Abstract
Health problems in some animal models remain unexplained, rendering in vivo studies ethically challenging, especially when experimental animals are prone to sudden death. Over the last 3 decades, the myelin-deficient (md) rat, a strain with severe dysmyelination due to mutant proteolipid protein, has been key to important discoveries in mechanisms of myelination and glial cell biology. The usefulness of this mutant rat, however, has been limited by sudden death during the fourth week of life. Timely euthanasia has been difficult because the cause of these mortalities remains unexplained and the endpoint not determined. In this clinicopathologic study, we determined that sudden onset of hindlimb paralysis inevitably leads to paralysis of the urinary bladder and then breathing difficulties because of severe injury to the spinal cord in the midthoracic region with concurrent narrowing of the vertebral canal due to fracture of a vertebral body. Sudden onset of hindlimb paralysis likely is related to seizures and severe muscle spasms that begin to occur at the end of the third week of life. Once seizure activity begins, we recommend frequent monitoring of md rats for hindlimb paralysis and distention of the urinary bladder as indication of endpoints mandating prompt euthanasia.
Abbreviations: md, myelin-deficient
Animal models with challenging health problems continue to be essential in scientific research, but ethical concerns regarding using such models may be formidable, particularly if endpoints are not well defined. Rodents with dysmyelination (that is, lacking myelin in the CNS) have been used to study cellular mechanisms, biochemistry, and genetic mechanisms of myelination3,10,12,16,27,30 and as recipients of implanted cells in remyelination experiments.13,14,17,25,26,31 Lack of myelin prevents correct arrangement of ion channels in and around the node of Ranvier,1,33 resulting in incorrect, diffuse, and slow conduction of the action potential rather than saltatory, fast transmission that uses ion channels localized in and around nodes of Ranvier in normally myelinated axons. Animals with dysmyelination have therefore been useful in studying the function of ion channels in relation to presence or absence of myelin,11,15,18,20 important in elucidation of neurologic dysfunction in diseases of myelin loss such as multiple sclerosis and CNS injury. These models likely will be key for testing for efficacy of novel therapeutic approaches, whether they involve implanted cells or pharmaceutical compounds designed to restore neurologic function to demyelinated axons. With the use of sophisticated and powerful imaging systems such as MRI, interpretation of changes in the CNS including demyelination requires thorough understanding of correlations between the in vivo appearance of myelin-lacking tissue with neuropathology of demyelination, and recent studies involving dysmyelinated rodents have contributed to this understanding.2,4
Myelin is a powerful inhibitor of regrowth of cut axons that occur during CNS injury, effectively preventing recovery of neurologic function in normally myelinated animals and human patients. However, animals that lack myelin, especially those that survive beyond the first month of life, such as shiverer (shi) mouse and Long Evans Shaker (LES), have not been used to study regeneration of CNS tissue (neuroregeneration) in general or axonal regeneration in particular.
The myelin-deficient (md) rat, first described in 1979,6 has been used extensively in several laboratories to study the formation of CNS myelin and biology of glial cells.9,19,34 These rats have a single-base substitution in the proteolipid protein gene, on the X chromosome, affecting its expression and leading to severe dysmyelination.32 Rare, thin myelin sheaths are abnormal, in that the intraperiod line, an electrolucent space between 2 electrodense lines, is absent.8,21 Male md rats develop high-frequency intention whole-body tremors noticeable at 12 d of age and seizure activity with severe muscle convulsions starting at 17 d.6 These rats die suddenly during the 4h week of age, and despite numerous studies, the cause of death remains unknown, and endpoints useful for humane euthanasia are not established. A substrain of md rats that carries an identical mutation in the PLP gene but survives for as long as 80 d has been identified,10 but the cause of death in that strain has not yet been established either.
We performed clinicopathologic analysis of md rats to determine the cause of their deaths and to describe an endpoint useful for future studies using these rats. We determined that md rats develop sudden-onset hindlimb paralysis that results from hyperflexion of the spinal column and fracture of vertebral bodies in the midthoracic region. The resulting severe damage to the spinal cord is associated with hindlimb paralysis and likely with paralysis of the intercostal muscles and urinary bladder, both of which likely contribute to fatality. We propose frequent monitoring of seizuring md rats for sudden-onset hindlimb paralysis and their prompt euthanasia if paralysis occurs.
