Abstract
Dark-phase light contamination can significantly disrupt chronobiologic rhythms, thereby potentially altering the endocrine physiology and metabolism of experimental animals and influencing the outcome of scientific investigations. We sought to determine whether exposure to low-level light contamination during the dark phase influenced the normally entrained circadian rhythms of various substances in plasma. Male Sprague–Dawley rats (n = 6 per group) were housed in photobiologic light-exposure chambers configured to create 1) a 12:12-h light:dark cycle without dark-phase light contamination (control condition; 123 µW/cm2, lights on at 0600), 2) experimental exposure to a low level of light during the 12-h dark phase (with 0.02, 0.05, 0.06, or 0.08 µW/cm2 light at night), or 3) constant bright light (123 µW/cm2). Dietary and water intakes were recorded daily. After 2 wk, rats underwent 6 low-volume blood draws at 4-h intervals (beginning at 0400) during both the light and dark phases. Circadian rhythms in dietary and water intake and levels of plasma total fatty acids and lipid fractions remained entrained during exposure to either control conditions or low-intensity light during the dark phase. However, these patterns were disrupted in rats exposed to constant bright light. Circadian patterns of plasma melatonin, glucose, lactic acid, and corticosterone were maintained in all rats except those exposed to constant bright light or the highest level of light during the dark phase. Therefore even minimal light contamination during the dark phase can disrupt normal circadian rhythms of endocrine metabolism and physiology and may alter the outcome of scientific investigations.
Abbreviation: FFA, free fatty acid; SCN, suprachiasmatic nuclei; TFA, total fatty acid
The critical effect of lighting cycles on the synchronization of animal circadian rhythms has been investigated for many years.2-5,14,15,18,25,41,44,48,51 Even minor deviations in the intensity and duration of environmental light at a given time of day can alter or disrupt various chronobiologic rhythms.11,25,52,53 Therefore, knowledge and appropriate maintenance of controlled lighting in laboratory animal facilities is important to biomedical researchers and animal care personnel alike. Over time, animal facilities worldwide have incorporated doors with open or translucent windows connecting to nighttime lighted corridors or the outside environment, doors lacking light-tight seals, and even lighted equipment within the animal quarters.20 Such designs may compromise adherence to laboratory animal controlled lighting cycles and protocols, as is recommended in The Guide.44 If animal facilities do not maintain light-tight housing environments, an important consideration becomes identifying levels of light contamination that will not compromise diurnal rhythms of animal physiology and metabolism.
Visually perceived light influences animal physiology and behavior.3,5,9-11,18,25,48,54 Light that is detected by the eyes activates the neural pathway of the primary optic tract, leading to vision. However, some profound effects of light appear to occur by means of the nonvisual neural pathway of the retinohypothalamic tract.6,26 In laboratory rodents and in mammals in general, the retinohypothalamic tract interfaces with a circadian timing system that regulates and maintains all daily rhythms.12,53,55 This system consists of a central molecular circadian clock, located in the suprachiasmatic nuclei (SCN) of the hypothalamus,53 that is entrained (that is, synchronized) by regular alterations in the light–dark cycle. Light entering the eyes reaches specialized photoreceptors in the retina that stimulate specific neurons of the retinohypothalamic tract, which extends to the SCN.6,12,21,26,30,55 Fibers then pass from the SCN to the pineal gland by means of a multisynaptic pathway through the paraventricular nucleus, upper thoracic intermediolateral cell column, and superior cervical ganglia. The light–dark cycle entrains the rhythmic synthesis and production of the pineal neurohormone melatonin.54 This effect depends on the specific wavelength (459 to 520 nm; blue-green, most potent),10 intensity,9 and duration11 of the dark-phase light exposure.
The pacemaker activity of the SCN drives a complex series of molecular events, resulting in pineal gland production of the principal chronobiotic neurohormone melatonin (N-acetyl-5-methoxytryptamine) during the night.9,28,41 The daily rhythmic melatonin signal contributes to the temporal coordination of normal behavioral and physiologic functions, including sleep–wake cycle,3,48 locomotor activity,2,61 feeding behavior,2,46 retinal physiology,5,6 seasonal reproductive cycles,51 immune function,13,35 cardiovascular function,33,42 bone metabolism,37 and intermediary metabolism.32 Exposure of animals to light at night suppresses melatonin production, which may contribute to several diseases in rodents52 and alter various normal functions associated with maturation,25 reproductive development,53 adrenal hormone cycling,23 anabolic activity of the brain and brain protein synthesis,60 and redox enzyme activity in the CNS.49
Previous studies from our laboratory8,14,15 provided the first direct link between exposure of laboratory animals to low-intensity light during dark phase and suppression of nighttime pineal production of melatonin, leading to disruptions in diurnal patterns of animal metabolism and stimulation of rodent and human tumor growth in rats. We determined that exposure to as little light (white polychromatic) as 0.20 lx (0.08 μW/cm2 at rodent eye level) during an otherwise normal dark phase resulted in a marked increase in rodent hepatoma and MCF7 steroid-receptor–negative human breast cancer growth and metabolism, with a magnitude rivaling that provoked by exposure to constant bright light. This effect was mediated by light-induced suppression of the normal nighttime melatonin production, which allowed diurnally continuous tumor uptake of linoleic acid and its metabolism to the mitogenic agent, 13-(S)-hydroxyoctadecadienoic acid.7,57 Linoleic acid is a tumor growth promoter and the most prominent fatty acid in the human Western diet and in most laboratory animal chows.7,56 Nighttime melatonin tumor growth suppression occurred by means of melatonin receptor-mediated inhibition of cAMP-dependent signal transduction pathways.7,8,57 These studies provided the first laboratory-derived experimental evidence in support of subsequent epidemiologic findings showing that, in humans, alternating night-shift work, as a surrogate for exposure to light at night, significantly increases the risk of breast cancer.16,24,58
In the course of our previous work, we observed that during normal or light-contaminated dark phases, the diurnal changes in plasma lipid levels associated with normal feeding cycles were not disrupted, unlike the situation in rats exposed to a constant bright-light environment. This finding suggested that the existence of a minimal response threshold to light between 0.20 lx (0.08 µW/cm2) and a constant bright-light environment (300 lx; 123.0 µW/cm2) with regard to whether these changes in plasma lipid concentrations occur. The current study used laboratory rats to compare dark-phase exposure to minimal intensity light with bright light exposure. The study evaluated nocturnal melatonin production and alterations in the normal chronobiologic rhythms of markers of physiology and metabolism that are altered in metabolic disorders such as glucose intolerance,34,36 insulin resistance,4,29,32 and obesity50 and diseases such as cardiovascular disease,17,18 type II diabetes,34,36 and cancer.7,8,14,15
Materials and Methods
Animals, housing conditions, and diet.
