not trivial to individuals suffering from insomnia. In a recent study based on focus group discussions, participants tended to emphasize to a greater extent waking problems such as fatigue, irritability and performance impairments, instead of sleep difficulties per say, when asked to describe their insomnia experience (4). The daytime problems associated with insomnia are also among the main motives for seeking treatment to improve sleep (1) and the perceived lack of understanding from health professionals and relatives regarding the daytime impact of insomnia appears to add up to the feelings of isolation and frustration (4, 5).
The most consistent daytime complaint associated with insomnia is fatigue (3, 6–8). Because sleep is usually acknowledged as an effective countermeasure against fatigue (9, 10), the primary cause of fatigue in individuals with insomnia is often presumed to reside in a lack of appropriate restoration of resources due to disturbed sleep. However, this assumed relationship between sleep and fatigue has been very scarcely studied in the literature. One study conducted in healthy individuals without sleep complaints reported that fatigue was predicted by subjective sleep quality, depressive symptoms and somatization, all of which were better predictors than quantitative sleep parameters such as sleep latency, number of nightly awakenings or frequency of early morning awakenings (11). In another study conducted among both good and poor sleepers, fatigue was significantly correlated to sleep duration, time spent awake during the night and sleep efficiency, but also to sleep-related tension and distress as well as depression and anxiety (12). While these studies tend to support the presumed relation between fatigue and some aspects of sleep disturbances, they also suggest that poor sleep may not be the only pathway to increased feelings of fatigue in individuals with insomnia and that different patterns of relations between sleep disturbances and fatigue may arise. However, previous studies examining these relations have relied on correlations, which are only sensitive to linear relationships between symptoms and consequently prevent identification of individuals presenting different patterns of symptoms. Moreover, these studies are limited by the exclusive use of subjective and retrospective measures of sleep. Perceived sleep is not always concordant with the actual amount and continuity of sleep (13), and subjective sleep estimates can be further influenced by perceived feelings of tiredness experienced during the day, thus potentially leading to an artificially inflated association between subjectively-defined sleep and fatigue. A more refined analysis of the relations between fatigue and sleep disturbances in individuals with insomnia may bring a better understanding of its mechanisms and lead to more targeted interventions.
The objective of this study was to explore the relations between sleep, fatigue and other indicators of daytime functioning in a sample of individuals with insomnia. The first step was to describe different patterns of associations between objective PSG-defined sleep disturbances and fatigue, and then contrast clusters of individuals presenting those different patterns on demographic variables, subjective sleep disturbances and indicators of health-related quality of life. The next step was to identify specific domains of impairment among several indicators of fatigue and health-related quality of life and to investigate predictors of the different domains identified.
Methods
Participants
Participants were individuals with persistent insomnia taking part in a treatment study (14). Only data derived from baseline measures were used for this analysis of daytime symptoms. Inclusion criteria were: (a) being aged 30 years or older and (b) meeting diagnostic criteria for primary insomnia based on the following combined criteria from the DSM-IV-TR (15) and the ICD-10 (16): difficulties initiating and/or maintaining sleep at least 3 nights per week for more than 6 months, accompanied by significant distress or functional impairment.
Exclusion criteria involved : (a) presence of an active and progressive physical illness or neurological degenerative disease; (b) use of medication known to alter sleep; (c) lifetime diagnosis of any psychotic or bipolar disorder; (d) current diagnosis of major depression, dysthymia, or anxiety disorders, unless currently in remission; (e) more than two past episodes of major depression; (f) history of suicide attempt/contemplation within the past year; (g) alcohol or drug abuse within the past year; (h) evidence of sleep apnea or periodic limb movements during sleep; and (i) night-shift work or irregular sleep pattern. The large majority of patients enrolled in this clinical trial suffered from primary insomnia, although patients with comorbid anxiety or affective disorders were also included if these co-existing conditions were not the primary causes of insomnia, and only if they were treated and in remission. Prospective patients using sleep-promoting agents on an occasional basis were enrolled after they had been withdrawn from their medication.
This study was approved by the local ethic committee. Informed written consent was obtained from all participants.
Procedure
Participants were recruited through newspaper advertisements and referrals from outpatient clinics. They underwent a multi-step screening evaluation consisting of a telephone interview, followed by a face-to-face evaluation including the Insomnia Interview Schedule (17) and the Structured Clinical Interview for DSM-IV (SCID-I) (18), as well as a medical history and a physical examination. Sleep diaries and several questionnaires were initially sent by mail after the telephone interview and completed in the two weeks preceding the face-to-face assessment. Participants meeting inclusion and exclusion criteria were then invited in the laboratory for polysomnographic recordings as soon as possible, usually within the following one to three weeks.
Measures
Polysomnography (PSG)
Participants underwent three consecutive nights of PSG recordings. Bedtime and arising time in the sleep laboratory were kept within 30 minutes of the participants’ habitual sleep schedule at home. A standard PSG montage was used and sleep stages were scored by experienced technicians according to standard criteria (19). To rule out sleep apnea and periodic limb movements, respiration and anterior tibialis EMG were also monitored during the first night.
Sleep diaries
Participants kept daily sleep diaries for a two-week period, as well as during the nights spent in the lab.