Materials and Methods
Animals.
Two female Wistar rats (Rattus norvegicus) that were carriers of the md phenotype were obtained from Dr Judith B Grinspan (Children's Hospital of Philadelphia, University of Pennsylvania). They were crossed with Wistar male rats (Charles River Laboratories, Montreal, Quebec, Canada), and male offspring had whole-body intention tremor noticeable by 12 to 14 d of age. The rats were housed free of endoparasites, fur mites, and Sendai virus, pneumonia virus of mice, sialodacryoadenitis virus, Kilham rat virus, H1 virus, parvovirus NS1, rat parvovirus, reovirus, rat minute virus, rat theilovirus, and Mycoplasma pulmonis according to ELISA testing performed by Charles River Laboratories. Rats were housed in a conventional rodent facility (Central Animal Facility, McMaster University, Hamilton, Ontario, Canada) and given rodent chow (Lab Diets, PMI Feeds, St Louis, MO) and tap water ad libidum. The Animal Research Ethics Board of McMaster University approved the breeding and care protocols involving these rats, and these procedures were conducted according to guidelines by the Canadian Council of Animal Care.5 Newborn pups in 4 litters were observed daily by one of the authors (JMK) until their euthanasia. Pups with whole-body tremors were designated as md rats (n = 13). The clinical abnormalities previously described6 included high-frequency intention body-tremor noticeable at 10 to 12 d and seizure activity with tonic–clonic convulsions with onset at approximately 3 wk of age. Spontaneous hindlimb paralysis and urinary bladder distended with hemorrhagic discharge were considered endpoints, and affected rats were euthanized in a CO2 chamber.
Pathology.
Complete necropsy was performed on euthanized md rats, and specimens of soft tissues and spine were collected in 10% phosphate-buffered formalin (pH 7.4). After 3 to 5 d, spines were placed in a Surgipath Decalcifier I (Surgipath Medical Industries, Richmond, IL) containing 10% formaldehyde, 8% formic acid, 1% methanol (%wt) until the bone became soft (approximately 10 to 15 d). Spines were trimmed sagitally or coronally. All tissues were dehydrated in increasing concentrations of ethanol (50% to 100%) followed by xylene, embedded in paraffin, and sliced into 5-μm sections, which were mounted on glass slides, stained with hematoxylin and eosin, and coverslipped. The prepared slides were analyzed under light microscopy (Eclipse 50i, Nikon, Tokyo, Japan) and remarkable changes photographed.
Results
Clinical assessment of the endpoint.
Whole-body tremors did not appear to affect the ambulatory ability of the md rats greatly. However, the rats suddenly developed seizures starting at the end of the third week of age. The seizures coincided with severe muscle convulsions followed by a period of immobility. Hindlimb paralysis occurred in all md rats suddenly during the fourth week of life. Rats with hindlimb paralysis (n = 13) often had noticeably increased breathing frequency and labored breathing. These rats had markedly distended urinary bladders, which were difficult to express in some cases. In some rats, urine was moderately to severely discolored with hemorrhagic exudate. Myelin-deficient rats with hindlimb paralysis and distended urinary bladders were considered to be at endpoint and were euthanized. The mean age of md rats at endpoint was 26 d (1 SD, 2.8 d; n = 13).
Pathologic analysis.