Weanling (35 to 50 g), male, SPF Sprague–Dawley rats (Hsd:Sprague–Dawley SD) were purchased from Harlan (Indianapolis, IN). These rats were maintained in environmentally controlled rooms (23 °C; 45% to 50% humidity) in microisolation units (Thoren Caging Systems, Hazleton, PA) in an AAALAC-accredited facility in accordance with the Guide for the Care and Use of Laboratory Animals.42 This investigation was approved by Bassett Research Institute of the Mary Imogene Bassett Hospital IACUC, where the study was conducted, and by the Tulane University School of Medicine IACUC, where all procedures were IACUC-approved and the manuscript was written. Rats were maintained in autoclaved polycarbonate cages (Thoren Caging Systems; model 4; 12.13 in. × 12.13 in. × 7.37 in.; 2 rats per cage) by using hardwood maple bedding (no. 7090M, Sanichips, Harlan Teklad, Madison, WI; 2 bedding changes weekly). To ensure that all study animals remained uninfected with bacterial or viral agents during the course of this study, serum samples from sentinel rats, housed solely on the combined soiled bedding from other cages in the same housing unit, were tested by ELISA (Sendai virus, pneumonia virus of mice, sialodacryoadenitis virus, Kilham rat virus, Toolan H1 virus, Theiler murine encephalomyelitis virus, reovirus, Myocoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus 1 and 2, Hantaan virus, Encephalitozoon cuniculi, cilia associated respiratory bacillus, parvovirus NS1, rat parvoviruses, and rat murine virus; Comprehensive Health Monitoring Program, Charles River Laboratories, Kingston, NY). Lighting was diurnal, 12:12-h light:dark (lights on 0600 to 1800; 300 lx; 123 µW/cm2); rats were completely protected from exposure to any light at night. Rats had free access to autoclaved, acidified, municipal tap water and an essential-fatty-acid-replete diet (Prolab RMH 1000 formula, Agway, Syracuse, NY) assayed as described previously.14,15 Analysis of the fatty acid content of the diet revealed a total fatty acid (TFA; mean ± 1 SD) content of 49.9 ± 1.3 mg/g, composed of 1.3% ± 0.2% myristic (C14:0), 12.5% ± 0.2% palmitic (C16:0), 2.4% ± 0.1% palmitoleic (C16:1, n-7), 4.1% ± 0.1% stearic (C18:0), 21.7% ± 0.4% oleic (C18:1, n-9), 56.1% ± 0.8% linoleic (C18:2, n-6), and 0.2% ± 0.1% arachidonic (C20:4, n-6) acids. Minor additional fatty acids comprise approximately 1.7%. More than 93% of the fatty acids were present as triglycerides and phospholipids; more than 6% were present as free fatty acids (FFA).
Lighting regimens.
After a 1-wk acclimation period, rats were randomized into 6 designated groups of 6 animals each (2 per cage) and placed in photobiologic light exposure chambers, as previously described.8 Briefly, each chamber was illuminated by 2 separate, solid-state, electromagnetic fluorescent ballasts with rapid-start, cool-white lamps connected to separate 24-h timers. One ballast–lamp system (GE Watt-Miser, F34CW-RS-WM, 34-W bulb, GE, Cleveland, OH) in each chamber provided a steady, light stimulus at eye level (300 lx; 123 µW/cm2), an accepted light intensity that reduces the potential for light-induced retinal degeneration in albino rodents,11,44 particularly Sprague–Dawley rats.44 The second ballast–lamp system (GE Starcoat, F32T8-SP-11, 32-W bulb, GE) was adjustable with a combination of neutral density filter material (CINEGEL 3403, N-9; Roscoe Laboratories, Stanford, CT) and electric dimmer modules to emit steady, indirect light uniformly at rodents’ eye level during the dark phase. At the end of the 1-wk acclimation period, animals were maintained under either the control condition (group 1; 12 h light [300 lx; 123 µW/cm2, lights on 0600; 12 h of dark without light contamination or an experimental condition with low intensity light at night) or with 0.02 µW/cm2 (group 2), 0.05 µW/cm2 (group 3), 0.06 µW/cm2 (group 4), or 0.08 µW/cm2 (group 5) light contamination during the 12-h dark phase. A final group (group 6) was exposed to constant bright light (123 µW/cm2, 300 lx). All light intensities were measured and regularly monitored (model 1L 1400a Radiometer–Photometer, International Light, Newburyport, MA) in the center of each cage at animals’ eye level. As a matter of convention, in terms of human or animal environments, the terminology ‘luminous flux’ (that is, lux) is used to describe the amount of light falling on a surface to stimulate the optic system. Although lux is the standard unit used nationally to specify lighting in both human and animal facilities,11,20,44 we also here report light intensities in radiometric terms (µW/cm2) for added usefulness to the research community. Rats were exposed to the various lighting intensities for the remainder of the study (a period of 6 wk). Cage positions were rotated daily during the light phase within the chambers to minimize the potential effects of slight variations in the reflected light intensity within each chamber.
Arterial blood collection.
After 2 wk of exposure to the described lighting regimens, rats underwent a series of 6 low-volume blood draws by means of cardiocentesis to collect heart blood, as described previously.8,14,15 Blood was collected over a period of 30 d. Briefly, blood collections were designated at 4-h intervals over the 24-h day; each animal was tested only once every 5 d to reduce effects of blood collection on feeding, stress, and potential mortality. Animals were lightly anesthetized by CO2 inhalation (70% CO2, 30% air); 1-mL samples were taken from the left ventricle by cardiocentesis (less than 5% total blood volume) by using a tuberculin syringe (25 gauge, 3/8 in.; Becton-Dickinson, Franklin Lakes, NJ) moistened with sodium heparin (1000 U/mL; Elkin-Sinn, Cherry Hill, NJ). Blood sampling during the dark phase (that is, at 2000, 2400, and 0400) was accomplished for groups 1 and 2 by using a safelight red lamp (120 V, 15 W; catalog no.152 1517, Kodak 1A, model B, Kodak, Rochester, NY) to preserve the nocturnal melatonin surge.8,11,14,15,28,41
Melatonin analysis.
