Abstract
Objectives
We compared Inter alpha Inhibitor proteins (LALP) levels in infants with proven necrotizing enterocolitis (NEC) and with infants who had other, non-specific abdominal disorders.
Study design
A prospective observational study of infants in the NICU. NEC was diagnosed according to Bell’s staging criteria. Infants in the control group had non-specific abdominal disorders but no radiographic evidence of NEC and no disease progression. All infants with radiographic NEC were included. Plasma IaIp was quantitated using ELISA.
Results
Seventeen infants had confirmed NEC and 34 infants had non-specific disorders that improved rapidly. Gestational age, postnatal age, weight, sex, maternal obstetric variables, rupture of membranes and mode of delivery did not differ. Mean IaIp level in the NEC group was significantly lower (137±38 mg/L, 95% CI=118–157) than the control group (258±53 mg/L, 95%CI: 238–277), p < 0.0001.
Conclusions
The demonstration that IaIp are significantly reduced in neonates with NEC suggests LALP serve useful as a sensitive biomarker, allowing patients to be placed on appropriate therapy and reducing antibiotic overuse in infants with suspected but unproven NEC. Administration of LALP may significantly reduce the severity of systemic inflammation and associated tissue injury.
Keywords: Necrotizing enterocolitis, inter alpha inhibitor, serine protease inhibitors, serpins
Necrotizing enterocolitis (NEC) is an acute inflammatory condition of the gastrointestinal tract resulting in intestinal necrosis, systemic sepsis and multi-system organ failure found mostly in infants born prematurely (1–3). Despite advances in medical care of low birth weight infants over the past decades, the etiology remains elusive and morbidity and mortality are still unacceptably high. Clinically, NEC poses major diagnostic challenges because early warning signs and symptoms are often nonspecific. The search for useful biomarkers based on cytokines or other mediators continues as none have shown sufficient sensitivity and specificity for clinical use (4).
Inter-alpha LALP are a family of structurally related serine protease inhibitors found in relatively high concentration in human plasma (5). Substantial evidence suggests that IaIp play an important regulatory role in systemic inflammation (6). The high level of IaIp circulating normally in plasma suggests that the proteins are essential. No person with complete absence of IaIp has ever been detected (7). There is a profound decrease in plasma IaIp levels in both adult and newborn patients with clinically proven sepsis and the levels correlated with the disease severity and frequency of mortality (5,8–10). Our most recent studies demonstrate that IaIp is a reliable diagnostic marker with high sensitivity, specificity and predictive value in detection of neonatal sepsis (9). The markedly decreased plasma levels during sepsis and concomitant increase of IaIp-related fragments in the urine demonstrate that these proteins are ‘consumed’ and rapidly cleared from the systemic circulation during sepsis. Likewise, hepatic IaIp biosynthesis is down regulated during severe inflammation. As such, IaIp is considered a typical negative acute phase protein (10). Since systemic host inflammatory responses are strongly involved in NEC, we hypothesized that, if inflammatory responses are involved in the pathogenesis, IaIp levels might be a useful biomarker in the diagnosis of NEC. We sought, therefore, to examine IaIp levels in infants with proven NEC and to compare the levels with infants who had other, non-specific abdominal disorders.
METHODS
This was a prospective, cohort-based observational study carried out in the neonatal intensive care unit (NICU) at Women and Infants Hospital of Rhode Island from September 2007 to January 2009 with approval of the Institutional Review Board. During the study period, there were 1817 patients admitted to the NICU. Of these, 136 were less than 1500 g birth weight. The infants in the control group consisted of neonates who presented with non-specific abdominal findings including feeding intolerance, increased gastric aspirates, abdominal distention, and/or abdominal tenderness. The initial clinical presentations were worrisome enough to warrant cessation of feedings, performance of abdominal radiographs, and obtaining blood for a complete blood count (CBC) and blood culture. An independent pediatric radiologist who was unaware of the group assignment interpreted all abdominal radiographs. Clinical manifestations and radiographic findings were used to establish the stage of NEC according to the modified Bell’s staging criteria (10).For these staging criteria, the radiographic findings of peumatosis intestinalis with significant intestinal dilation and ileus with or without portal vein gas or ascites are defined as NEC stage II. The additional findings of ascites and/or bowel perforation is defined as advanced NEC, stage III. In stage I, radiographic findings are limited to mild intestinal dilation and dysmotility. All newborn infants demonstrated to have Stage II or III NEC were included in the “NEC” group.
