Abstract
Background: Desonide is a low-potency corticosteroid recently formulated in a novel aqueous gel (hydrogel) formulation. Currently US Food and Drug Administration approved for use in the treatment of mild-to-moderate atopic dermatitis, this hydrogel formulation may offer aesthetic advantages over traditional vehicles. Objective: To conduct a pilot study evaluating efficacy, tolerability, and patient preference of desonide hydrogel 0.05% for the treatment of scalp and facial seborrheic dermatitis. Methods:Subjects treated affected areas on the face or scalp twice daily for four weeks. Evaluations of pruritus, target area scaling, induration and erythema; static global assessments; and photography were conducted. Results: Ten subjects aged 13 to 73 years with mild scalp or facial seborrheic dermatitis completed the study. Statistically significant reductions in pruritus, target area scaling, erythema, and induration, and significant improvements in static global assessments were demonstrated over Baseline (all P<0.05). Conclusion:Desonide hydrogel 0.05% may provide an effective, well-tolerated, and cosmetically elegant treatment option for scalp and facial seborrheic dermatitis.
Seborrheic dermatitis (SD) is a common inflammatory skin condition characterized by scaly patches of skin that may present with different degrees of erythema and pruritus. Although the exact cause of SD is not well understood, proliferation of the yeast Malassezia furfur is believed to play an important role in the condition's pathophysiology.1 As a result, antifungals, such as ketoconazole or zinc pyrithione, are commonly used to treat SD. Topical corticosteroids have also been successfully used for SD treatment by addressing the skin's inflammatory reaction to M. furfur.2,3
Desonide is a low-potency corticosteroid with an excellent safety profile, and has been widely used as a topical treatment of inflammatory dermatoses for more than 30 years.4,5 Advances in formulation technology have enabled the development of desonide into a novel aqueous gel (hydrogel) that is alcohol-free and disappears quickly without leaving a greasy residue.6,7 Unlike older alcohol-based gel formulations, which could be drying to dermatitic skin, the hydrogel vehicle has been shown to increase epidermal moisturization.8 Currently US Food and Drug Administration (FDA) approved for use in mild-to-moderate atopic dermatitis, this hydrogel formulation may offer aesthetic advantages over traditional vehicles that can be messy and time consuming to apply. This is especially important since patient preference for an aesthetically pleasing formulation may help to increase therapy compliance.9
To evaluate the potential suitability of a topical treatment, the application site needs to be considered. SD frequently involves the scalp, eyebrows, nasolabial folds, and mid-chest. Since these areas are highly visible and frequently hair bearing, patients may benefit from the use of the hydrogel formulation, which has been shown in other studies as a suitable treatment for multiple body areas, including glabrous areas.10
The purpose of this four-week, open-label, pilot study was to explore the potential suitability of desonide hydrogel 0.05% for the treatment of scalp and facial SD by evaluating efficacy, tolerability, and patient preference.
Methods
Study population. The criteria for study participation included male or female subjects aged 12 years or older with a baseline SD severity score of two (mild) or three (moderate) as determined by an Investigator's Static Global Assessment (ISGA).
Subjects were excluded from the study if they had used systemic antifungals, steroids, retinoids, or immunosuppressive therapy within four weeks of the study or if they had used topical antifungals, steroids, retinoids, calcineurin inhibitors, and shampoos containing tar or salicylic acid within two weeks of the study. Subjects with any medical condition that would contraindicate their study participation were also excluded.
Study design. Institutional review board (IRB) approval was obtained for this open-label, single-center, pilot study. The study was conducted according to ethical and regulatory principals from the International Conference on Harmonization and good clinical practices. Prior to treatment, the subject or a parent/guardian (if the subject was younger than 18 years old) provided informed consent. If applicable, an assent was obtained for minors 13 to 17 years.