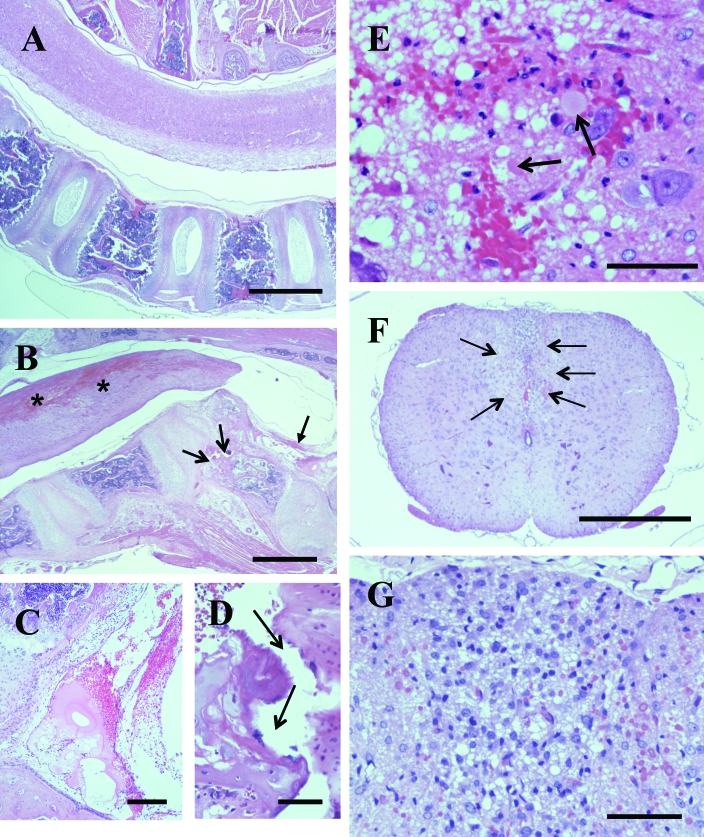
The inline arrangement of the vertebrae creates a spacious vertebral canal in normal rats (Figure 1 A). In md rats diagnosed with hindlimb paralysis, severe hemorrhages in the spinal cord were associated with focal narrowing of the spinal cord that corresponded to narrowing of the vertebral canal (Figure 1 B). In narrowed areas of the vertebral canal, which typically were present in the midthoracic region, a vertebral body was fractured and protruding upward. Mechanical disruption of the cancellous bone was associated by hemorrhage in the surrounding bone and marked hemorrhage and fibrin deposition in the epidural space above the fracture site (Figure 1 C, D). Large patchy to diffuse areas of hemorrhage were localized predominantly to the dorsal column and extended for a distance of 2 to 3 vertebrae rostrally and caudally from the fracture site, with gradual reduction of the severity of hemorrhage. Histologic analysis revealed patchy to diffuse hemorrhage encompassing and surrounded by marked microvacuolation and scattered swollen axons and neutrophils (Figure 1 E). In 3 of the 13 rats examined, the vertebral fracture site was associated with regional marked myositis with interstitial edema, fibroplasia, and infiltration by mononuclear phagocytes in the spinal muscles. In the adjacent spinal cord, a large area in the dorsal column had diffuse microvacuolation and marked infiltration by mononuclear phagocytic cells (Figure 1 F, G). There were small patchy areas of hemorrhage. Spinal lesions in 3 rats with severe phagocytic myelitis and regional myositis were considered to be subacute, whereas the hemorrhagic lesions in the remaining 10 rats were considered to be acute. All 13 rats examined had severe generalized pulmonary congestion and patchy alveolar edema and urinary bladders markedly distended with urine, with hemorrhagic exudate in a proportion of rats (not shown).
Figure 1.
Spine with spinal cord in myelin-deficient (md) rat. (A) Sagittal section of the spine including intact spinal cord. Bar, 1000 μm. (B) Vertebral canal is markedly narrowed over the angular protrusion of a vertebrae with its body fractured (open arrows). There is marked fibrinohemorrhagic exudate in the adjacent area of the vertebral canal (solid arrow). The spinal cord has large interconnected hemorrhages predominantly in the dorsal area (asterices). Bar, 1000 μm. (C) Higher magnification of the fibrinohemorrhagic exudate in the lumen of the vertebral canal indicated by the solid arrow in panel B. Bar, 200 μm. (D) Higher magnification of the fracture in the vertebral body (open arrows) in panel B. Bar, 50 μm. (E) Histologic detail of the area with hemorrhage in the spinal cord in panel B. There is patchy hemorrhage, marked vacuolation of the neuropil, scattered swollen axons (arrows), and scattered neutrophils. Bar, 50 μm. (F) Cross section of the thoracic spinal cord, with a large hypercellular area in the dorsal column (arrows). Bar, 500 μm. (G) Higher magnification of the area of hypercellularity in the dorsal column in panel F, showing diffuse vacuolation of neuropil and infiltration by mononuclear cells, presumably macrophages. Bar, 50 μm.