All patients had blood collected at the time of initial presentation and clinical evaluation. Plasma IaIp levels were measured quantitatively on residual blood using a competitive enzyme-linked immunosorbent assay with a monoclonal antibody against human IaIp (MAb 69.26), as previously described (5,8,9). The assay has a sensitivity of 50 mg/L and a linear dynamic range up to 750 mg/L. The intra-assay variability (CV) is less than 3% and the inter-assay CV is less than 7%. Clinical and demographic variables were recorded for the infants including gestational age, postnatal age, weight, and sex. All maternal medical and obstetrical variables were recorded including hypertensive disease of pregnancy, prolonged rupture of membranes (defined as >24 hours), and mode of delivery.
Statistical Analysis
The two groups were compared using unpaired t tests, Mann-Whitney U tests, or χ2 tests when appropriate. Statistical calculations were performed using StatisticaTm and MedCalcTm software (Belgium, vers 10.4)
RESULTS
Seventeen infants had a confirmed diagnosis of NEC (Bell Stage II or III) and 34 infants, who presented with non-specific abdominal disorders that were worrisome enough to withhold feedings and obtain an abdominal radiograph, later improved rapidly (Table I). There were no significant differences between the groups in these or gestational age at birth weight or other clinical variables. There was a tendency for more male infants in the NEC group (p=0.08). Among the infants with documented NEC, only one had had a transfusion within 48 hours of onset. The remaining infants had either none or remote transfusions early in the neonatal course.
Table 1.
Clinical characteristics the study patients.
| Variable | NEC group (n=17) | Control group (n=34) | P value |
|---|---|---|---|
| Gestational age (weeks) | 27 (23–39) | 29 (23–41) | 0.70 |
| Age of onset (Days) | 16 (4–165) | 17 (1–89) | 0.88 |
| Body weight (grams) | 1360 (460–5000) | 1200 (400–5400) | 0.57 |
| Male Sex n(%) | --(77%) | --(47%) | 0.08 |
| Maternal Hypertension, n (%) | 9 (26) | 4 (23%) | 0.9 |
| Prolonged rupture of membranes, n (%) | 4 (23.5%) | 3 (8.8%) | 1 |
| C-section, n (%) | 21 | 12 | 0.75 |
| 1 min Apgar ≤3, n (%) | 1 (5.8%) | 5(14.7%) | 0.23 |
| 5 min Apgar ≤ 3, n (%) | 0 (0%) | 1 (2.9%) | 0.48 |
| RDS, n (%) | 26 (76%) | 13 (76% ) | 0.72 |
| PDA, n (%) | 4 (11) | 3 (17) | 0.88 |
| IUGR, n (%) | 8 (23%) | 4 (23) | 0.7 |
Clinical manifestations in the confirmed NEC group included: gross blood in the stool (82%), abdominal distension (76%) and increased gastric residuals (65%) (Table II). Seven infants with confirmed NEC (41%) required increased respiratory support, five (29%) developed hypotension treated with fluid boluses and vasopressors, and three (17%) developed disseminated intravascular coagulation, treated with transfusion of blood products. Of the 17 infants with confirmed NEC, five (29%) had positive blood cultures (Staphylococcus epidermidis [n = 3] and Escherichia coli [n = 2]). Six (35%) had placement of surgical drain and/or exploratory laparotomy, five (29%) developed a subsequent intestinal stricture, and three (17%) died. All initial abdominal radiographs of the confirmed NEC group were interpreted as abnormal. Radiographic findings included pneumatosis intestinalis (n = 17), and intestinal perforation and/or ascites (n = 6).