Eligible subjects were treated twice daily with desonide hydrogel 0.05% for four weeks. Subjects were instructed to apply the study medication to the affected areas on their face or scalp once in the morning and once in the evening. Investigator and subject evaluations of efficacy and tolerability were conducted at Baseline, Week 2, and Week 4. Photographs of the target area were also taken at all visits. Investigator evaluations included target area assessments for scaling, induration, and erythema as well as an ISGA. Subjects evaluated their pruritus at each visit and also completed a product-assessment questionnaire at the end of the study.
Study endpoints. Efficacy. The investigator selected a target area lesion located on either the face or scalp for scaling, induration, and erythema assessments. Both scaling and induration were rated on a 5-point scale: 0 (none), 1 (minimal), 2 (mild), 3 (moderate), and 4 (severe). Target area erythema was assessed on a scale of 0 (normal), 1 (faint), 2 (light red), 3 (moderate red), and 4 (dusky to deep red). Overall scalp and facial lesions were assessed by an ISGA based on a 5-point scale from 0 (normal), 1 (minimal), 2 (mild), 3 (moderate), and 4 (severe).
Evaluations of pruritus included a subject assessment regarding the amount of itching experienced over the past 24-hour period. The subjects rated pruritus on a 5-point scale of 0 (no itching), 1 (minimal, rarely aware of itching), 2 (mild, only aware of itching at times, only present when relaxing, not present when focused on other activities), 3 (moderate, often aware of itching, annoying, sometimes disturbs sleep and daytime activities), and 4 (severe, constant itching, distressing, frequent sleep disturbance, interferes with activities).
Safety. Tolerability of the study medication was assessed at all visits by the reporting of adverse events.
Product attribute assessment. Subjects completed a product-assessment questionnaire at Week 4, asking them to rate the study product attributes based on their usage experience. Subjects answered 14 questions using a scale of 1 (excellent), 2 (good), 3 (fair), 4 (poor), and 5 (very poor).
Statistical Analysis
