Minocycline extended-release (ER) tablets are approved by the Food and Drug Administration (FDA) for the treatment of acne vulgaris based on efficacy and safety results from Phase 2 and Phase 3 clinical studies.1 Until the approval of this formulation for acne vulgaris, no oral antibiotic has been FDA approved for this indication based on Phase 2 and Phase 3 pivotal studies. The efficacy and safety of the formulation was evaluated using weight-based dosing, demonstrating that a daily dose of 1mg/kg/day demonstrated efficacy (inflammatory lesion reduction) equivalent to 2mg/kg/day and 3mg/kg/day, with a markedly lower incidence of acute vestibular adverse events.1,2 Additionally, the pharmacokinetic profile of minocycline ER tablets demonstrates that as compared to minocycline immediate-release (IR) formulations, minocycline ER is associated with a delayed time-to-peak plasma concentration (Tmax), reduced peak plasma concentration (Cmax), and reduced systemic exposure to minocycline over time, defined as reduced area-under-the curve (AUC0-24) (Figure 1).3
Figure 1.
Pharmacokinetic properties of extended-release minocycline tablets.
The finding that increasing the dose of minocycline ER once daily beyond 1mg/kg does not increase efficacy, coupled with the pharmacokinetic data, supports the following concepts. First, the high lipophilicity of minocycline suggests that this agent is capable of achieving high concentrations within the lipid-rich follicle, with steady accumulation over time as administration is continued. Second, clinical efficacy in acne vulgaris, especially with a highly lipophilic agent like minocycline, would be expected to correlate with a dose-response curve, which is dependent on a progressive increase in follicular concentration over time, rather than the need to achieve high serum levels that are more immediate in onset. Third, the ability of minocycline ER to be dosed by weight allows for a reduction in both peak serum levels and cumulative exposure, which correlate with a lower risk of adverse events.
This article discusses the background information on the extended-release formulation and weight-based dosing with minocycline with the objective of exploring potential clinical significance.
Is there a correlation between minocycline release from specific oral formulations and potential for vestibular side effects?
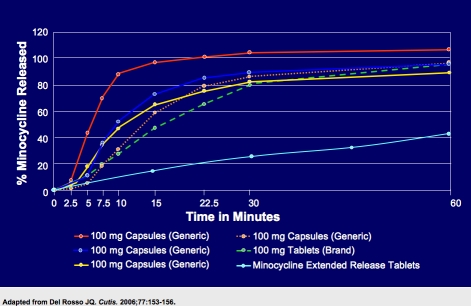
In a blinded crossover study of female subjects (n=32), generic minocycline IR formulations, which released the drug rapidly (100% release over 15 minutes) and were associated with a rapid rise to peak serum level, produced a fivefold greater risk of vestibular adverse reactions as compared to a brand minocycline IR formulation, which released 90 percent of the drug over 45 minutes (P=.0003 on Day 5).4 The release of minocycline from the ER tablets is slower and more prolonged than with IR formulations: >30% to <53% in one hour, >53% to <84% in two hours, and >85% in four hours.
What is the potential clinical significance of minocycline drug-release characteristics?
Figure 2 compares the drug-release characteristics of minocycline ER versus multiple minocycline IR formulations, suggesting that the extended-release properties of minocycline ER tablets contribute a lower potential risk for vestibular side effects.
Is there a correlation between weight-based dosing of minocycline extended-release tablets and potential for acute vestibular adverse reactions?
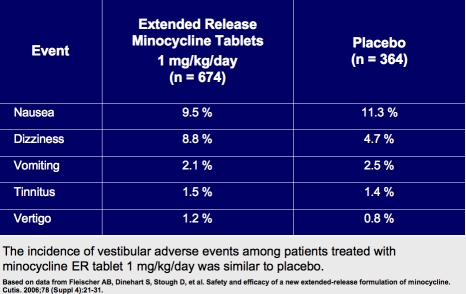
A Phase 2, double-blind, randomized, 12-week trial (N=241) of minocycline ER tablets administered once daily in subjects ages 12 to 30 years with moderate-to-severe acne vulgaris demonstrated that the incidence of acute vestibular adverse events (AVAEs)—defined as dizziness, nausea, tinnitus, vertigo, or vomiting noted within the first five days of treatment—appeared to be dose related, occurring in 28.3 percent, 23.7 percent, and 10.2 percent of patients in the 3mg/kg, 2mg/kg, and 1mg/kg treatment groups, respectively. The incidence of AVAEs in the 1mg/kg/day group was lower than that reported in the placebo group (10.2% and 16.4%, respectively).1,2
The combined data from Phase 2 and 3 clinical trials showed that the percent of subjects experiencing individual vestibular adverse reactions were comparable among those treated with minocycline ER 1mg/kg/day (n=674) and placebo (n=364).1,2
Does weight-based dosing of minocycline extended-release tablets produce a clinically significant reduction in vestibular side effects?