TABLE 2.
Clinical presentation of the confirmed NEC group and the control group
| Clinical Presentation | NEC group n=17, (%) | Control group n=34, (%) |
|---|---|---|
| Gastric residuals | 11 (64%) | 25 (65.5%) |
| Abdominal Distention | 13 (76%) | 22 (59%) |
| Abdominal Tenderness | 12(70%) | 2 (7%) |
| Bloody Stools | 14 (82%) | 0 (0%) |
| Vomiting | 0 (0%) | 7 (14%) |
| Increased respiratory support | 7 (41%) | 0 (0%) |
| Hypotension | 5 (29.5%) | 0 (0%) |
| DIC | 3 (17%) | 0 (0%) |
In the control group, the most common initial gastrointestinal manifestation was increased gastric residuals (65%) and abdominal distention (59%). None of these infants developed NEC. Their abdominal radiographs were read as unremarkable/non specific abdominal distension and none showed progression on subsequent radiographs 12 to 24 hours later.
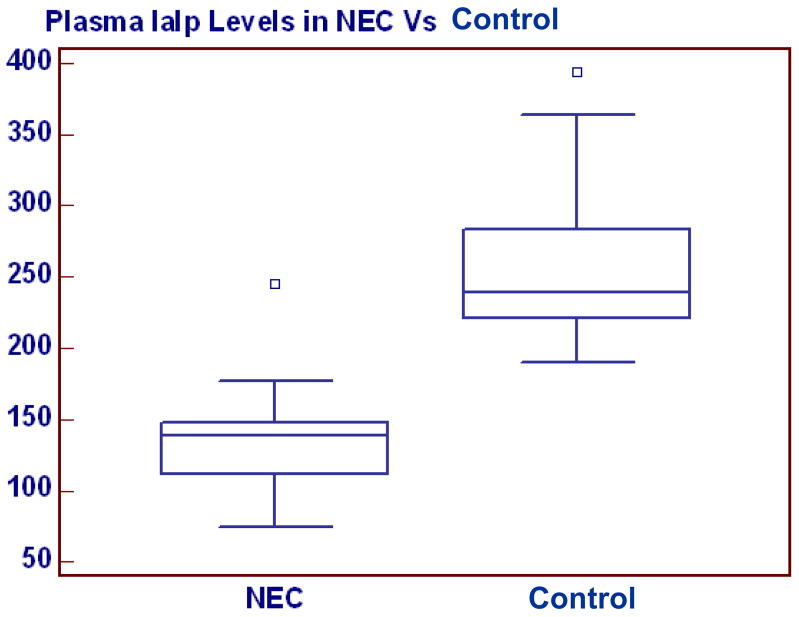
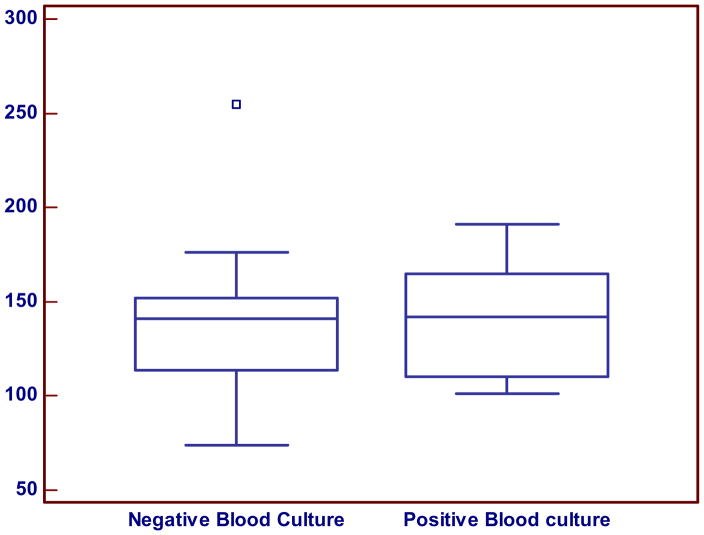
The mean IaIp level in the confirmed NEC group was significantly lower (137±38 mg/L, 95% CI=118–157) compared with control group (258±53 mg/L, 95%CI: 238–277), p < 0.0001, (Figure 1). With the exception of one patient, there was no overlap between the IaIp values in the NEC and the control groups. A single patient in the NEC group presented with abdominal distention and gastric residuals with an initial IaIp level of 240 mg/dL (Figure 1). This patient had pneumatosis on initial radiograph that disappeared on a subsequent film within 12 hours. The patient was treated for NEC and subsequently recovered rapidly without complications. Among the patients with NEC, the mean IaIp levels did not differ between those with NEC stage II (132±29, 114–150, n=12) and stage III (138±34, 95–181, n=4) and were similar in all patients with NEC regardless of an associated positive blood culture (Figure 2).