Insomnia Severity Index (ISI)
The ISI (17) is a 7-item questionnaire assessing the severity of sleep disturbances in the previous month, including sleep-onset, sleep-maintenance, and early morning awakening problems, satisfaction regarding sleep, perceived interference of sleep difficulties with daytime functioning, noticeability of sleep problems by others and distress caused by sleep disturbances. A 5-point Likert scale (“0” = not at all, “4” = extremely) is used to rate each of these items, yielding a total score ranging from 0 to 28.
Multidimensional Fatigue Inventory (MFI)
The MFI (20) includes 20 statements for which participants must indicate, on a 5-point Likert scale, the extent to which the particular item applies to their situation in recent times. The questionnaire covers five dimensions of fatigue: general fatigue, physical fatigue, reduced activity, reduced motivation and mental fatigue. For each scale, the score varies between 4 and 20, with higher scores indicating a higher level of fatigue.
SF-36 Health Survey (SF-36)
The SF-36 (21) includes 36 items based on dichotomous and Likert scales. Individual items are combined to evaluate eight health-related domains: general health, mental health, physical functioning, social functioning, bodily pain, vitality, and restriction of usual activities due to physical problems (role physical) or emotional problems (role emotional). Instructions for this questionnaire do not specify a precise time frame. Total scores range from 0 to 100 for each subscale, with higher scores indicating better functioning.
Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI)
The BDI (22) and BAI (23) each contain 21 items rating depression or anxiety symptoms experienced during the previous week on a 5-point Likert scale (0–4). Total scores range from 0 to 63, with higher scores suggesting higher levels of depression or anxiety symptoms.
Other variables
The presence and nature of past and present psychiatric conditions were assessed in the baseline screening interviews. In addition, number of medical conditions and frequency of physical activity (per week), as reported by participants during screening interview were also used as dependent variables.
Results
Data were entered by two independent research assistants. Distributions were examined for outliers and missing data were investigated using standard procedures (24). No data imputation was done. All analyses were performed using Statistical Analysis System (25). Alpha level was set at 5% bilateral.
Sample description
The final sample included 160 participants (97 women, 63 men) with a mean age of 50.3 years old (SD = 10.1; range, 30–72) and a mean education of 14.7 years (SD = 3.5). The majority (73.8%) reported mixed insomnia (i.e., sleep-onset, maintenance and/or terminal). The average insomnia duration was 16.4 years (SD = 13.6). Twenty-four participants (15%) had at least one psychiatric diagnosis at baseline (75% with anxiety disorders), 62 (38.8%) had at least one past psychiatric diagnosis (68% with major depressive episodes) and 92 (57.5%) presented at least one comorbid medical disorder (most commonly a cardio-vascular condition). Average scores on the MFI and the SF-36 subscales were all within one standard deviation from published normative values (26), although the means were slightly elevated for the general fatigue, mental fatigue and decreased motivation subscales of the MFI and slightly decreased for the role physical, vitality, social functioning and mental health subscales of the SF-36.
Patterns of associations between sleep and fatigue
In order to identify patterns of association between sleep and fatigue, a data reduction step was first necessary to derive a rating of the relative severity of each participant’s symptoms for: (a) subjective fatigue and (b) PSG-defined sleep disturbance. To this end, two composite z-scores were created for each participant. In order to compute the composite z-score for fatigue, individual raw scores on each of the five MFI subscales were standardized into z-scores against the whole sample means. The composite fatigue z-score was then obtained by averaging z-scores of the five MFI subscales. To derive the composite z-score for sleep disturbance, individual raw scores for mean sleep onset latency (SOL), wake after sleep onset (WASO) and total sleep time (TST) for the three nights spent in the lab were standardized into z-scores against the whole sample mean (TST z-scores were reversed so that a positive z-score reflected a shorter sleep duration). Z-scores for SOL, WASO and TST were then averaged into a composite sleep disturbance z-score. A greater z-score indicates greater disturbance (either more severe fatigue or more severe sleep disturbance).