Plasma melatonin levels measured by radioimmunoassay by using the sensitive melatonin rat 125I radioimmunoassay kit (catalog no. BA 3500, Labor Diagnostika Nord, Nordham, Germany) and analyzed (Cobra 5005 Automated Gamma Counter, Packard, Palo Alto, CA), as previously described.8 The minimal detection level for the assay was 1 to 2 pg/mL plasma.
Fatty acid extraction and analysis.
Plasma FFA, triglycerides, phospholipids, and cholesterol esters were extracted from 0.1-mL samples, as previously described.14,15,56 Prior to extraction, heptadecanoic acid (100 µg), tripentadecanoin (105 µg), diheptadecanoyl phosphatidyl choline (145 µg), and cholesterol heptadecanoate (244 µg), which all had been dissolved in chloroform (Fisher Scientific, Fair Lawn, NJ), were added as internal standards. Separation of the lipid fractions was achieved through thin-layer chromatography by using silica gel G plates (250 µM; no. 01011, Analtech, Newark, DE) coated with rhodamine B in ethanol (Sigma Scientific, St Louis, MO). Methyl esters of fatty acids were analyzed by using a gas chromatograph (model 5890A, Hewlett Packard) fitted with a flame ionization detector (model 7673 A, Hewlett Packard), autoinjector (model 7673 S, Hewlett Packard), and integrator (model 3396A). All separations were performed by using a 0.25-mm × 30-m capillary column (model 2380; Supelco, Bellefonte, PA) at 190 °C, with helium as the carrier gas (linear rate, 20 cm/s; split 100:1). The injection port and detector were adjusted to 220 °C. All methyl esters were identified on the basis of their retention time, compared with that of known standards. The minimal detectable limit for the assay was 0.05 µg/mL.
Enzymatic analysis of glucose, lactic acid, and corticosterone.
Plasma samples were analyzed for glucose and lactic acid concentrations (Roche Diagnostics, Roswell, GA) by spectrophotometry (Cobas MIRA Plus, Roche, Branchburg, NJ), as previously described.8 Levels are reported here as mg/dL plasma. Plasma was prepared in duplicate for measurement of corticosterone levels (catalog no. TKRC1, bulletin no. 2006-12-29, Coat-A-Count Rat Corticosterone Kit, Siemens Medical Solutions Diagnostics, Los Angeles, CA), a solid-phase 125I radioimmunoassay, as described previously.22 Briefly, at room temperature and in duplicate, 50 µL plasma was placed in rat corticosterone antibody-coated tubes (TRC1, Roche) and incubated for 2 h at room temperature (15 to 28 °C). Tubes were then decanted thoroughly, dried, and analyzed (Cobra 5005 Automated Gamma Counter, Packard). Corticosterone concentrations were calculated based on known standards (calibrator A–H, 0 to 2000 ng/mL; Roche) and reported as ng/mL plasma. The lower limit of the assay was 1.0 ng/mL, and the coefficient of variation of the immunoassay was less than 4%.
Statistical analysis.
All data are presented as mean ± 1 SD unless otherwise noted, based on 6 rats per group, and were compared by using one-way ANOVA followed by Student–Neuman–Keul multiple comparison test to evaluate differences. Differences among group means were considered statistically different at a P value of less than 0.05.
Results
Illumination in light-exposure chamber.
Mean daytime radiometric illuminance measurements for each of the groups (including constant-light group 6) were not significantly different (P > 0.05) and are reported here as a combined mean value of 300 ± 1 lx (123.25 ± 0.43 µW/cm2). Mean dark-phase light-at-night illuminance measurements for each of the groups were 0 (0; group 1), 0.05 (0.022 ± 0.002; group 2), 0.12 (0.05 ± 0.004; group 3), 0.15 (0.062 ± 0.004; group 4), and 0.20 (0.08 ± 0.004; group 5) lx (µW/cm2), respectively (n = 24 measurements per chamber).
Dietary and water intake and animal growth rate measurements.
Daily dietary and water intakes and body growth rates over the 6-wk course of this study did not vary significantly (P > 0.05) between the groups exposed to the various lighting regimens and controls. The mean daily dietary intake was 4.78 ± 0.50 g per 100 g body weight daily; mean water intake was 12.1 ± 1.9 mL per 100 g body weight daily; and average body weight gain measured 5.36 ± 0.38 g daily (n = 42).
Plasma melatonin and lipid values.
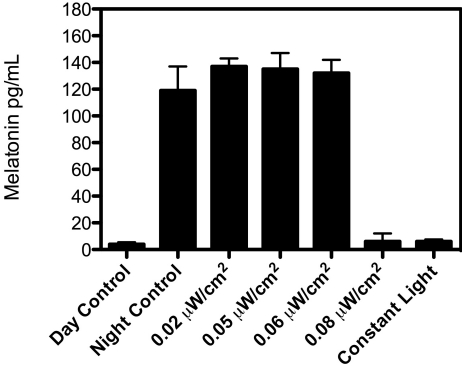
Diurnal rhythms in concentrations of plasma melatonin and lipids were measured in rats from all groups (Figure 1). Plasma melatonin levels in groups 1 to 4, which were high during the dark phase (131 ± 8 pg/mL) and low during the light phase (6.3 ± 0.2 pg/mL), were consistently low throughout the 24-h period light and dark phases of rats in groups 5 and 6. Melatonin values for and diurnal rhythms in rats from groups 1 through 4 were in good agreement with those reported for other strains of laboratory rats maintained in similar lighting environments. 8,14,15
Figure 1.
sPlasma melatonin levels (pg/mL; mean ± 1 SD) of Sprague–Dawley rats (n = 6 per group) maintained for 6 wk on either a control 12:12-h light:dark cycle (300 lx; 123 μW/cm2; lights on, 0600; group 1); experimental light-at-night (LAN) lighting cycles (that is, a 12:12-h light:dark cycle with light contamination during the dark phase) with 0.02 (group 2); 0.04 (group 3), 0.06 (group 4), 0.08 (group 5) μW/cm2 during the dark phase; or constant bright light (group 6). Plasma samples for controls were obtained at either daytime (1600, day control) or nighttime (0400, night control) and for experimental groups (2 through 6) during the dark phase at 0400. Melatonin levels in the control group at 0400 and 1600 differed significantly (P < 0.05); melatonin levels for groups 1 (1600), 5 (0400), and 6 (0400) did not differ between each other or with those of groups 2 through 4 (0400).