Figure 1.
Plasma IaIp levels in neonates with confirmed NEC and control. IALP was significantly decreased in the NEC group.
Figure 2.
IALP levels in patients with NEC with negative and positive blood culture. There was no significant difference in IALP levels in the blood culture positive (142 ± 31 mg/L, 95 % CI; 107–177, n=5) compared with the culture negative neonates with proven NEC ( 141 ± 28, 95% CI; 112–170, n= 12) P=0.96
DISCUSSION
NEC is an acute inflammatory condition of the gastrointestinal tract resulting in intestinal necrosis, systemic sepsis and multi-system organ failure. NEC is the most common neonatal gastrointestinal emergency (1) with a relatively high mortality rate among affected infants (3,4). The morbidity and mortality have not changed appreciably in several decades (1–4). Early warning signs and symptoms of NEC are nonspecific, often inconspicuous and may be difficult to distinguish from other non-specific abdominal disorders such as gastrointestinal dysmotility and/or acute exacerbations of bronchopulmonary dysplasia (4). Thus, early identification of NEC in premature infants remains a major diagnostic challenge. No reliable early biomarkers have demonstrated sufficient positive and negative predictive values to be clinically useful (4). We now show that IaIp levels are significantly lower in infants with documented NEC than those later confirmed to have non-specific abdominal disorders. With the exception of a single infant, there was no overlap of the measured levels in the two groups.
Although the precise etiology remains enigmatic, the pathogenesis of NEC appears to involve a pathway that includes the endogenous production of inflammatory mediators involved in the development of intestinal injury (11). Endotoxin/Lipopolysaccharide (LPS), platelet-activating factor (PAF), tumor necrosis factor alpha (TNF-a), IL-8, prostaglandins, leukotrienes, protease/anti-protease balance and nitric oxide, are thought to be involved in NEC pathogenesis (12–15). Recent studies indicate that an unbalanced pro-inflammatory response contributes to the predilection towards NEC in animal studies and in premature infants (16,17). Up-regulation of pro-inflammatory mediators occurs early in the inflammatory response, and, as studies have demonstrated alterations in NFkB and IL-8 activation, these data suggest that identification of alterations in circulating levels as early biomarkers of NEC is possible (4,14). In addition to contributing to the balance of the inflammatory response, inhibitors of serine proteases (serpins) were shown to protect against epithelial cell necrosis (15). C. elegans harboring a serpin knockout gene develop exaggerated cell death in response to hypotonic shock, cold exposure, and other forms of stress. These data suggest that lower anti-protease levels might not only be an early biomarker, but could promote mucosal cell death and contribute to the pathophysiology of neonatal NEC. Differences in IL-6, CRP, calprotectin, procalcitonin, and I-FABP (13,14,18–22) have been examined but clinical studies are limited, and the results have not demonstrated sufficient reliability (4). Since no current method has been shown to be both sensitive and specific in identifying infants with definite NEC early in the course of disease, we examined IaIp as a candidate molecule.