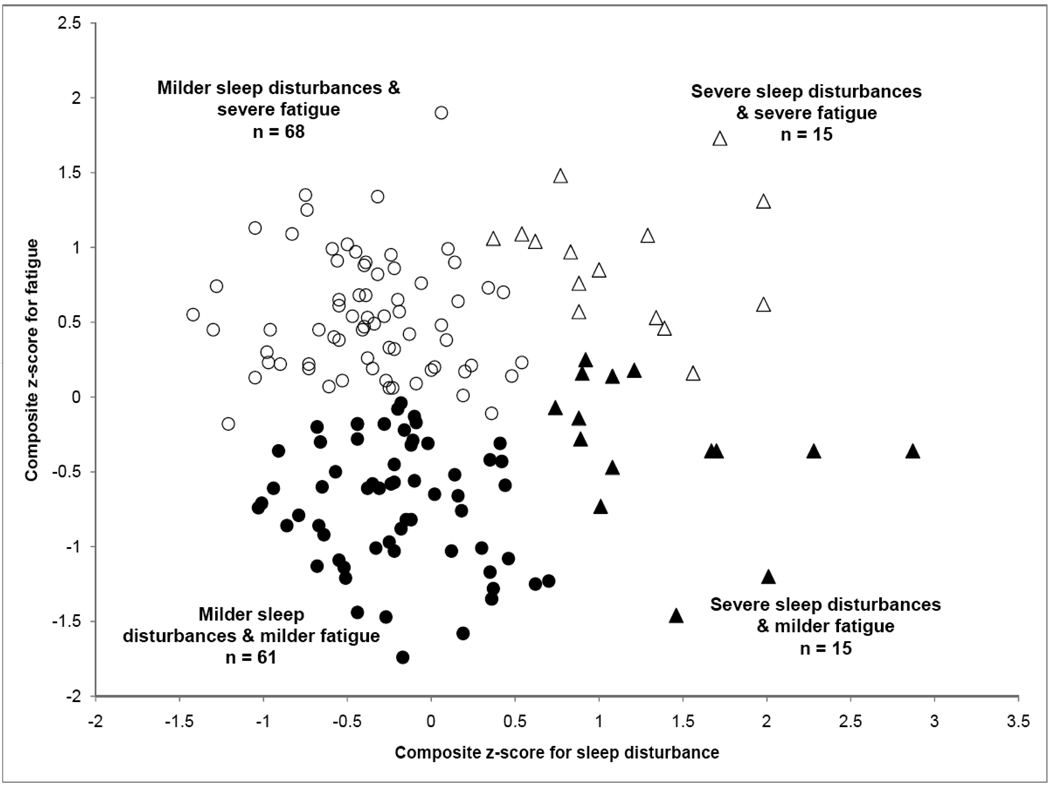
The next step was to identify natural groupings of participants presenting similar patterns of association between fatigue and PSG-defined sleep disturbance. Fatigue composite z-scores and sleep disturbance composite z-scores were entered into a hierarchical cluster analysis using Ward’s method. This was preferred over a median split approach because it has the advantage of allowing identification of natural subgroups of different sizes and variability. Solutions ranging from two to six clusters were investigated. A four-cluster solution was retained based on cluster sizes, parsimony and clinical significance of the solution. These four clusters were mutually exclusive so that individuals were classified as having either (a) both severe sleep disturbance and severe fatigue (n = 15); (b) severe sleep disturbance but milder fatigue (n = 15); (c) milder sleep disturbance but severe fatigue (n = 68) or (d) both milder sleep disturbance and milder fatigue (n = 61). The distribution of participants according to their composite sleep disturbance z-score, composite fatigue z-scores and clusters is illustrated in Figure 1.
Figure 1.
Data distribution and clusters according to the composite sleep disturbance and fatigue z-scores.
Demographic, sleep and health-related quality of life differences between clusters
Clusters were compared on demographics, sleep and health-related quality of life measures using one-way ANOVAs and chi-square tests. Post-hoc comparisons were completed when appropriate using Ryan-Einot-Gabriel-Welsh (REG-W) tests. Means and standard deviations for demographic variables for each of the four clusters are shown in Table 1. Clusters did not differ on age, gender distribution, insomnia duration or insomnia subtype, but were significantly different on occupation.
Table 1.
Descriptive data of sociodemographic, sleep and fatigue variables by cluster.
| Milder sleep disturbance & milder fatigue (n = 61) n (%) |
Milder sleep disturbance & severe fatigue (n = 68) n (%) |
Severe sleep disturbance &milder fatigue (n = 15) n (%) |
Severe sleep disturbance & severe fatigue (n = 15) n (%) |
|||
|---|---|---|---|---|---|---|
| Gender | x2 (3, N=159) = 0.69 | p = .89 | ||||
| Female | 35 (57.4%) | 43 (63.2%) | 9 (60.0%) | 10 (61.0%) | ||
| Male | 26 (42.6%) | 25 (36.8%) | 6 (40.0%) | 5 (33.3%) | ||
| Occupation | x2 (3, N=159) = 8.88 | p = .03* | ||||
| Working | 44 (74.6%) a, b | 55 (82.1%) b | 8 (53.3%) a | 8 (53.3%) a | ||
| Not working / Retired | 15 (25.4%) a, b | 12 (17.9%) b | 7 (46.7%) a | 7 (46.7%) a | ||
| Marital Status | x2 (9, N=159) = 3.55 | p = .93 | ||||
| Single | 6 (8.8%) | 4 (6.6%) | 2 (13.33%) | 2 (13.33%) | ||
| Married / Common-law relationship |
45 (66.2%) | 43 (70.5%) | 10 (66.7%) | 11 (73.33%) | ||
| Divorced / Separated | 12 (17.7%) | 10 (16.4%) | 3 (16.4%) | 2 (13.33%) | ||
| Widowed | 5 (7.4%) | 4 (6.6%) | 0 (0%) | 0 (0%) | ||
| Type of insomnia | x2 (9, N=159) = 4.56 | p = .87 | ||||
| Initial | 3 (4.5%) | 1 (1.5%) | 0 (0%) | 0 (0%) | ||
| Middle | 13 (21.3%) | 18 (26.5%) | 2 (13.3%) | 3 (20.0%) | ||
| Late | 1 (1.6%) | 1 (1.5%) | 0 (0%) | 0 (0%) | ||
| Mixed | 44 (72.1%) | 48 (70.6%) | 13 (86.7%) | 12 (80.0%) | ||
| M (SD) | M (SD) | M (SD) | M (SD) | |||