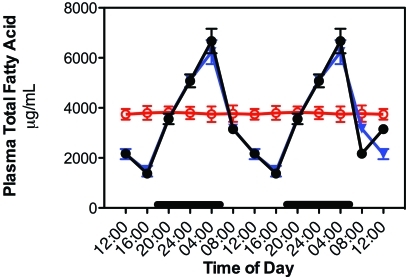
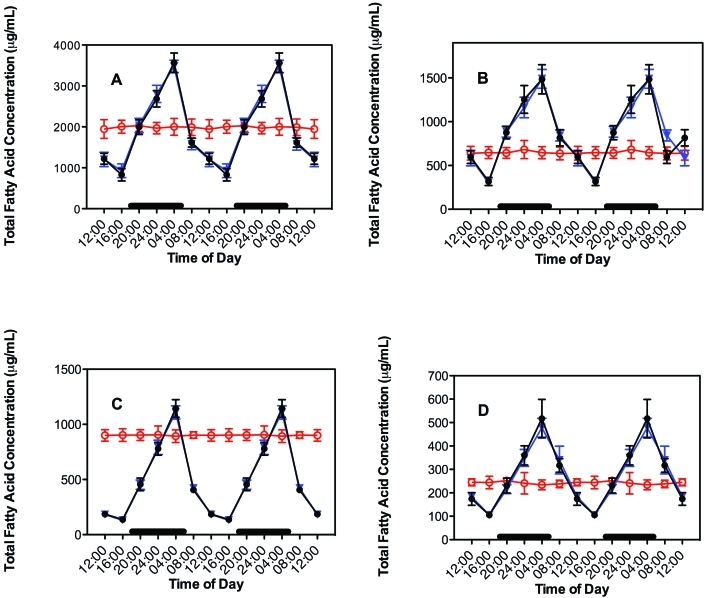
The diurnal rhythms for plasma concentrations TFA and linoleic acid from rats with free access to the diet are shown in Figure 2 for groups 1 through 6. TFA were analyzed further as various major lipid fractions (Figure 3). The plasma lipid levels in groups 1 through 5 paralleled that of normal feeding activity, as reported previously.8,14,15 Values of fatty acid and lipid fraction concentrations measured during the dark phase and light phases were significantly (P < 0.001) different, with peak levels occurring at 2400 to 0400 and decreasing to a nadir between 1200 to 1600. No diurnal variation occurred in rats maintained in the constant bright-light environment (group 6); plasma TFA levels in these rats remained continuously elevated throughout the entire day. Despite the presence of a rhythm in groups 1 through 5 and the lack of a rhythm in group 6, the daily combined mean TFA and linoleic acid values for all 6 groups was not significantly different from each other, with mean values of 3678 ± 1525 and 1313 ± 524 µg/mL plasma, respectively. Plasma concentrations of the 4 major fatty acid lipid classes (triglycerides > FFA > phospholipid > cholesterol ester) did not differ among the 6 study groups, reflecting similar daily dietary intake. Plasma FFA and triglycerides revealed an elevated supply of palmitic and oleic acids, whereas all lipid classes showed substantial quantities (> 30%) of linoleic acid, particularly in the FFA fraction (data not shown). Arachidonic acid, highest in phospholipid and cholesterol ester fractions, was lowest in FFA and triglyceride fractions, in agreement with previous observations in plasma of groups 1, 5, and 6 rats of the inbred Buffalo rat strain (BUF/CrCrl).14,15,56
Figure 2.
Diurnal changes in plasma total fatty acids in the arterial blood of animals in groups 1 through 4 (closed circles), 5 (triangles), and constant bright light (open circles, group 6) fed normal chow ad libitum. Animals were subjected to dark-phase lighting cycles (as described in the legend to Figure 1) from 1800 to 0600. Values are expressed as μg/mL plasma, and total fatty acid values are the sums of myristic, palmitic, palmitoleic, stearic, oleic, linoleic, and arachidonic acids. Each point represents mean ± 1 SD (n = 6 per group). Data are plotted twice. Concentrations without asterisks are different (P < 0.05) from concentrations with asterisks.
Figure 3.
Diurnal changes in the blood plasma lipid concentrations in the arterial blood of adult male Sprague–Dawley rats fed normal chow ad libitum. Animals were subjected to dark-phase lighting cycles (as described in the legend to Figure 1) from 1800 to 0600 (indicated by dark bars). Total fatty acid values (μg/mL plasma; mean ± 1 SD; n = 6 per group) for groups 1 through 4 (closed circles), 5 (triangles), and 6 (constant bright light; open circles) were the sums of myristic, palmitic, palmitoleic, stearic, oleic, linoleic, and arachidonic acids in the different lipid classes of (A) triglycerides, (B) free fatty acids, (C) phospholipids, and (D) cholesterol esters collected at the various time points. Data are plotted twice. Concentrations without asterisks are different (P < 0.05) from concentrations with asterisks.
Plasma glucose, lactic acid, and corticosterone values.
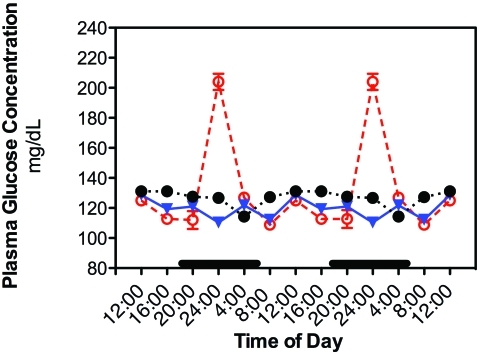
Daily rhythms of plasma glucose concentrations are depicted in Figure 4. The values for every 4-h time point in groups 1 through 4 were not significantly different, and therefore were averaged together. Phase shifts were determined by comparing peak values from experimental groups 5 and 6 with the peak values of group 1. A ‘phase advance’ was defined as a shift in a group peak level to an earlier time (that is, from 0400 to 2400), whereas a ‘phase delay’ was defined as a peak shifting to a later time (that is, from 0400 to 0800 h). In groups 1 through 4, the rhythm of plasma glucose concentration was somewhat higher (P < 0.05) during light phase (peak at 1200 h), decreasing to a nadir at middark phase (2400 h). A second, but lower-amplitude, peak occurred at 0400 followed by a second nadir at 0800. In contrast, in group 5 rats, plasma glucose levels showed phase shifts, in which the peak concentration occurred at 1600 h and the nadir at 0400. Groups 1 through 5 were normoglycemic throughout the entire 24-h period, with average daily concentrations of 124 ± 1 mg/dL (groups 1 through 4) and 120 ± 8 mg/dL (group 5). Plasma glucose levels of group 6 macaques (exposed to constant bright light) were significantly (P < 0.05) elevated at 1600 to 2400 compared with those of groups 1 through 5, with peak concentrations nearly 2-fold higher occurring at 2400. Overall, circulating plasma glucose concentrations across the 24-h day in rats of group 6 were nearly 2-fold increased and at hyperglycemic levels of 198 ± 20 mg/dL (P > 0.05 compared with groups I to V).