IALP include a family of structurally related serine protease inhibitors found at relatively high concentrations in human plasma (5). Unlike other inhibitor molecules, this family of inhibitors is composed of a combination of covalently linked polypeptide chains. IaIp play roles in inflammation, wound healing and cancer metastasis (7). They are known to inhibit several serine proteases, such as trypsin, HLE, plasmin, cathepsin G and granzyme K. Upon forming a stable complex with TSG-6, one of the possible ligands of IaIp, the inhibitory activity of IaIp toward plasmin is enhanced significantly (6). Plasmin is a serine protease involved in the activation of matrix metalloproteinases that are part of the proteolytic cascade associated with inflammation. The liver is the major source of heavy and light chains of IaIp (26).
The protective effects of exogenously administered IaIp can be explained through inhibition of destructive serine proteases such as elastase, plasmin, cathepsin G, granzyme K, and furin (7). The inhibitory activity of IaIp against neutrophil-induced elastase activity may be of considerable pathophysiologic importance in severe sepsis (29, 30). The inhibition of granzyme K may contribute to apoptotic signaling by cytotoxic T cells in severe sepsis. Inhibition of plasmin activity attenuates plasmin-mediated activation of matrix metalloproteinases, which may cause tissue injury in systemic inflammatory states (6). IaIp are also potent inhibitors of furin (31), an endogenous cell membrane-associated serine endoprotease that plays a role in partial proteolytic activation of a variety of bacterial toxins including Pseudomonas exotoxin A, diphtheria toxin, lethal toxin and edema toxin formation by Bacillus anthracis, and a variety of other microbial exotoxins (31). IaIp also operate through down-regulation of pro-inflammatory cytokines such as TNF-alpha, and IL-6 (26) and by blocking excess complement activation and generation of circulating C5a (31–32). Administration of IaIp (30 mg/kg) to 2-day-old mice within hours of inducing neonatal sepsis with either E. coli or Group B beta hemolytic streptococci significantly improves survival (abstract).
In adult patients with sepsis, we and others have shown that plasma levels of IaIp are decreased significantly (by 20% 90%) and inversely correlated with unfavorable outcome (5). In neonates, we previously studied a cohort of newborn infants with gestational age between 24–42 weeks (8). We measured IaIp level in umbilical cord blood, postnatal blood and in samples obtained at the time of evaluation for sepsis. We showed that IaIp levels were endogenously produced, independent of maternal levels, independent of gestational age, independent of postnatal age and similar to the levels seen in adults (8). We also showed that IaIp levels were significantly decreased in neonatal sepsis. In a larger study of their diagnostic utility, we showed that IaIp are a more reliable diagnostic marker for neonatal sepsis than other currently available tests (9). Moreover, in several adult and newborn animal models of sepsis, IaIp treatment to normalize the decreased systemic levels has been demonstrated to significantly improve the sepsis related mortality (29, 30).
In summary, in the present study we demonstrated that IaIp are significantly decreased in patients with NEC stage II/III compared with a control group with non-specific abdominal disorders. We hypothesize that IaIp are involved in the pathogenesis of NEC. As one of the critical acute phase reactants during the host response to inflammation and tissue injury, it appears to rapidly decrease as the molecule is “consumed” during the inflammatory process and rapidly excreted through the kidneys. Measurement of IALP may serve useful as a sensitive biomarker. The ability to detect NEC in the early stages will allow patients to be placed on appropriate therapy and may provide an objective means for reducing antibiotic overuse in infants with suspected but unproven NEC.