| Age (years) | 52.4 (9.4) | 47.5 (10.2) | 53.3 (9.3) | 51.7 (10.2) | F (3, 155) = 3.30 | p = .02* |
| Education (years) | 14.6 (3.8) a, b | 14.8 (3.2) a, b | 16.4 (2.4) a | 13.1 (4.9) b | F (3, 154) = 2.28, | p = .08 |
| Insomnia duration (years) | 15.62 (13.29) | 16.40 (13.12) | 18.20 (18.10) | 16.52 (13.45) | F (3, 155) = 0.14 | p = .93 |
| PSG variables | ||||||
| SOL | 14.78 (6.39) a | 13.83 (7.50) a | 26.39 (12.04)b | 33.28 (16.90) c | F (3, 155) = 26.47 | p < .001** |
| WASO | 54.67 (22.41) a | 50.07 (25.19) a | 118.61 (36.14) c | 96.19 (39.57) b | F (3, 155) = 35.93 | p < .001** |
| TST | 367.54 (29.26) a | 379.51 (33.57) a | 285.04 (45.92) c | 316.31 (31.51) b | F (3, 155) = 43.16 | p < .001** |
| % SE | 83.70 (5.33) a | 84.76 (5.40) a | 65.25 (7.26) c | 69.56 (6.14) b | F (3, 155) = 74.38 | p < .001** |
| NWAK | 12.52 (4.02) a | 10.88 (4.28) a | 12.91 (3.84) a | 17.01 (5.07) b | F (3, 155) = 8.96 | p < .001** |
| % Stage 1 | 5.34 (2.66) a | 4.61 (2.63) a | 8.27 (5.99) b | 6.68 (3.61) a,b | F (3, 155) = 6.27 | p < .001** |
| % Stage 2 | 64.90 (5.65) | 63.79 (7.45) | 63.67 (6.14) | 66.50 (4.76) | F (3, 155) = 0.91 | p = .44 |
| % Stages 3–4 | 5.92 (5.50) | 8.01 (7.12) | 8.68 (9.88) | 6.91 (5.29) | F (3, 155) = 1.33 | p = .27 |
| % REM | 12.52 (4.02) a | 10.88 (4.28) a | 12.91 (3.84) a | 17.01 (5.07) b | F (3, 155) = 7.68 | p < .001** |
| Composite z-score | −0.21 (0.42) a | −0.37 (0.44) a | 1.38 (0.62) b | 1.14 (0.51) b | F (3, 155) = 94.71 | p < .001** |
| MFI subscales | ||||||
| General fatigue | 9.75(2.41) a | 14.40 (2.44) b | 10.73 (3.19) a | 15.47 (1.77) b | F (3, 155) = 48.97 | p < .001** |
| Physical fatigue | 6.79(2.35) a | 10.79 (2.61) b | 7.00 (2.20) a | 12.80 (3.23) c | F (3, 155) = 40.90 | p < .001** |
| Reduced activity | 6.66 (1.78) a | 10.38 (2.03) c | 8.60 (2.53) b | 13.80 (2.86) d | F (3, 155) = 62.48 | p < .001** |
| Reduced motivation | 6.98 (2.14) a | 10.59 (2.59) c | 8.60 (2.53) b | 9.93 (2.52) b,c | F (3, 155) = 24.90 | p < .001** |
| Mental fatigue | 7.92 (2.64) a | 12.56 (3.18) b | 9.27 (2.68) a | 12.93 (2.91) b | F (3, 155) = 31.73 | p < .001** |
| Composite z-score | −0.72 (−0.41) a | 0.54 (0.40) b | −0.34 (.49) c | 0.91 (0.41) d | F (3, 155) = 21.74 | p < .001** |
p < .05
p < .01
Note. Different letters indicate significant differences between clusters.
Means and standard deviations of PSG variables and sleep disturbance composite z-score as well as MFI subscales scores and fatigue composite z-score are also displayed in Table 1. As expected given the method used for cluster identification, differences among clusters were obtained for many of these variables, hence validating the classification procedure.
Means and standard deviations for sleep diary variables, health-related quality of life ratings on the SF-36 and perceived degree of interference of insomnia with daytime functioning for each cluster are presented in Table 2. One-way ANOVAs indicated the presence of significant differences among clusters for diary-derived SOL, WASO, TWT, TST, percent SE and subjective sleep quality ratings. Clusters did not differ regarding early morning awakenings (EMA) or time in bed (TIB). Overall, participants classified within the severe (PSG-defined) sleep disturbances and severe fatigue cluster presented greater subjective sleep disturbances compared to the other three clusters.
Table 2.