Statistical analyses were conducted on the per-protocol (PP) population, which included all subjects that completed the study. Statistics for the observed and change from baseline were performed for the ISGA scores; target area scaling, erythema and induration scores, and subject pruritus assessment scores. Confidence intervals (95%) were determined based upon a t distribution, and the P values were determined using a Wilcoxon signed-rank test.
Statistics for the observed scores were also conducted for the subject product-assessment questionnaire. Confidence intervals (95%) were determined based upon a t distribution. P values, where applicable, were determined using both Wilcoxon signed-rank and binomial probability distribution tests.
Results
Ten subjects, 13 to 73 years old, with mild scalp or facial SD, were enrolled in the study. All 10 subjects completed the four-week study. Table 1 shows subject demographics and target area location.
Table 1.
Subject demographics and target area location
| N | % | |
|---|---|---|
| Gender | ||
| Female | 5 | 50% |
| Male | 5 | 50% |
| Aqe (years) | ||
| Mean | 48 | |
| Min | 13.1 | |
| Max | 73.6 | |
| Ethnicity | ||
| White | 9 | 90% |
| Black | 1 | 10% |
| Target Area Location | ||
| Nasolabial Fold | 3 | 30% |
| Posterior Scalp | 4 | 40% |
| Medial eyebrow | 1 | 10% |
| Center Forehead | 1 | 10% |
| Center Scalp (Apex) | 1 | 10% |
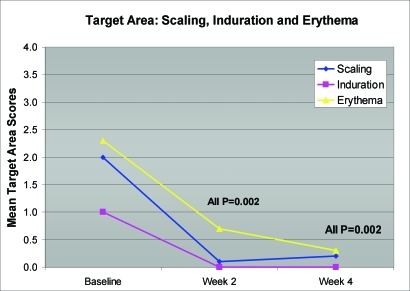
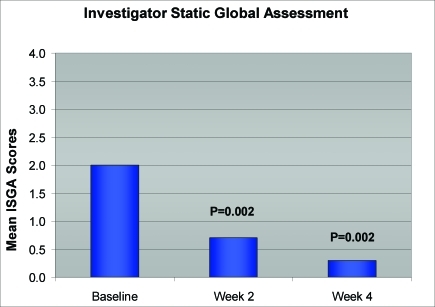
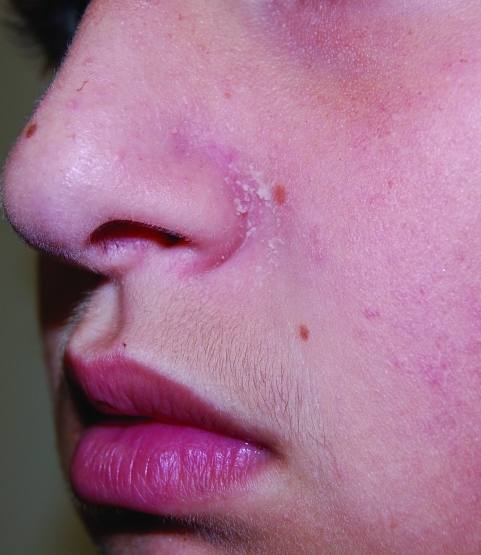
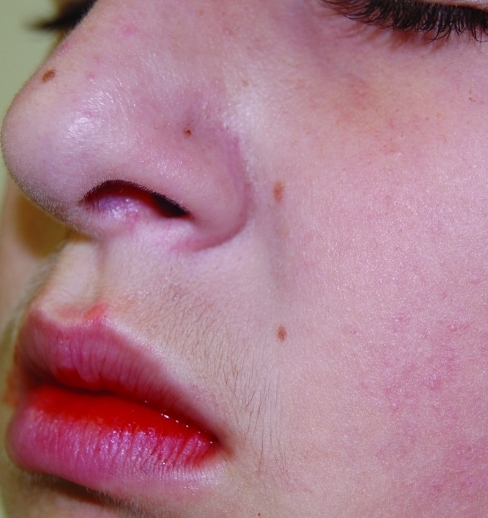
Statistically significant reductions in mean target area scaling, induration, and erythema were seen at Week 2 (first follow-up visit) and extended through Week 4 (all P=0.002) (Figure 1). In addition, significant improvements in ISGA scores for all subjects were also observed as early as Week 2 and continued through Week 4 (all P=0.002) (Figure 2). Improvement in target area locations, including nasolabial folds, medial eyebrows, center forehead, and center scalp, is depicted in Figures 3 and 4.
Figure 1.
Mean reductions in target area scaling, induration, and erythema (N=10)
Figure 2.
Improvement in ISGA scores (N=10)
Figure 3a.
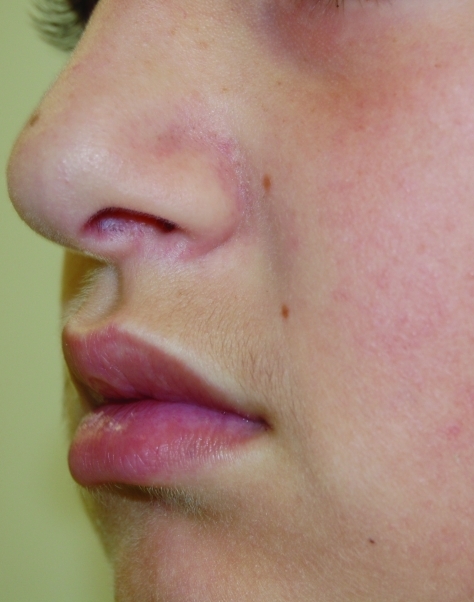
Thirteen-year-old male patient with SD on left nasolabial fold treated with desonide hydrogel 0.05% for four weeks. Baseline photo.
Figure 3b.
The same patient after two weeks of treatment
Figure 3c.
The same patient after four weeks of treatment
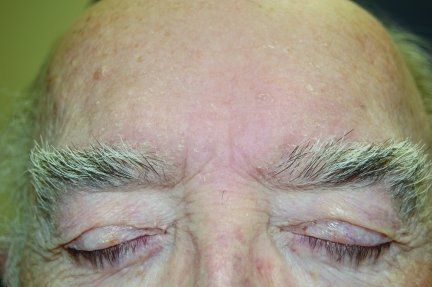
Figure 4a.