Figure 4.
Diurnal changes in plasma glucose levels (mg/dL; mean ± 1 SD; n = 6 per group) in the arterial blood of rats in groups 1 through 4 (closed circles), 5 (triangles), and 6 (constant bright light; open circles). Rats were exposed to dark-phase lighting cycles (as described in the legend to Figure 1) from 1800 to 0600 (dark bars). Data are plotted twice. Concentrations without asterisks are different (P < 0.05) from concentrations with asterisks.
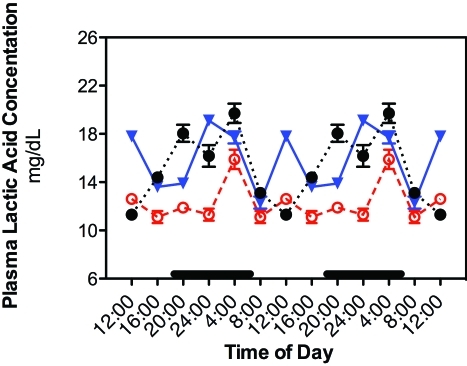
In addition, similar phase-shifts occurred in daily rhythms of plasma concentrations of lactic acid (Figure 5). In groups 1 through 4, lactic acid concentrations began to increase from lowest levels at the beginning of the light phase (0800) to a peak 4 h later at 1200. A subsequent decrease in lactic acid concentration to low levels during the end of the light phase and beginning of the dark phase was followed by an increase to a second peak at middark phase (2400). Plasma lactic acid peaks in group 5 rats were phase-advanced by 4 h, with peak levels at 2000 and again at 0400; a nadir in lactic acid concentration occurred during the middle of the light phase (1200). Plasma lactic acid concentrations in group 6 rats had lower integrated levels (1.37 ± 0.05 mM) over the 24-h period as compared with groups 1 to 5 (1.82 ± 0.01 mM; P < 0.05). The smaller amplitude peak at 0400 was also significantly (P < 0.05) lower than peak values for groups 1 through 5.
Figure 5.
Diurnal changes in plasma lactic acid concentrations (mg/dL; mean ± 1 SD; n = 6 per group) in the arterial blood of rats in groups 1 through 4 (closed circles), 5 (triangles), and 6 (constant bright light; open circles). Rats were exposed to dark-phase lighting cycles (as described in the legend to Figure 1) from 1800 to 0600 (dark bars). Data are plotted twice. Concentrations without asterisks are different (P < 0.05) from concentrations with asterisks.
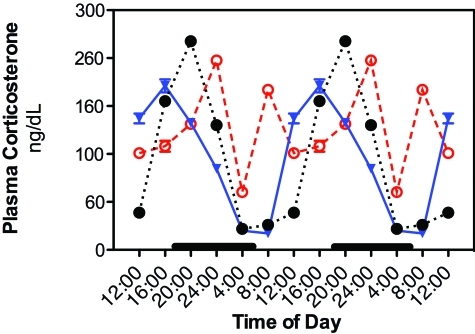
Plasma corticosterone levels revealed clear differences among groups 1 through 4, 5, and 6 with regard to daily rhythms and integrative concentrations (Figure 6). In groups 1 through 4, plasma corticosterone levels began to increase near the beginning of the light cycle, peaking at 1600 before decreasing throughout the dark-phase hours to lowest levels at 0400 through 0800. A similar pattern occurred for group 5 rats; however, the peak amplitude was phased-delayed by 4 h, compared with that for groups 1 through 4, peaking significantly (P < 0.05) higher at 2 h into the dark phase (2000). In contrast to levels for groups 1 through 5, plasma corticosterone in group 6 showed a phase-advanced peak during subjective night (2400) and a second peak at the 0800 time point, with overall levels elevated compared with those for groups 1 through 5. Average 24-h mean plasma corticosterone concentrations for groups 1 through 4, 5, and 6, respectively, were 112 ± 1 ng/mL (325 ± 4 nmol/L), 125 ± 2 ng/mL (361 ± 7 nmol/L), and 187 ± 2 ng/mL (540 ± 5 nmol/L), with group 6 levels consistently elevated throughout the 24-h period and significantly (P < 0.05) different from that of groups I through 5.
Figure 6.
Diurnal changes in plasma corticosterone concentrations (ng/dL; mean ± 1 SD; n = 6 per group) in the arterial blood of rats in groups 1 through 4 (closed circles), 5 (triangles), and 6 (constant bright light; open circles). Rats were exposed to dark-phase cycles (as described in the legend to Figure 1) from 1800 to 0600 (dark bars). Data are plotted twice. Concentrations without asterisks are different (P < 0.05) from concentrations with asterisks.