Weight-based dosing with the minocycline ER tablet formulation administered at 1mg/kg once daily was associated with a lower risk of acute vestibular side effects than higher doses administered once daily without loss of clinical efficacy. The risk of vestibular side effects with minocycline ER 1mg/kg once daily appears to be comparable to what occurs with placebo.
Is there a correlation between weight-based dosing of minocycline extended-release tablets and reduction in inflammatory acne lesions?
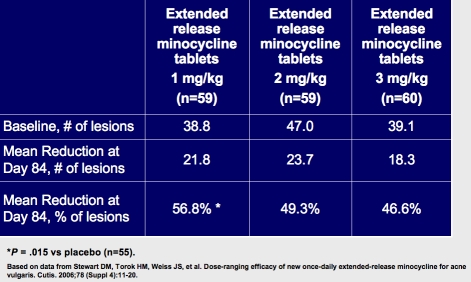
A Phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-ranging study evaluated the efficacy of the minocycline ER tablet formulation once daily in subjects with moderate-to-severe acne vulgaris (N=233). The results, depicted in Figure 4, demonstrate that 1mg/kg once daily was as effective as 2mg/kg once daily or 3mg/kg once daily.2
What is the clinical significance of efficacy data with weight-based dosing of minocycline extended-release tablets?
Weight-based dosing using minocycline ER tablets allows for once-daily administration and demonstrates that “more is not necessarily better” in terms of efficacy when treating acne vulgaris with oral antibiotic therapy. However, the results demonstrated with minocycline ER tablets cannot necessarily be extrapolated to other antibiotics or formulations, as inherent properties of the antibiotic, and the pharmacokinetic characteristics of a given formulation, may modify clinical activity and/or dose response.
Is there correlation of variability in drug absorption with extended-release versus immediate-release minocycline formulations?
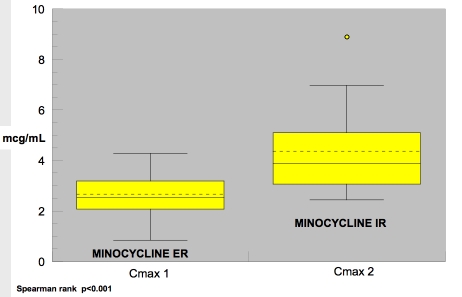
The availability of pharmacokinetic data allows for an assessment of potential variability in gastrointestinal (GI) drug absorption among a population of study subjects. The pharmacokinetic data collected with minocycline ER tablets represents the first attempt in dermatology, with an oral antibiotic agent used to treat acne vulgaris, to establish dose-response correlation with efficacy and safety and to assess potential variability in drug absorption among treated subjects. A randomized, active-controlled, crossover study (N=30) was completed to compare the steady-state systemic exposure with minocycline ER tablets and minocycline IR capsules. Each group, using a crossover design with appropriate washout between study phases, received minocycline ER 135mg daily or minocycline IR 100mg twice daily over six days. Both the Cmax and AUC0-24 were determined for both the minocycline ER and minocycline IR groups. Using FDA-based criteria, the minocycline IR was adjusted to the 135-mg dose of minocycline ER and data was log transformed for analysis. Figure 5 depicts the use of box plots to describe the ranges achieved with the pharmacokinetic data, which in this study included Cmax and AUC0-24. This method of data presentation depicts results centered around the 50th (median) percentile, allowing for an assessment of the variability of the parameters and the presence of minor and extreme outliers.
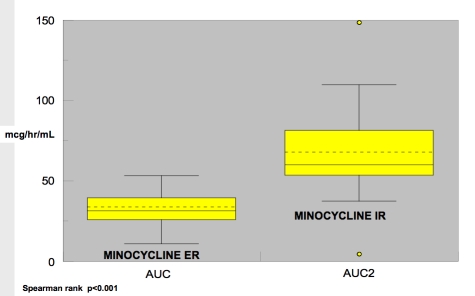
Figures 6 and 7 depict the comparative results for Cmax and AUC0-24 with both the minocycline-ER and minocycline-IR formulations. Figure 6 shows the variability in Cmax with both drugs; however, the variability range is less (“tighter”) with minocycline ER. In Figure 7, the range in AUC is much tighter with minocycline ER, demonstrating lower variability.