Discussion
Despite its short lifespan (approximately 4 wk), the myelin-deficient (md) rat is a valuable model of dysmyelination and has been widely used by neurobiologists specializing in abnormalities of CNS myelin.6 Review of the literature revealed 2 recent studies that addressed the possible effect of mutation in proteolipid protein gene on premature and sudden death in md rats,28,29 but the cause of death has remained unknown, thereby rendering these rats of limited use for in vivo experimentation. The unexplained and sudden mortality of md rats has complicated appropriate timing of euthanasia, thus presenting an ethical problem for the users of these rats. The present study was performed to elucidate the cause of death in md rats in attempt to determine a humane endpoint.
In another line of rats with dysmelination, Long Evans Shaker (LES) rats,7,24 sudden onset of hindlimb paralysis and distention of the urinary bladder with hemorrhagic urine have been used as endpoints.22 We used the same approach to establishing the endpoint for md rats. Although onset of hindlimb paralysis occurs at 5 to 15 wk of age in LES rats, rather than the fourth week of life as in md rats, the syndrome of hindlimb paralysis in either rat model is related to similar pathology and probably results from seizure activity and severe convulsion of the spinal muscles. We speculate that severe convulsion of spinal muscles causes hyperflexion of the spinal column and thus severe damage to the spinal cord. Spinal cord injury typically is associated with fractured vertebral bodies in the md rats (this study) and LES rats.22,23 We do not know why a large proportion of LES rats escape sudden hindlimb paralysis to live as long as a normal laboratory rat,22 whereas all md rats (except for the so-called ‘aged md rat’ substrain10) succumb to this fatal syndrome within the fourth week of age.
In the midthoracic area adjacent to the vertebral fracture, damage to the spinal cord is severe, results in hemorrhages obliterating the dorsal column and large areas of adjacent gray matter and lateral and ventral columns suggesting damage to the local vascular supply. The hemorrhages extend caudally and rostrally from the lesion in a tapering fashion indicating that damage to the nervous supply of the intercostal muscles and breathing difficulties may inevitably follow. Damage to the nervous supply of the urinary bladder can result in its distention with urine and rapid proliferation of microbial flora resulting in inflammatory damage to transitional epithelium and severe hemorrhagic cystitis. A proportion (n = 3) of md rats analyzed in this study had marked infiltration of the spinal cord injury area by mononuclear phagocytes, presumably macrophages suggesting that in some md rats, a less severe damage to the spinal cord may result in more protracted syndrome where a paralyzed md rat may experience a delayed onset of breathing difficulties and hemorrhagic cystitis. Furthermore, md rats with broken backs continue to have seizures which can result in additional injury to the already damaged area of the spinal cord due to repeated periods of hyperflexion of the spinal column at the site of vertebral fracture.
The sudden death of dysmyelinated mutants that are prone to seizures and convulsions, including md rats, creates a major ethical concern. We therefore recommend frequent monitoring of md rats and other dysmyelinated models for signs of hindlimb paralysis and distention of the urinary bladder once seizure activity has begun. Definition of a recommended endpoint likely will facilitate the use of this difficult animal model in research projects requiring survival until endpoint. The evidence of pulmonary congestion and hemorrhagic cystitis in md rats with hindlimb paralysis indicate that these rats survive for several hours after the traumatic damage to the spinal cord, thus providing an opportunity to apply the endpoint and euthanize the affected animal. We recommend frequent monitoring of md rats by an observer familiar with clinical signs of hindlimb paralysis as an integral part of any research project using the md rat and other dysmyelinated and seizuring animal models.
Acknowledgment
We thank Dr Judith Grinspan of Children's Hospital of Philadelphia, University of Pennsylvania for providing heterozygous female Wistar rats that were carriers of the md mutation.