Seventy-year-old male patient with SD on center forehead treated with desonide hydrogel 0.05% for four weeks. Baseline photo.
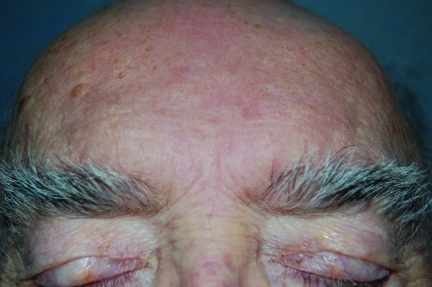
Figure 4b.
The same patient after two weeks of treatment
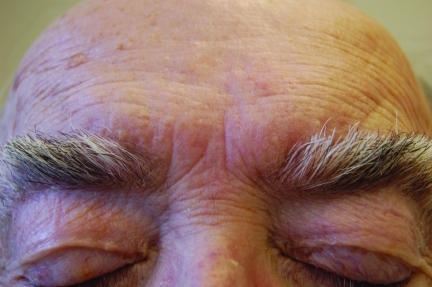
Figure 4c.
The same patient after four weeks of treatment
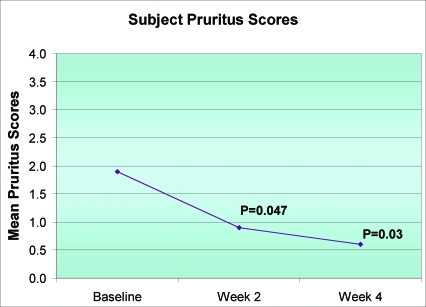
Mean pruritus severity scores demonstrated a significant decrease at Week 2 and through the end of the study (all P<0.05) (Figure 5). At study completion, the majority of subjects (8/10) rated all 14 product attributes of desonide hydrogel as excellent or good (all P≤0.04). Subject responses to the product-assessment questionnaire are summarized in Table 2.
Figure 5.
Reduction in pruritus severity scores (N=10)
Table 2.
Product-attribute assessment: Subjects were asked to rate the study product's attributes.
| Product Attribute | % of Subjects |
|---|---|
| Stain-free | 100 |
| Not greasy on skin | 100 |
| Suitable for use on multiple body areas (face, scalp, etc.) | 90 |
| Does not dry out my skin | 80 |
| Feels soothing | 100 |
| Fragrance-free | 100 |
| Comfortable to wear under make-up and/or cosmetics | 100 |
| Easy to spread | 100 |
| Disappears quickly | 80 |
| Low residue | 100 |
| Feels moisturizing | 90 |
| Easy to use on hair-bearing skin | 90 |
| Easy to apply/ easy to use | 100 |
| My overall satisfaction with treatment | 80 |
The study medication was very well tolerated, and there were no treatment-related adverse events reported during the study.
Discussion
This four-week pilot study demonstrates that desonide hydrogel 0.05% provided an effective treatment option for patients with mild scalp and facial SD. The improvements observed with desonide hydrogel treatment are consistent with other SD studies where a low-potency corticosteroid was used.3,4 However, unlike these other treatments, desonide hydrogel offers additional aesthetic advantages that may be important in enhancing therapy compliance. In particular, these advantages include being stain free and suitable for use on multiple body areas, including hair-bearing skin, such as the face and scalp. In addition, the low-residue hydrogel formulation spreads easily and feels moisturizing, and SD subjects found it easy to apply/use. In fact, at least 90 percent of subjects in the study rated these desonide hydrogel characteristics as excellent or good (all P≤0.01).