What is the potential clinical significance of variability in drug absorption?
Low absorption of minocycline may potentially result in a diminished response to treatment, and may occur in some “low-end” absorption outliers treated with minocycline ER tablets. The majority of subjects treated with minocycline ER exhibit a narrow range of variability in both peak-serum concentration and systemic exposure over time, suggesting overall a more predictable pharmacokinetic profile than with minocycline IR. The minocycline IR formulation demonstrated a broader range in peak-serum concentration and systemic exposure over time and a less predictable pharmacokinetic profile. High absorbers of minocycline would have a greater potential to experience adverse events, especially vestibular side effects, with the minocycline IR formulation. Adverse events that are serum-concentration dependent and/or related to greater cumulative drug exposure would be anticipated to occur more often with the minocycline IR formulation than with minocycline ER tablets.
What can be concluded from pharmacokinetic and clinical data completed with minocycline formulations and with weight-based dosing with minocycline ER tablets?
Weight-based dosing of minocycline using the ER formulation allows for equivalent efficacy using 1mg/kg once daily as compared to use of higher doses (up to 3mg/kg once daily). Adverse reactions, such as acute vestibular side effects, have been shown to correlate with the rate and extent of drug release in the GI tract from its formulation.
Using minocycline ER tablets, weight-based dosing has been shown to reduce the risk of vestibular side effects. GI absorption characteristics vary markedly among minocycline formulations, and within a given formulation there are individual variations in absorption. Minocycline ER tablets exhibit a more narrow range in peak-serum concentration, cumulative-systemic exposure, and a more predictable pharmacokinetic profile than minocycline IR.
When faced with a lack of efficacy or side-effect concern, variation in absorption should be considered. In such cases, changes in dosing may be warranted as a subset of individuals may be either “low end” or “high end” GI absorption outliers. Data of this type would be useful for other drugs as variability in GI absorption may affect clinical outcomes.
Figure 3.
Figure 2.
Comparative drug-release data of minocycline formulations.
Figure 4.
Phase 2 dose-ranging evaluation reduction in inflammatory acne lesions.
Figure 5.
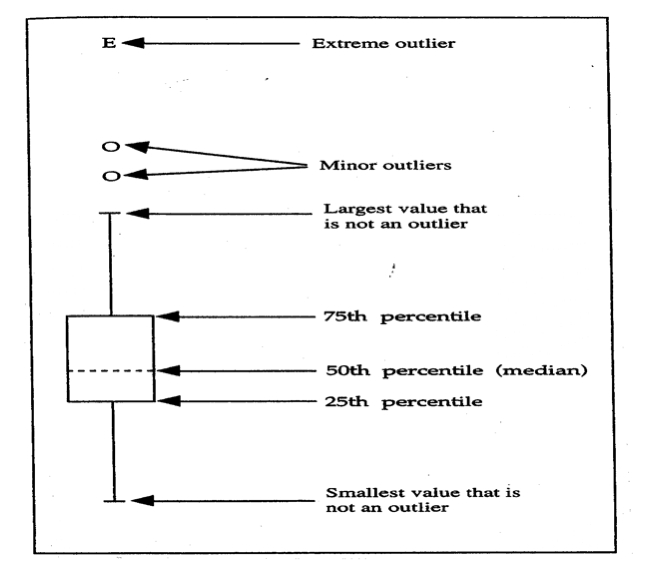
Description of box plots used for data analysis to assess ranges of Cmax and AUC0-24.
Figure 6.
Box plot depiction of Cmax results for minocycline ER and IR formulations.
Figure 7.
Box plot depiction of AUC0-24 results for minocycline ER and IR formulations.
References
- 1.Fleischer AB, Dinehart S, Stough D, et al. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(Suppl 4):21–31. [PubMed] [Google Scholar]
- 2.Stewart DM, Torok HM, Weiss JS, et al. Dose-ranging efficacy of new once-daily extended-release minocycline for acne vulgaris. Cutis. 2006;78(Suppl 4):11–20. [PubMed] [Google Scholar]
- 3.Plott RT, Wortzman MS. Key bioavailability features of a new extended-release formulation of minocycline hydrochloride tablets. Cutis. 2006;78(Suppl 4):6–10. [PubMed] [Google Scholar]
- 4.DelRosso JQ. Clinical significance of brand versus generic formulations: focus on oral minocycline. Cutis. 2006;77:153–156. [PubMed] [Google Scholar]