References
- 1.Arroyo EJ, Xu T, Grinspan J, Lambert S, Levinson SR, Brophy PJ, Peles E, Scherer SS. 2002. Genetic dysmyelination alters the molecular architecture of the nodal region. J Neurosci 22:1726–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar-Shir A, Duncan ID, Cohen Y. 2009. QSI and DTI of excised brains of the myelin-deficient rat. Neuroimage 48:109–116 [DOI] [PubMed] [Google Scholar]
- 3.Beesley JS, Lavy L, Eraydin NB, Siman R, Grinspan JB. 2001. Caspase 3 activation in oligodendrocytes from the myelin-deficient rat. J Neurosci Res 64:371–379 [DOI] [PubMed] [Google Scholar]
- 4.Biton IE, Duncan ID, Cohen Y. 2006. High b-value q-space diffusion MRI in myelin-deficient rat spinal cords. Magn Reson Imaging 24:161–166 [DOI] [PubMed] [Google Scholar]
- 5.Canadian Council on Animal Care 1993. Guide to the care and use of experimental animals, vol 1, 2nd ed Ottawa (Canada): Canadian Council of Animal Care [Google Scholar]
- 6.Csiza CK, de Lahunta A. 1979. Myelin deficiency (md): a neurologic mutant in the Wistar rat. Am J Pathol 95:215–224 [PMC free article] [PubMed] [Google Scholar]
- 7.Delaney KH, Kwiecien JM, Wegiel J, Wisniewski HM, Fletch AL. 1995. Familial dysmyelination in a Long Evans rat mutant. Lab Anim Sci 45:547–553 [PubMed] [Google Scholar]
- 8.Duncan ID, Hammang JP, Trapp BD. 1987. Abnormal compact myelin in the myelin-deficient rat: absence of proteolipid protein correlates with a defect in the intraperiod line. Proc Natl Acad Sci USA 84:6287–6291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan ID, Jackson KF, Hammang JP, Marren D, Hoffman R. 1993. Development of myelin mosaicism in the optic nerve of heterozygotes of the X-linked myelin-deficient (md) rat mutant. Dev Biol 157:334–347 [DOI] [PubMed] [Google Scholar]
- 10.Duncan ID, Nadon NL, Hoffman RL, Lunn KF, Csiza C, Wells MR. 1995. Oligodendrocyte survival and function in the long-lived strain of the myelin deficient rat. J Neurocytol 24:745–762 [DOI] [PubMed] [Google Scholar]
- 11.Eftekharpour E, Karimi-Abdolrezaee S, Sinha K, Velumian AA, Kwiecien JM, Fehlings MH. 2005. Structural and functional alterations in spinal cord axons in adult Long Evans shaker (LES) dysmyelinated rats. Exp Neurol 193:334–349 [DOI] [PubMed] [Google Scholar]
- 12.Espinosa de los Monteros A, Kumar S, Zhao P, Huang CJ, Pan T, Nazarian R, Pan T, Scully S, Chang R, de Vellis J. 1999. Transferrin is an essential factor for myelination. Neurochem Res 24:235–248 [DOI] [PubMed] [Google Scholar]
- 13.Espinosa de los Monteros A, Zhao P, Huang C, Pan T, Chang R, Nazarian R, Espejo D, de Vellis J. 1997. Transplantation of CG4 oligodendrocyte progenitor cells in the myelin-deficient rat brain results in myelination of axons and enhanced oligodendroglial markers. J Neurosci Res 50:872–887 [DOI] [PubMed] [Google Scholar]
- 14.Fanarraga ML, Griffiths IR, Zhao M, Duncan ID. 1998. Oligodendrocytes are not inherently programmed to myelinate a specific size of axon. J Comp Neurol 399:94–100 [PubMed] [Google Scholar]
- 15.Felts PA, Black JA, Waxman SG. 1995. Expression of sodium channel α and β subunits in the nervous system of the myelin-deficient rat. J Neurocytol 24:654–666 [DOI] [PubMed] [Google Scholar]
- 16.Grinspan JB, Coulalaglou M, Beesley JS, Carpio DF, Scherer SS. 1998. Maturation-dependent apoptotic cell death of oligodendrocytes in myelin-deficient rats. J Neurosci Res 54:623–634 [DOI] [PubMed] [Google Scholar]