Descriptive data of demographic, sleep and fatigue variables by cluster.
| Milder sleep disturbance & milder fatigue (n = 61) M (SD) |
Milder sleep disturbance & severe fatigue (n = 68) M (SD) |
Severe sleep disturbance & milder fatigue (n = 15) M (SD) |
Severe sleep disturbance & severe fatigue (n = 15) M (SD) |
|||
|---|---|---|---|---|---|---|
| Sleep diary (14 days) | ||||||
| SOL | 30.13 (31.58) a | 28.77 (22.65) a | 36.71 (30.06) a | 64.79 (49.79) b | F (3, 155) = 6.27 | p < .001** |
| WASO | 57.06 (29.24) a | 59.23 (28.69) a | 76.74 (47.57) b | 95.12 (65.81) b | F (3, 155) = 5.56 | p = .001** |
| EMA | 57.31 (37.82) | 63.16 (40.28) | 46.08 (42.67) | 65.93 (67.83) | F (3, 155) = 0.82 | p = .48 |
| TWT | 144.80 (61.58) a | 151.02 (55.49) a | 159.51 (65.77) a | 225.84 (121.84) b | F (3, 155) = 6.08 | p < .001** |
| TIB | 495.13 (42.00) | 499.05 (35.00) | 504.70 (67.05) | 504.70 (43.15) | F (3, 155) = 0.81 | p = .49 |
| TST | 350.98 (57.93) a | 352.51 (65.31) a | 346.42 (59.40) a | 293.73 (116.53) b | F (3, 155) = 3.23 | p = .02* |
| % sleep efficiency | 70.96 (11.80) a | 69.59 (11.83) a | 68.67 (12.18) a | 55.65 (23.21) b | F (3, 155) = 5.52 | p = .001** |
| Sleep quality | 2.92 (0.52) a | 2.71 (0.54) a, b | 3.06 (0.60) a | 2.46 (0.61) b | F(3, 155) = 4.67 | p = .003** |
| SF-36 subscales | ||||||
| General health | 85.91 (13.09) a | 74.62 (16.11) b | 80.20 (12.04) a, b | 60.67 (21.63)c | F (3, 155) = 13.07 | p < .001** |
| Physical functioning | 93.43 (6.57) a | 86.47 (12.81) a | 93.00 (7.02) a | 70.37 (29.63) b | F (3, 155) = 15.40 | p < .001** |
| Role physical | 85.66 (27.56) a | 52.94 (38.52) b | 76.67 (30.57) a | 29.44 (36.17) c | F (3, 155) = 16.66 | p < .001** |
| Bodily pain | 77.82 (15.89) a | 67.25 (20.32) a | 74.80 (13.20) a | 52.60 (22.89) b | F (3, 155) = 8.91 | p < .001** |
| Vitality | 65.22 (15.24) a | 38.82 (15.77) b | 57.33 (15.68) a | 36.00 (13.40) b | F (3, 155) = 37.23 | p < .001** |
| Social functioning | 86.89 (19.15) a | 66.54 (19.73) b, c | 76.67 (17.59) a, b | 59.17 (26.50) c | F (3, 155) = 14.32 | p < .001** |
| Role emotional | 89.62 (26.20) a | 66.92 (34.40) b | 73.33 (42.16) a, b | 44.44 (43.03) c | F (3, 155) = 9.45 | p < .001** |
| Mental health | 76.67 (12.47) a | 67.20 (12.50) b | 71.47 (14.80) a, b | 63.46 (13.56) b | F (3, 155) = 11.69 | p < .001** |
| Insomnia Severity Index | ||||||
| Interference of insomnia | 2.07 (0.77) a | 2.85 (0.72) b, c | 2.40 (0.91) a, b | 2.93 (0.73) c | F(3, 155) = 12.90 | p < .001** |
p < .05
p < .01
Note. Different letters indicate significant differences between clusters.
Regarding health-related quality of life, one-way ANOVAs indicated significant cluster differences for each of the eight subscales of the SF-36. Clusters also differed regarding the perceived degree of interference of insomnia with daytime functioning as measured by an item from the ISI. Participants classified within either cluster with severe fatigue generally exhibited lower health-related quality of life and reported a greater interference of their insomnia with their daily activities compared to participants included in clusters with milder fatigue.
Domains of daytime impairment among indicators of fatigue and health-related quality of life
An exploratory factor analysis was performed on 14 indicators of daytime functioning (five MFI subscales; eight SF-36 subscales; ISI interference item). In order to standardize the direction of scores on these indicators, scores on the SF-36 subscales were reversed to insure that higher scores would indicate greater impairment. To allow for interdependency between factors, the promax oblique rotation method was preferred over traditional varimax method. Factorial structures involving two, three, and four factors were investigated and a three-factor structure was selected based on parsimony (Thurstone’s simple structure), clinical interpretability, and percentage of variance explained by each factor. Semi-partial correlations (controlling for shared variance between factors) between the indicators and each of the three factors, as well as communalities and explained variance, are presented in Table 3.
Table 3.
Semi-partial correlations between indicators of fatigue or quality of life and each of the three factors identified with the exploratory factor analysis
| Indicator | F1a | F2 | F3 | h2 |
|---|---|---|---|---|
| MFI general fatigue | .66 | .02 | .01 | .81 |
| MFI physical fatigue | .49 | .32 | −.16 | .72 |
| MFI mental fatigue | .35 | −.01 | .15 | .33 |
| MFI reduced activities | .41 | .15 | .00 | .45 |
| MFI reduced motivation | .37 | −.17 | .28 | .42 |
| SF-36 physical functioning | −.07 | .66 | .01 | .54 |
| SF-36 role physical | .17 | .31 | .26 | .48 |
| SF-36 bodily pain | −.05 | .50 | .07 | .34 |
| SF-36 general health | .13 | .52 | −.01 | .54 |
| SF-36 vitality | .62 | −.03 | .09 | .75 |
| SF-36 social functioning | .26 | .09 | .37 | .53 |
| SF-36 role emotional | .02 | .07 | .61 | .52 |
| SF-36 mental health | .08 | .00 | .56 | .49 |
| ISI interference | .39 | −.03 | .19 | .41 |
| Percent of variance b | 12.57 | 8.64 | 7.64 |
Factor labels: F1 = fatigue; F2 = physical health; F3 = mental health.