Acknowledgments
Supported in part by a grant from the NIH NCRR P20 RR018728. Y.-P.L. has equity in the company ProThera Biologics where the IaIp protein assays used in these experiments were carried out. The other authors declare no conflicts of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma R, Tepas J, Jr, Hudak ML, Mollitt DL, Wludyka PS, Tseng RJ, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42:454–461. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 2.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 3.Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6:6–23. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young C, Sharma R, Handfield M, Mai V, Neu J. Biomarkers for Infants at Risk for Necrotizing Enterocolitis: Clues to Prevention? Pediatric Research. 2009 doi: 10.1203/PDR.0b013e31819dba7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, et al. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis. 2003;188:919–926. doi: 10.1086/377642. [DOI] [PubMed] [Google Scholar]
- 6.Wisniewski HG, Hua JC, Poppers DM, Naime D, Vilcek J, et al. TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-alpha-inhibitor and exerts a strong anti-inflammatory effect in vivo. J Immunol. 1996;156:1609–1615. [PubMed] [Google Scholar]
- 7.Fries E, Blom AM. Bikunin--not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000;32(2):125–37. doi: 10.1016/s1357-2725(99)00125-9. [DOI] [PubMed] [Google Scholar]
- 8.Baek YW, Brokat S, Padbury JF, Pinar H, Hixson DC, et al. Inter-alpha inhibitor proteins in infants and decreased levels in neonatal sepsis. J Pediatr. 2003;143:11–15. doi: 10.1016/S0022-3476(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 9.Chaaban H, Singh K, Huang J, Siryaporn E, Lim YP, Padbury JF. The role of inter-alpha inhibitor proteins in the diagnosis of neonatal sepsis. J Pediatr. 2009;154:620–622. e621. doi: 10.1016/j.jpeds.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Daveau M, Rouet P, Scotte M, Faye L, Hiron M, Lebreton JP, Salier JP. Human inter-alpha-inhibitor family in inflammation: simultaneous synthesis of positive and negative acute-phase proteins. Biochem J. 1993;292(Pt 2):485–92. doi: 10.1042/bj2920485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holman RC, Stoll BJ, Clarke MJ, Glass RI. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997;87(12):2026–2031. doi: 10.2105/ajph.87.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Sem Fet Neonatal Med. 2006;13:35–43. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]
- 13.Pourcyrous M, Korones SB, Yang W, Boulden TF, Bada HS. C-reactive protein in the diagnosis, management, and prognosis of neonatal necrotizing enterocolitis. Pediatrics. 2005;116:1064–1069. doi: 10.1542/peds.2004-1806. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland AD, Gearry RB, Frizelle FA. Review of fecal biomarkers in inflammatory bowel disease. Dis Colon Rectum. 2008;51:1283–1291. doi: 10.1007/s10350-008-9310-8. [DOI] [PubMed] [Google Scholar]
- 15.Luke CJ, Pak SC, Askew YS, Naviglia TL, Askew DJ, Nobar SM, Vetica AC, Long OS, Watkins SC, Stolz DB, Barstead RJ, Moulder GL, Brömme D, Silverman GA. An intracellular serpin regulates necrosis by inhibiting the induction and sequelae of lysosomal injury. Cell. 2007 Sep 21;130(6):1108–19. doi: 10.1016/j.cell.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claud EC, Savidge T, Walker WA. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr Res. 2003 Mar;53(3) doi: 10.1203/01.PDR.0000050141.73528.AD. [DOI] [PubMed] [Google Scholar]
- 17.De Plaen IG, Liu SX, Tian R, Neequaye I, May MJ, Han XB, Hsueh W, Jilling T, Lu J, Caplan MS. Inhibition of nuclear factor-kappaB ameliorates bowel injury and prolongs survival in a neonatal rat model of necrotizing enterocolitis. Pediatr Res. 2007 Jun;61(6):716–21. doi: 10.1203/pdr.0b013e3180534219. [DOI] [PubMed] [Google Scholar]