- 17.Hammang JP, Archer DR, Duncan ID. 1997. Myelination following transplantation of EGF-responsive neural stem cells into a myelin-deficient environment. Exp Neurol 147:84–95 [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Tokuriki M, Shibamori Y, Saito T, Njyo Y. 2004. Cochlear nerve demyelination causes prolongation of wave I latency in ABR of the myelin-deficient (md) rat. Hear Res 191:119–124 [DOI] [PubMed] [Google Scholar]
- 19.Jackson KF, Duncan ID. 1988. Cell kinetics and cell death in the optic nerve of the myelin-deficient rat. J Neurocytol 17:657–670 [DOI] [PubMed] [Google Scholar]
- 20.Kaplan MR, Meyer-Franke A, Lambert S, Bennet V, Duncan ID, Levison SR, Barres BA. 1997. Induction of sodium channel clustering by oligodendrocytes. Nature 386:724–728 [DOI] [PubMed] [Google Scholar]
- 21.Koeppen AH, Csiza CK, Willey AM, Ronne M, Barron KD, Dearborn RE, Hurwitz CG. 1992. Myelin deficiency in female rats due to a mutation in the PLP gene. J Neurol Sci 107:78–86 [DOI] [PubMed] [Google Scholar]
- 22.Kwiecien JM. 2010. Cellular compensatory mechanisms in the CNS of dysmyelinated rats. Comp Med 60:205–217 [PMC free article] [PubMed] [Google Scholar]
- 23.Kwiecien JM, Blanco M, Fox JG, Delaney KH, Fletch AL. 2000. Neuropathology of bouncer Long Evans, a novel dysmyelinated rat. Comp Med 50:503–510 [PubMed] [Google Scholar]
- 24.Kwiecien JM, O'Connor LT, Goetz BD, Delaney KH, Fletch AL, Duncan ID. 1998. Morphological and morphometric studies of the dysmyelinating mutant, the Long Evans shaker rat. J Neurocytol 27:581–591 [DOI] [PubMed] [Google Scholar]
- 25.Learish RD, Brustle O, Zhang SC, Duncan ID. 1999. Intraventricular transplantation of oligodendrocyte progenitors into a fetal myelin mutant results in widespread formation of myelin. Ann Neurol 46:716–722 [PubMed] [Google Scholar]
- 26.Li D-W, Duncan ID. 1998. The immune status of the myelin-deficient rat and its immune responses to transplanted allogeneic glial cells. J Neuroimmunol 85:202–211 [DOI] [PubMed] [Google Scholar]
- 27.Lipsitz D, Goetz BD, Duncan ID. 1998. Apoptotic glial cell death and kinetics in the spinal cord of the myelin-deficient rat. J Neurosci Res 51:497–507 [DOI] [PubMed] [Google Scholar]
- 28.Mayer CA, Macklin WB, Avishai N, Balan K, Wilson CG, Miller MJ. 2009. Mutation in the myelin proteolipid protein gene alters BK and SK channel function in the caudal medulla. Respir Physiol Neurobiol 169:303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller MJ, Haxhiu MA, Georgiadis P, Gudz TI, Kangas CD, Macklin WB. 2003. Proteolipid gene mutation induces altered ventilatory response to hypoxia in myelin-deficient rat. J Neurosci 23:2265–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pringle NP, Nadon NL, Rhode DM, Richardson WD, Duncan ID. 1997. Normal temporal and spatial distribution of oligodendrocyte progenitors in the myelin-deficient (md) rat. J Neurosci Res 47:264–270 [DOI] [PubMed] [Google Scholar]
- 31.Schiff R, Rosenbluth J, Dou W-K, Liang W-L, Moon D. 2002. Distribution and morphology of transgenic mouse oligodendroglial-lineage cells following transplantation into normal and myelin-deficient rat CNS. J Comp Neurol 446:46–57 [DOI] [PubMed] [Google Scholar]
- 32.Simons R, Riordan JR. 1990. The myelin-deficient rat has a single base substitution in the 3rd exon of the myelin proteolipid gene. J Neurochem 54:1079–1081 [DOI] [PubMed] [Google Scholar]
- 33.Struckhoff G, Przyrembel C, Bahr M, Goht A. 1997. Fate of developing astrocytes in the optic nerve of the myelin-deficient rat. J Comp Neurol 378:105–116 [DOI] [PubMed] [Google Scholar]
- 34.Yanagisawa K, Duncan ID, Hammang JP, Quarles RH. 1986. Myelin-deficient rat: analysis of myelin proteins. J Neurochem 47:1901–1907 [DOI] [PubMed] [Google Scholar]