Favorable results were similarly reported in atopic dermatitis subjects who rated the desonide hydrogel formulation significantly higher when asked to sample and compare the vehicle attributes of hydrogel, foam, cream, ointment, and oil.11 These same atopic subjects significantly preferred desonide hydrogel over these other vehicles when requesting a prescription in the future.11 Since poor adherence to topical medications may contribute to poor treatment outcomes, the selection of a preferred vehicle may help to optimize clinical results and patient satisfaction. It has been shown that the vehicle attributes may impact therapy compliance and therefore influence efficacy.12
Another important observation from these SD cases, one agent for both scalp and face, such as the hydrogel due to its unique properties, would replace separate or multiple formulations. Additionally, since all subjects found desonide hydrogel to be stain free and nongreasy, the treatment may be suitable for patient use in SD-affected areas beyond the face and scalp. It is useful to have a versatile treatment option available for patients since a simplified therapy regimen may also help to enhance compliance, which, in return, will increase efficacy.
Overall, the results from this pilot study suggest that desonide hydrogel 0.05% can be an effective, well-tolerated, and cosmetically elegant treatment option for scalp and facial SD. Additional larger studies are needed to establish the efficacy and safety of desonide hydrogel 0.05% for the treatment of SD.
References
- 1.Bergbrant IM. Seborrhoeic dermatitis and Pityrosporum yeasts. Curr Top Med Mycol. 1995;6:95–112. [PubMed] [Google Scholar]
- 2.Katsambas A, Antoniou C, Frangouli E, et al. A double-blind trial of treatment of seborrhoeic dermatitis with 2% ketoconazole cream compared with 1% hydrocortisone cream. Br J Dermatol. 1989;121(3):353–357. doi: 10.1111/j.1365-2133.1989.tb01429.x. [DOI] [PubMed] [Google Scholar]
- 3.Stratigos JD, Antoniou C, Katsambas A, et al. Ketoconazole 2% cream versus hydrocortisone 1% cream in the treatment of seborrheic dermatitis. A double-blind comparative study. J Am Acad Dermatol. 1988;19(5 Pt 1):850–853. doi: 10.1016/s0190-9622(88)70244-3. [DOI] [PubMed] [Google Scholar]
- 4.Jorizzo J, Levy M, Lucky A, et al. Multicenter trial for long-term safety and efficacy comparison of 0.05% desonide and 1% hydrocortisone ointments in the treatment of atopic dermatitis in pediatric patients. J Am Acad Dermatol. 1995;33(1):74–77. doi: 10.1016/0190-9622(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 5.Wong VK, Fuchs B, Lebwohl M. Overview on desonide 0.05%: a clinical safety profile. J Drugs Dermatol. 2004;3(4):393–397. [PubMed] [Google Scholar]
- 6.Kircik L, Del Rosso J. A novel hydrogel vehicle formulated for the treatment of atopic dermatitis. J Drugs Dermatol. 2007;6(7):718–722. [PubMed] [Google Scholar]
- 7.Trookman NS, Rizer RL, Ford R, Gotz V. The importance of vehicle properties to atopic dermatitis patients: a preference study with a novel desonide hydrogel treatment. Poster #617 presented at: 66th Annual Meeting of the American Academy of Dermatology; February 1-5, 2008; San Antonio, TX.
- 8.Trookman NS, Rizer RL, Ford R, Trancik RJ. Topical vehicle preferences in atopic dermatitis patients evaluating a novel hydrogel vehicle. Poster #725 presented at: 65th Annual Meeting of the American Academy of Dermatology; February 2-6, 2007; Washington, DC.
- 9.Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55(4):607–613. doi: 10.1016/j.jaad.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Hebert A, Cook-Bolden F, Basu S, Calvarese MS, Trancik R. Safety and efficacy of desonide hydrogel 0.05% in pediatric subjects with atopic dermatitis. J Drugs Dermatol. 2007;6(2):175–181. [PubMed] [Google Scholar]
- 11.Yentzer B, Fountain J, Clark A, et al. Vehicle preference in patients with atopic dermatitis. Poster presented at: 26th Annual Convention of the Dermatology Nurses' Association; March 25-28, 2008; Las Vegas, NV.
- 12.Feldman SR, Housman TS. Patients' vehicle preference for corticosteroid treatments of scalp psoriasis. Am J Clin Dermatol. 2003;4(4):221–224. doi: 10.2165/00128071-200304040-00001. [DOI] [PubMed] [Google Scholar]