Discussion
Circadian rhythms in a diverse group of physiologic and behavioral processes are found in all vertebrates; they are under the control of the SCN, the intrinsic master biologic clock.55 Without pronounced SCN signals, circadian rhythms of peripheral tissues become decoupled, resulting in disorganization of cellular physiology and metabolism and increased risk of disease.11,20,38,44,54 Light is arguably one of the most important parameters in our environment. The present study shows that diurnal rhythms in plasma glucose, lactic acid, and corticosterone concentrations in rats are disrupted by exposure to low-level light (as little as 0.2 lx in group 5) during the dark phase. Because these rhythms are disrupted by exposure to dim light at night, they appear to be independent from and not coupled to the SCN-generated rhythms in dietary and water intake or fatty acid and lipid fractions concentrations (triglyceride > FFA > phospholipid > cholesterol ester). These results are in agreement with previous findings in our laboratory for the inbred Buffalo rat strain.14,15 Studies using mutant strains of mice that have meager or no nocturnal melatonin rhythm but normal feeding and activity patterns also corroborate our findings.32 Dietary and water intake and animal growth rate measurements in the present study are in good agreement with those determined for healthy young, male Sprague–Dawley rats.43,50 Plasma glucose levels peaked during the middle of the light phase in groups 1 through 5, followed by a decline to their lowest levels during the middle of the dark phase, prior to the animals’ main active period. By altering normal dietary regimens of or inducing hypothalamic lesions in rats, other investigators have shown similar SCN-generated glucose and insulin rhythms that are independent of both daily locomotor activity rhythms and feeding cycles.19,34,36,61
Like the rats exposed to dim light during the dark phase in the present study, pinealectomized rats, lacking the circadian melatonin signal, no longer exhibit circadian rhythms in blood glucose and lactic acid,19,34 insulin,19,36,40 and corticosterone levels.59 Although we did not measure insulin levels in our study, glucose concentrations remained at hyperglycemic levels, and levels of lactic acid and corticosterone were elevated markedly in groups 5 and 6, suggesting a critical role for the dark-phase melatonin signal in diurnal metabolic homeostasis. Furthermore, removing the dark-phase availability of melatonin in rats causes a decrease in hepatic and muscular glycogenesis53 and increases plasma concentrations of glucose and glucagon.24 Although the inhibitory effects of melatonin on glucose and lactic acid metabolism have been observed in both humans62 and rodents,19,34 the mechanism of action is unknown. One possibility is that melatonin may act directly on its target cells (hepatocytes and pancreatic β cells), which contain the melatonin receptors MT1 and MT2.1,47 In addition, the absence of the daily melatonin signal induces glucose intolerance and impairs insulin secretion and action,4,31,34,36 possibly through downregulation of expression of the insulin-dependent GLUT4 glucose transporter gene.36
Elevated corticosterone levels in rodents have long been associated with a variety of stressors, including anxiety, fear, pain, hemorrhage, infections, low blood glucose, and starvation.45 Corticosterone acts on muscle, liver, and adipose tissues to alter metabolism, thus providing the organism with the necessary fuels to better cope with stress. In adipose tissue, this glucocorticoid stimulates the release of fatty acids, including linoleic acid, from triglyceride fat stores. Fatty acids exported to other tissues serve as a source of fuel, whereas the glycerol released is used in gluconeogenesis by the liver. In addition, corticosterone functions to break down muscle proteins into amino acids, including glutamine, which function as the precursors for gluconeogenesis in the liver. The overall effect of these metabolic changes is to restore blood glucose to normal levels, increase glycogen stores, counterbalance the effects of insulin, and reduce stress.
Daily variations in plasma corticosterone levels in the laboratory rat were first reported in 1959 23 and subsequently confirmed over the years by others.22,57 Under the normal conditions of a 12:12-h light:dark cycle for laboratory rats (group 1), as in the present report, circulating corticosterone levels show a pronounced increase during the light phase, peaking just before dark onset. Subsequently, corticosterone concentrations fall throughout the dark phase, reaching their lowest levels at the dark–light transition. Rats in our experimental groups 2 through 4, all of which had similar, normal nighttime melatonin levels to those of the group 1 controls, exhibited identical diurnal profiles of corticosterone levels. For groups 5 and 6, overall daily corticosterone levels were elevated compared with those of groups 1 through 4, and peak levels were phase-delayed by 4 h (group 5) and 8 and 12 h (group 6). Others22 have demonstrated that peak amplitudes vary somewhat depending on animal strain and sex, with female rats having overall higher daily concentrations but male rats showing a more consistent rhythm. To our knowledge, ours is the first report demonstrating a direct association between a minimal-threshold light exposure during dark-phase, abrogation of the normal nighttime melatonin signal, and disruption of the corticosterone rhythm in any mammalian species, and more specifically, in rodents.
The consistency and quality of lighting intensity, wavelength, and duration, administered during well-controlled photoperiods in laboratory animal facilities, are of paramount importance maintaining stable animal models, in that variations in lighting will affect virtually every biologic response associated with animal physiology and metabolism. Factors such as lighting malfunctions that result in inappropriate illumination during the dark phase, light contamination from in-room equipment such as biosafety cabinets and lighted electric circuits or alarms, ‘leaky’ door jams and frames, and translucent door windows and disregard for proper lighting protocols may have long-lasting biologic and behavioral consequences. As an alternative to complete darkness during the dark phase, some investigators have suggested the use of sodium lighting (bichromatic wavelength, 570 nm) to monitor the dark-phase behavior of laboratory animals, in light of findings that exposure to this illumination did not alter normal wakefulness, alertness, and locomotor activity in certain strains of mice.39 However, others 27 have reported that even exposure to intermittent red light (650 nM) during the dark phase over as little as 2 wk can alter locomotor activity in C57BL/6 and DBA/2 mutant mice, much like full-spectrum lighting.3 However, the studies that used mutant mice32 did not report melatonin concentrations, which vary significantly among strains,32 or other measures of SCN-controlled circadian rhythms of metabolism and physiology. Furthermore, the effects of light threshold intensity and duration were not considered. The authors of that study32 concluded that locomotor activity, due to its complexity, was an unsuitable parameter for detection of subtle effects of the lighting on behavior. In more extensive studies in rodents10,32 and humans,12 measurements of the action spectra for melatonin regulation included both sodium- and red-light measurements of threshold intensity and dark-phase light exposure duration, and revealed the critical importance of all 3 parameters in relation to SCN-controlled metabolic and physiologic circadian rhythms.
Our study provides compelling evidence that exposure of rats to dim light during the dark phase, as sometimes occurs in laboratory animal facilities, suppresses melatonin production and results in chronobiologic disruptions that potentially can influence the outcome of scientific investigations. We believe that the data presented contribute to our understanding of the influence of environmental light at night on mammalian circadian rhythms of metabolism and physiology and will support improved laboratory animal facility design and enhanced lighting protocols.
Acknowledgments
This work was supported in part by American Association for Laboratory Animal Science 2007-2008 Grant for Laboratory Animal Science (GLAS, to RTD), Johns Hopkins University Center for Alternatives to Animal Testing 2007 Animal Welfare Enhancement Award (AWE 2007-50, RTD), and the Stephen C Clark Foundation (DEB).