Because of the use of the oblique rotation method, an additional 23.36% of explained variance is unaccounted for in the reported percentages of variance and can be attributed to shared variance between factors.
Note. Variables in each factor are those displayed in larger bold font under the factor’s column. Prior to the analysis, SF-36 subscales were reversed, higher scores indicative of greater impairment, as it is the case for the MFI subscales and the ISI item.
Factor 1, labeled “fatigue”, included all five MFI subscales, the SF-36 vitality subscale, and the ISI interference item. Factor 2 included four SF-36 subscales: physical functioning, role physical, bodily pain, and general health and was labeled “physical health”. The remaining three SF-36 subscales, social functioning, role emotional, and mental health, formed Factor 3, labeled “mental health”. Inter-factor correlation coefficients (r) were .55 between fatigue and physical health factors, .49 between fatigue and mental health, and .22 between physical and mental health.
Predictors of daytime impairment domains
An aggregate standardized score was derived for each factor. Standard linear regressions were then performed using the aggregate standardized score of each factor as dependent variables. Potential predictors were: age, gender, occupation, insomnia duration, sleep disturbance composite z-score from sleep diary (i.e., combining SOL, WASO, EMA, and reversed TST), TIB from sleep diary, sleep disturbance composite z-score from PSG (i.e., combining SOL, WASO, and reversed TST), presence of past, and current psychiatric diagnoses, BDI and BAI total scores, number of medical conditions, and frequency of physical activity. These predictors were selected based either on their previous association with fatigue and health-related quality of life in the literature (e.g., indicators of sleep disturbance, depression and anxiety symptoms) or because they could potentially represent coping behavior in reaction to fatigue or decreased health-related quality of life (e.g., longer/shorter TIB, higher/lower frequency of physical activity). Three separate standard linear regressions were performed with the three factors as dependent variables. Results of these analyses are displayed in Table 4. Percentages of variance explained by the linear regression models (derived from Adjusted-R2) were 43.2% for the regression with fatigue (factor 1) as the dependent variable, 33.7% for physical health (factor 2), and 42.0% for mental health (factor 3). For the fatigue dimension, a higher level of depressive symptoms on the BDI, younger age, and more severe sleep disturbance on the sleep diary significantly predicted a higher level of fatigue. Three variables significantly predicted the composite score of the physical health factor: age, number of medical conditions, and BAI total score. Four predictors were marginally significant: BDI total score (p = .05), TIB from sleep diary (p = .05), past psychiatric diagnosis (p = .06), and occupation (p = . 06). The only negative relations were with age and occupation, meaning that a younger age and being unemployed and not studying were associated with a higher level of physical health impairment. Finally, for the mental health factor, four significant predictors were found: BDI total score, age, number of medical conditions, and frequency of physical activity, while BAI total score was marginally significant (p = .09). Only age had a negative relationship with the dependent variable.
Table 4.
Results of the standard linear regressions performed on each factor of the exploratory factor analysis
| Regression for F1 | Regression for F2 | Regression for F3 | ||||
|---|---|---|---|---|---|---|
| Β | 95% C.I. | β | 95% C.I. | Β | 95% C.I. | |
| Sleep disturbance composite z-score (diary) | .17** | .03 – .32 | .09 | −.07 – .25 | .08 | −.06 – .23 |
| TIB (diary; minutes) | .10 | −.04 – .25 | .15 * | .00 – .31 | .11 | −.03 – .26 |
| Sleep disturbance composite z-score (PSG) | .08 | −.07 – .22 | .10 | −.06 – .26 | .06 | −.09 – .21 |
| BAI total score | .12 | −.06 – .29 | .21 ** | .02 – .40 | .15 * | −.02 – .33 |
| BDI total score | .41 **a | .24 – .58 | .18 *b | .00 – .36 | .37 **a | .20 – .54 |
| Insomnia duration (years) | .00 | −.14 – .14 | −.10 | −.25 – .05 | −.11 | −.25 – .03 |
| Frequency of physical activity (# days/week) | .04 a | −.10 – .18 | −.03 a | −.18 – .13 | .21 **b | .06 – .35 |
| Number of medical conditions | .12 | −.03 – .27 | .26 ** | .10 – .42 | .26 ** | .11 – .41 |
| Age (years) | −.43 ** | −.62 – −.24 | −.37 ** | −.57 – −.17 | −.33 * | −.52 – −.14 |
| Gender (male) | −.09 | −.24 – .06 | .00 | −.16 – .16 | −.07 | −.22 – .08 |
| Occupation (working/studying) | −.06 ab | −.26 – .13 | −.20 *a | −.41 – .01 | .01 b | −.19 – .21 |
| Current psychiatric diagnosis (having one) | .10 | −.04 – .24 | −.02 | −.18 – .13 | .11 | −.03 – .25 |
| Past psychiatric diagnosis (having one) | .10 | −.04 – .24 | .15 * | .00 – .30 | .02 | −.12 – .16 |
Significant predictors at **p < .05;
p < .10.