- 18.D'Inca R, Dal Pont E, Di Leo V, Benazzato L, Martinato M, Lamboglia F, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007–2014. doi: 10.1111/j.1572-0241.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 19.Josefsson S, Bunn SK, Domellöf M. Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2007;44:407–413. doi: 10.1097/MPG.0b013e3180320643. [DOI] [PubMed] [Google Scholar]
- 20.Corfield DA, Spicer R, Cairns P. Faecal calprotectin concentrations and diagnosis of necrotising enterocolitis. Lancet. 2003;361:310–311. doi: 10.1016/S0140-6736(03)12333-1. [DOI] [PubMed] [Google Scholar]
- 21.Yang Q, Smith PB, Goldberg RN, Cotten CM. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology. 2008;94:267–271. doi: 10.1159/000151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guthmann F, Borchers T, Wolfrum C, Wustrack T, Bartholomaus S, Spener F. Plasma concentration of intestinal- and liver-FABP in neonates suffering from necrotizing enterocolitis and in healthy preterm neonates. Mol Cell Biochem. 2002;239:227–234. [PubMed] [Google Scholar]
- 23.Derikx JP, Evennett NJ, Degraeuwe PL, Mulder TL, van Bijnen AA, van Heurn Lw, et al. Urine based detection of intestinal mucosal cell damage in neonates with suspected necrotising enterocolitis. Gut. 2007;56:1473–1475. doi: 10.1136/gut.2007.128934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enghild JJ, Salvesen G, Thogersen IB, Valnickova Z, Pizzo SV, Hefta SA. Presence of the protein-glycosaminoglycan-protein covalent cross-link in the inter-alpha-inhibitor-related proteinase inhibitor heavy chain 2/bikunin. J Biol Chem. 1993;268(12):8711–6. [PubMed] [Google Scholar]
- 25.Morelle W, Capon C, Balduyck M, Sautiere P, Kouach M, Michalski C, Fournet B, Mizon J. Chondroitin sulphate covalently cross-links the three polypeptide chains of inter-alpha-trypsin inhibitor. Eur J Biochem. 1994;221(2):881–8. doi: 10.1111/j.1432-1033.1994.tb18803.x. [DOI] [PubMed] [Google Scholar]
- 26.Garantziotis S, Hollingsworth JW, Ghanayem RB, Timberlake S, Zhuo L, et al. Inter-alpha-trypsin inhibitor attenuates complement activation and complement- induced lung injury. J Immunol. 2007;179:4187–4192. doi: 10.4049/jimmunol.179.6.4187. [DOI] [PubMed] [Google Scholar]
- 27.Salier J-P, Rouet P, Raguenez G, Daveau M. The inter-α-inhibitor family: from structure to regulation. Biochem J. 1996;351:1–9. doi: 10.1042/bj3150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potempa J, Kwon K, Chawla R, Travis J. Inter-alpha-trypsin inhibitor. Inhibition spectrum of native and derived forms. J Biol Chem. 1989;264(25):15109–14. [PubMed] [Google Scholar]
- 29.Wu R, Cui X, Lim YP, Bendelja K, Zhou M, et al. Delayed administration of human inter-alpha inhibitor proteins reduces mortality in sepsis. Crit Care Med. 2004;32:1747–1752. doi: 10.1097/01.ccm.0000132903.14121.0e. [DOI] [PubMed] [Google Scholar]
- 30.Yang S, Lim YP, Zhou M, Salvemini P, Schwinn H, Josic D, Koo DJ, et al. Administration of human inter-alpha-inhibitors maintains hemodynamic stability and improves survival during sepsis. Crit Care Med. 2002 Mar;30(3):617–22. doi: 10.1097/00003246-200203000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:652–676. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 32.Neijens JH, Abbink JJ, Wachtfogel YT, et al. Plasma elastase alpha 1-antitrypsin and lactoferrin in sepsis: Evidence for neutrophils as mediators in fatal sepsis. J Lab Clin Med. 1992;119:159–168. [PubMed] [Google Scholar]
- 33.Gordon V, Klimpel K, Arora N, et al. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immum. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]