References
- 1.Acuna-Castroviejo D, Reiter RJ, Menedez-Pelaez A, Pablos MI, Burgos A. 1994. Characterization of high-affinity melatonin binding sites in the purified cell nuclei of rat liver. J Pineal Res 16:100–112 [DOI] [PubMed] [Google Scholar]
- 2.Aschoff J. 1960. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol 25:11–28 [DOI] [PubMed] [Google Scholar]
- 3.Aschoff J. 1981. Handbook of behavioral neurobiology, biological rhythms, p 1–581 New York (NY): Plenum Press [Google Scholar]
- 4.Bailey CJ, Atkins TW, Matty AJ. 1974. Melatonin inhibition of insulin secretion in the rat and mouse. Horm Res 5:21–28 [DOI] [PubMed] [Google Scholar]
- 5.Bellhorn RW. 1980. Lighting in the animal environment. Lab Anim Sci 30:440–450 [PubMed] [Google Scholar]
- 6.Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070–1073 [DOI] [PubMed] [Google Scholar]
- 7.Blask DE, Dauchy RT, Sauer LA, Holowachuk E, Ruhoff M, Kopff H. 1999. Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism receptor-mediated signal transduction events. Cancer Res 59:4693–4701 [PubMed] [Google Scholar]
- 8.Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA, Lynch DT, Rollag MD, Zalatan F. 2005. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res 65:11174–11184 [DOI] [PubMed] [Google Scholar]
- 9.Brainard GC, Richardson BA, King TS, Matthews SA, Reiter RJ. 1983. The suppression of pineal melatonin content and N-acetyl-transferase activity by different light irradiances in the Syrian hamster: a dose–response relationship. Endocrinology 113:293–296 [DOI] [PubMed] [Google Scholar]
- 10.Brainard GC, Richardson BA, King TS, Reiter RJ. 1984. The influence of different light spectra on the suppression of pineal melatonin content in the Syrian hamster. Brain Res 294:333–339 [DOI] [PubMed] [Google Scholar]
- 11.Brainard GC. 1989. Illumination of laboratory animal quarters: participation of light irradiance and wavelength in the regulation of the neuroendocrine system, p 69–74 : Guttman HN, Mench JA, Simmonds RC. Science and animals: addressing contemporary issues. Bethesda (MD): Scientists Center for Animal Welfare [Google Scholar]
- 12.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glicman G, Gerner E, Rollag MD. 2001. Action spectrum for melatonin regulation in humans: evidence for melatonin a novel circadian photoreceptor. J Neurosci 21:6405–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. 2005. A review of the multiple actions of melatonin on the immune system. Endocrine 27:189–200 [DOI] [PubMed] [Google Scholar]
- 14.Dauchy RT, Sauer LA, Blask DE, Vaughan GM. 1997. Light contamination during the dark phase in ‘photoperiodically controlled’ animal rooms: effect on tumor growth and metabolism in rats. Lab Anim Sci 47:511–518 [PubMed] [Google Scholar]
- 15.Dauchy RT, Blask DE, Sauer LA, Brainard GC, Krause JA. 1999. Dim light during darkness-stimulated tumor progression by enhancing tumor fatty acid uptake and metabolism. Cancer Lett 144:131–136 [DOI] [PubMed] [Google Scholar]
- 16.Davis S, Mirrick DK, Stevens RG. 2001. Night-shift work, light at night, and the risk of breast cancer. J Natl Cancer Inst 93:1557–1562 [DOI] [PubMed] [Google Scholar]
- 17.De Boer SF, Van der Gugten J. 1987. Daily variations in plasma noradrenaline, adrenaline, and corticosterone concentrations in rats. Physiol Behav 40:323–328 [DOI] [PubMed] [Google Scholar]
- 18.Depres-Brummer P, Levi F, Metger G, Touitou Y. 1995. Light-induced suppression of the rat circadian system. Am J Physiol 268(5 Pt 2): R1111–R1116 [DOI] [PubMed] [Google Scholar]
- 19.Diaz B, Blazquez E. 1986. Effect of pinealectomy on plasma glucose, insulin, and glucagon levels in the rat. Horm Metab Res 18:225–229 [DOI] [PubMed] [Google Scholar]
- 20.Faith RE, Huerkamp MJ. 2008. Environmental considerations for research animals, p 59–83 In: Hessler JR, Lehner NDM. Planning and designing research animal facilities. Burlington (MA): Academic Press [Google Scholar]
- 21.Gooley JJ, Lu J, Fischer D, Saper CB. 2003. A broad role for melanopsin in nonvisual photoreception. J Neurosci 23:7093–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwosdow-Cohen A, Chen CL, Bosch EL. 1982. Radioimmunoassay (RIA) of serum corticosterone in rats. Proc Soc Exp Biol Med 170:29–34 [DOI] [PubMed] [Google Scholar]
- 23.Halberg F. 1959. [Physiologic 24-hour periodicity: general and procedural considerations with reference to the adrenal cycle]. Int Z Vitamin Hormone 10:225–296 [Article in German] [PubMed] [Google Scholar]
- 24.Hansen J. 2001. Increased breast cancer risk among women who work predominantly at night. Epidemiology 12:74–77 [DOI] [PubMed] [Google Scholar]
- 25.Hastings JW, Menaker M. 1976. Physiological and biochemical aspects of circadian rhythms. Fed Proc 35:2325. [PubMed] [Google Scholar]
- 26.Hattar S, Liao H-W, Takao M, Berson DM, Yau K-W. 2002. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295:1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofstetter JR, Hofstetter AR, Hughes AM, Mayeda AR. 2005. Intermittent long-wavelength red light increases the period of daily locomotor activity in mice. J Circadian Rhythms 3:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Illnerova H, Vanecek J, Hoffman K. 1983. Regulation of the pineal melatonin concentration in the rat (Rattus norvegicus) and in the Djungarian hamster (Phodopus sungorus). Comp Biochem Physiol A Comp Physiol 74:155–159 [DOI] [PubMed] [Google Scholar]
- 29.Kalsbeek A, Strubbe JH. 1998. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol Behav 63:553–558 [DOI] [PubMed] [Google Scholar]
- 30.Kalsbeek A, Teclemariam-Mesbah R, Cutrera RA, Perreau-Lenz S, Buijs RM. 2004. Neural pathways employed by the central pacemaker to transmit its rhythmic output. Proc Sym 2004 Light and Health: nonvisual effects. Commission Internationale de L'Eclairage 27:27–32 [Google Scholar]