Note. 95% C.I. = 95% confidence intervals for β values. β values on the same row with different subscripts differ significantly at p < .05.
Discussion
This study explored the relations between sleep disturbances, fatigue and other indicators of daytime functioning in a sample of individuals with chronic insomnia. Results revealed different patterns of associations between sleep and fatigue among individuals with insomnia, suggesting that more severe sleep disturbances, as defined by PSG, are not systematically associated with more severe fatigue. Participants with more severe fatigue exhibited lower health-related quality of life compared to those with milder fatigue. However, among participants with similar levels of fatigue, those with more severe sleep disturbances were more impaired on health-related quality of life. These results thus suggest a close relation between greater fatigue and decreased health-related quality of life.
The second step of this study was to examine the relationships among indicators of fatigue and health-related quality of life. Results suggest the existence of three general dimensions underlying self-reported daytime symptoms in our sample of individuals with insomnia: fatigue, physical health impairments and mental health impairments. Subjectively rated fatigue and health-related quality of life thus appear to be two inter-related but distinct constructs that seem associated with different sets of variables. Younger age and higher level of depressive symptoms were among the strongest predictors for all three domains of impairment. Subjective sleep quality predicted fatigue but did not predict either physical or mental health impairments, whereas anxiety and number of medical conditions predicted both physical and mental health impairments, but not fatigue. PSG-defined sleep disturbances did not predict impairment in any of the three areas of daytime symptoms, neither did insomnia duration, gender nor the presence of a current psychiatric diagnosis.
The presence of different patterns of relations between sleep and fatigue among individuals with insomnia suggests that fatigue may not be solely related to greater objective sleep disturbance: severe fatigue was found in individuals with both severe and milder sleep disturbances. In fact, in the sample studied, fatigue was not predicted by PSG-defined sleep but was instead related to greater subjective sleep disturbances. This finding is consistent with previous work suggesting that fatigue may be more closely related to subjective sleep quality rather than sleep duration and continuity. It could suggest that the perception of sleep quality is influenced by feelings of fatigue or exhaustion upon awakening or during the day. It may also be that sleep continuity and duration are not the most relevant determinants of fatigue complaints: other aspects pertaining to sleep quality may be useful as well. Indeed, differences in electrophysiological and metabolic brain activity during sleep have been found in individuals with insomnia, even in the absence of sleep disturbances on standard PSG. For example, one study suggested that wake-promoting brain structures failed to show the normal decline in metabolism from wakefulness to sleep in individuals with insomnia (27).This was paralleled by a finding of reduced metabolism in the prefrontal cortex during wakefulness, which could very well underlie complaints of fatigue during the day.
Alternatively, different patterns of associations between fatigue and sleep among individuals with insomnia could also reflect a manifestation of individual differences in reactivity to sleep loss. Such individual differences have been documented in sleep deprivation studies conducted with healthy individuals (28). Specific correlates of sleep disturbances may differ among individuals with insomnia as well. The present study thus emphasizes the need to investigate different possible pathways to fatigue among individuals with insomnia in order to reach a clearer understanding of its mechanisms. For example, the presence of fatigue in the absence of severely disturbed sleep has been hypothesized to result from increased arousal (29). Findings also suggest that greater impairments in all three domains of daytime functioning evaluated are associated to younger age and higher levels of depressive symptoms, but not to PSG-defined sleep, presence of a current psychiatric disorder or insomnia duration. While this is consistent with the multidimensional nature of fatigue and its complex relations to a large number of variables (11, 12), the contribution of individual reactivity to sleep loss, arousal level, depressive symptoms and other variables to fatigue and other daytime impairments experienced by individuals with insomnia warrants more research. Although insomnia phenotypes (psychophysiological, idiopathic and paradoxical) were not formally differentiated in this study, they may also be relevant to understand differences in patterns of associations between sleep and fatigue.
All participants included in this sample met the diagnostic criteria for primary insomnia, and all clusters exhibited average values for PSG-defined SOL, WASO and TST suggestive of significant sleep continuity disturbances. However, the distribution of sleep disturbance composite z-scores indicates that a small subset of participants experienced much more severe sleep disturbances compared to others. This finding could reflect the normal distribution of PSG-defined sleep disturbances among individuals with primary insomnia. Alternatively, it could also suggest the existence of a clinically distinct subgroup of individuals whose sleep continuity is significantly more impaired. More research will be necessary to clarify the meaning of this result.
Results from the present study are limited by the nature of daytime measures available for the analyses. For example, except for the ISI interference item, indicators used in the factor analysis were subscales from two validated measures (MFI and SF-36) and the factors identified remain largely similar, although not identical, to the subscales used in these questionnaires. While this has the merit of confirming these groupings of items in a sample of individuals with insomnia, results should nonetheless be replicated with measures covering a broader range of daytime functioning variables (e.g., cognitive functioning, absenteeism and reduced productivity, impaired social/familial relationships, sleepiness, etc.). The use of objective measures (e.g., neuropsychological tests) would also be very informative in this regard, as would prospective assessments, which have shown to be more closely related to sleep disturbances in a previous study (30). Similarly, the present study relied on PSG to quantify sleep disturbances. Sleep recorded during the three nights spent in the laboratory may not be representative of the sleep usually obtained at home and it is possible that different patterns of associations would have been obtained using subjective sleep assessments. While the decision to use PSG-defined sleep continuity arose from the wish to examine the relation between physiologically-defined sleep and fatigue, the use of both subjective and objective measures covering not only sleep continuity and quantity but also sleep quality may be necessary to reach a better understanding of the complex interplay between sleep disturbances and fatigue.