- 31.Karlsson B, Knutsson A, Lindahl B. 2001. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 58:747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennaway DJ, Voultsios A, Varcoe TJ, Moyer RW. 2002. Melatonin in mice: rhythms, response to light, adrenergic stimulation, and metabolism. Am J Physiol Regul Integr Comp Physiol 282:R358–R365 [DOI] [PubMed] [Google Scholar]
- 33.Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K. 1986. Increased risk of ischaemic heart disease in shift workers. Lancet 2:89–91 [DOI] [PubMed] [Google Scholar]
- 34.La Fleur SE, Kalsbeek A, Wortel J, van der Vliet J, Bujis RM. 2001. Role for the pineal and melatonin in glucose homeostatsis: pinealectomy increases night-time glucose concentrations. J Neuroendocrinol 13:1025–1032 [DOI] [PubMed] [Google Scholar]
- 35.Li JC, Xu F. 1997. Influence of light–dark shifting on the immune system, tumor growth, and life span of rats, mice and fruit flies as well as on the counteraction of melatonin. Biol Signals 6:77–89 [DOI] [PubMed] [Google Scholar]
- 36.Lima FB, Machado UF, Bartol I, Seraphim PM, Sumida DH, Moraes SM, Hell NS, Okamoto MM, Saad MJ, Carvalho CR, Cipolla-Neto J. 1998. Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am J Physiol 275(6 Pt 1): E934–E941 [DOI] [PubMed] [Google Scholar]
- 37.Maestroni GJ. 1995. T-helper-2 lymphocytes a peripheral target of melatonin. J Pineal Res 18:84–89 [DOI] [PubMed] [Google Scholar]
- 38.McEachron DL, Tumas KM, Blank KJ, Prytowsky MB. 1995. Environmental lighting alters the infection process in an animal model of AIDS. Pharmacol Biochem Behav 51:947–952 [DOI] [PubMed] [Google Scholar]
- 39.McLennan IS, Taylor-Jeffs J. 2004. The use of sodium lamps to brightly illuminate mouse houses during their dark phases. Lab Anim 38:384–392 [DOI] [PubMed] [Google Scholar]
- 40.Milcu SM, Nana-Ionescu I, Milcu I. 1971. The effect of pinealectomy on the plasma insulin in rats, p 345–357 : Woltensholme GEW, Knight J. The pineal gland. Edinburgh (UK): Churchill Livingston [Google Scholar]
- 41.Minneman KP, Lynch HJ, Wurtman RJ. 1974. Relationship between environmental light intensity and retina-mediated suppression of rat pineal serotonin-N-acetyltransferase. Life Sci 15:1791–1796 [DOI] [PubMed] [Google Scholar]
- 42.Mori W, Aoyama H, Mori W. 1989. Antihypercholesterolemic effect of melatonin in rats. Acta Pathol Jpn 39:613–618 [DOI] [PubMed] [Google Scholar]
- 43.Institute for Laboratory Animal Research 1995. Nutrient requirements of laboratory animals, p 11–79 Washington (DC): National Academies Press [Google Scholar]
- 44.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 45.Nelson DL, Cox MM. 2005. Hormonal regulation of food metabolism, p 881–922 : Lehninger biochemistry. New York (NY): WH Freeman and Company [Google Scholar]
- 46.O'Steen WK, Anderson KV. 1972. Photoreceptor degeneration after exposure of rats to incandescent illumination. Z Zellforsch Mikrosk Anat 127:306–313 [DOI] [PubMed] [Google Scholar]
- 47.Peschke E, Fautek JD, Musshoff U, Schmidt F, Beckmann A, Peschke D. 2000. Evidence for a melatonin receptor within pancreatic islets of neonate rats: functional, autoradiographic, and molecular investigations. J Pineal Res 28:156–164 [DOI] [PubMed] [Google Scholar]
- 48.Pittendrigh CS. 1965. On the mechanism of the entrainment of a circadian rhythm by light cycles, p 277–297 Aschoff J. Circadian clocks. Amsterdam: Elsevier [Google Scholar]
- 49.Radzialowski FM, Bousquet WF. 1968. Daily rhythmic variation in hepatic drug metabolism in the rat and mouse. J Pharmacol Exp Ther 163:229–238 [PubMed] [Google Scholar]
- 50.Rasmussen DD, Boldt BM, Wilkinson CW, Yellon SM, Matsumoto AM. 1999. Daily melatonin administration at middle age suppresses male rat visceral fat, plasma leptin, and plasma insulin levels to youthful levels. Endocrinology 140:1009–1012 [DOI] [PubMed] [Google Scholar]
- 51.Reiter RJ. 1973. Comparative effects of continual lighting and pinealectomy on the eyes, the Harderian glands, and reproduction in pigmented and albino rats. Comp Biochem Physiol A Comp Physiol 44:503–509 [DOI] [PubMed] [Google Scholar]
- 52.Reiter RJ. 1991. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev 12:151–180 [DOI] [PubMed] [Google Scholar]
- 53.Reiter RJ. 1991. Pineal gland: interface between photoperiodic environment and the endocrine system. Trends Endocrinol Metab 2:13–19 [DOI] [PubMed] [Google Scholar]
- 54.Reiter RJ. 2002. Potential biological consequences of excessive light exposure: melatonin suppression, DNA damage, cancer, and neurodegenerative diseases. Neuroendocrinol Lett 23:9–13 [PubMed] [Google Scholar]
- 55.Reppert SM, Weaver DR. 2002. Coordination of circadian timing in mammals. Nature 418:935–941 [DOI] [PubMed] [Google Scholar]
- 56.Sauer LA, Dauchy RT. 1988. Identification of linoleic and arachidonic acids as the factors in hyperlipemic blood that increases [3H]thymidine incorporation in hepatoma 7288CTC perfused in situ. Cancer Res 48:3106–3111 [PubMed] [Google Scholar]
- 57.Sauer LA, Dauchy RT, Blask DE, Armstrong BJ, Scalici S. 1999. 13-Hydroxyoctadienoic acid is the mitogenic signal for linoleic acid-dependent growth in rat hepatoma 7288CTC in vivo. Cancer Res 59:4688–4692 [PubMed] [Google Scholar]
- 58.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. 2001. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst 93:1563–1568 [DOI] [PubMed] [Google Scholar]
- 59.Seggie J, Shaw B, Uhlir I, Brown GM. 1974. Baseline 24-hour plasma corticosterone rhythm in normal, sham operated, and septally lesioned rats. Neuroendocrinology 15:51–61 [DOI] [PubMed] [Google Scholar]
- 60.Shapiro C, Girdwood P. 1981. Protein synthesis in rat brain during sleep. Neuropharmacology 20:457–460 [DOI] [PubMed] [Google Scholar]
- 61.Stephan FK, Zucker I. 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69:1583–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Cauter E. 1998. Putative roles of melatonin in glucose regulation. Therapie 53:463–472 [PubMed] [Google Scholar]