Aside from these limits, the current findings suggest that, in clinical practice, the assessment of daytime impairment associated with insomnia should cover many areas of functioning, including different aspects of fatigue, physical health and mental health. Similarly, as sleep disturbances do not seem to account entirely for daytime impairment, individuals with insomnia may benefit from treatments directly addressing daytime fatigue as well as impairments in physical and psychological functioning.
Acknowledgements
This research was supported by the National Institute of Mental Health (MH60413). The first and second authors are supported by the Canadian Institutes of Health Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Riedel BW, Lichstein KL. Insomnia and daytime functioning. Sleep Med Rev. 2000;4(3):277–298. doi: 10.1053/smrv.1999.0074. [DOI] [PubMed] [Google Scholar]
- 4.Carey TJ, Moul DE, Pilkonis P, Germain A, Buysse DJ. Focusing on the experience of insomnia. Behav Sleep Med. 2005;3(2):73–86. doi: 10.1207/s15402010bsm0302_2. [DOI] [PubMed] [Google Scholar]
- 5.Kyle SD, Espie CA, Morgan K. A qualitative analysis of daytime functioning and quality of life in persistent insomnia using focus groups and audio-diaries. Sleep. 2008;31:A243. Abstract Supplement: [Google Scholar]
- 6.Leger D, Stal V, Guilleminault C, Raffray T, Dib M, Paillard M. Les consequences diurnes de l'insomnie: impact sur la qualite de vie. Rev Neurol (Paris) 2001;157(10):1270–1278. [PubMed] [Google Scholar]
- 7.Moul DE, Nofzinger EA, Pilkonis PA, Houck PR, Miewald JM, Buysse DJ. Symptom reports in severe chronic insomnia. Sleep. 2002;25(5):553–563. [PubMed] [Google Scholar]
- 8.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999;22 Suppl 2:S354–S538. [PubMed] [Google Scholar]
- 9.Aaronson LS, Teel CS, Cassmeyer V, Neuberger GB, Pallikkathayil L, Pierce J, et al. Defining and measuring fatigue. Image J Nurs Sch. 1999;31(1):45–50. doi: 10.1111/j.1547-5069.1999.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 10.Aaronson LS, Pallikkathayil L, Crighton F. A qualitative investigation of fatigue among healthy working adults. West J Nurs Res. 2003;25(4):419–433. doi: 10.1177/0193945903025004007. [DOI] [PubMed] [Google Scholar]
- 11.Lavidor M, Weller A, Babkoff H. How sleep is related to fatigue. Br J Health Psychol. 2003 Feb;8(Pt 1):95–105. doi: 10.1348/135910703762879237. [DOI] [PubMed] [Google Scholar]
- 12.Alapin I, Fichten CS, Libman E, Creti L, Bailes S, Wright J. How is good and poor sleep in older adults and college students related to daytime sleepiness, fatigue, and ability to concentrate? J Psychosom Res. 2000;49(5):381–390. doi: 10.1016/s0022-3999(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 13.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976 Dec;133(12):1382–1388. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 14.Morin CM, Vallieres A, Guay B, Ivers H, Savard J, Merette C, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009 May 20;301(19):2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association (APA) Washington, DC: American Psychiatric Association; Diagnostic and statistical manual of mental disorders : DSM-IV-TR. (4th ed.) 2000
- 16.World Health Organization (WHO) The ICD-10 classification of mental and behavioral disorder: diagnostic criteria for research (10th revision) Geneva: World Health Organization; 1992
- 17.Morin CM. Insomnia : psychological assessment and management. New York: Guilford Press; 1993. [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) New York, NY: New York State Psychiatric Institute; 1996. [Google Scholar]
- 19.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human sleep subjects. U.S. Government Printing Office; 1968 doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed]
- 20.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Kosinski M, Keller SK. SF-36® Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 22.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988 Dec;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 24.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th edition ed. Boston: Allyn and Bacon; 2007. [Google Scholar]
- 25.Institute S. SAS/STAT 9.1 User's Guide. Cary, NC: SAS Institute; 2004. [Google Scholar]
- 26.Watt T, Groenvold M, Bjorner JB, Noerholm V, Rasmussen NA, Bech P. Fatigue in the Danish general population. Influence of sociodemographic factors and disease. J Epidemiol Community Health. 2000 Nov;54(11):827–833. doi: 10.1136/jech.54.11.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004 Nov;161(11):2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 28.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005 Apr 1;28(4):479–496. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 29.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1(2):97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 30.Buysse DJ, Thompson W, Scott J, Franzen PL, Germain A, Hall M, et al. Daytime symptoms in primary insomnia: a prospective analysis using ecological momentary assessment. Sleep Med. 2007 Apr;8(3):198–208. doi: 10.1016/j.sleep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]