Abstract
Recent events have heightened the need for the rapid development of vaccines directed against pandemic influenza H1N1 viruses circulating during 2009 to 2010. The current study was conducted to establish a virus challenge dose for a subsequent CA/04 vaccine efficacy study in 3-mo-old domesticated ferrets. An additional consideration in using CA/04 in ferrets is the selection of endpoints on which to base the challenge dose, given the potential nonlethality of this particular model. Four doses ranging from 104 to 107 TCID50 units of CA/04 per animal were administered by intranasal instillation to groups of male and female ferrets, and virus titers in nasal washes obtained 1, 3, and 5 d thereafter were determined in MDCK cells. Dosed ferrets developed clinically mild infections. Peak virus titers occurred on day 3 after instillation regardless of dose. Virus-treated ferrets had less weight gain than did untreated ferrets. In conclusion, 3-mo-old ferrets can be infected with doses as low as 104 TCID50 units of CA/04, and virus titers in nasal washes and decreased body weight gain can be used to assess the course of nonlethal infection of 3-mo-old ferrets by CA/04.
Abbreviations: MDCK, Madin–Darby canine kidney
The unexpected influenza pandemic of 2009 to 2010 has heightened concern for the rapid development of vaccines against novel influenza viruses with pandemic potential. The current study is part of a program to define an overall in vivo testing approach for H1N1 pandemic influenza virus vaccine development that can be implemented to satisfy the regulatory requirements for submission of an Investigational New Drug–Biologics License Application. The regulatory process for approval of an influenza vaccine typically includes a safety study followed by an efficacy–challenge study. Before a challenge study can be initiated, the viral challenge dose needs to be established. The current study was conducted to establish a virus challenge dose that will be used in a subsequent CA/04 virus vaccine efficacy study in ferrets. CA/04 was one of the first isolates of the 2009 H1N1 pandemic and was widely distributed for experimental studies and vaccine production; it was isolated from a 10-y-old boy who fully recovered from the infection.1 Domesticated ferrets (Mustela putorius furo) are an appropriate surrogate for humans6 for study of the pathogenicity2,8 and transmissibility4,5 of influenza viruses because they share many anatomic, metabolic, and physiologic features with humans, respond similarly (clinically, immunologically, and pathologically) to influenza virus infections, and are naturally susceptible to the same types of influenza viruses that affect humans. As in its original human host, CA/04 does not cause lethal infections in domesticated ferrets.7 Therefore, an additional consideration in using CA/04 in ferrets is the selection of endpoints on which to base the challenge dose, given the nonlethality of the model.
Morbidity in experimental studies of lethal influenza virus infections in ferrets (such as those caused by H5N1 viruses) typically is monitored by assessment of activity, body weight, hydration, body temperature, and hair coat appearance.2,6,8 However, in nonlethal models, with the possible exception of body weight, these commonly used endpoints may not be available for health assessment. In the absence of mortality, clinical observations such as activity level and coat appearance are subjective and dehydration difficult to determine accurately. Therefore, particularly in a nonlethal animal model, additional evaluation methods that are reliable and easily performed would be useful.
Whereas many studies with influenza virus have been conducted with older ferrets, we used 3-mo-old ferrets for the current influenza virus study. These younger ferrets have a fully developed immune system and, compared with older animals, are easier to house and work with because of their smaller size. Furthermore, the likelihood of their exposure to influenza viruses stemming from animal handlers is decreased. This consideration is important, given that to-date, barrier-raised SPF ferrets are not readily commercially available on a large scale.
The current study tested 3 hypotheses: 1) the replication of CA/04 would follow trends previously described for older ferrets,7 with active viral replication in the lungs peaking 2 to 3 d after infection and viral titers diminishing 4 to 5 d after infection; 2) body weight would be affected by infection with CA/04, and 3) mild clinical signs would be present in infected ferrets.
Materials and Methods
Viruses.
A low-passage culture of CA/04 grown in Madin–Darby canine kidney (MDCK) cells was obtained from the Centers for Disease Control (Atlanta, GA). Working stocks of CA/04 subsequently were prepared in MDCK cells.3 The identity of the virus was verified by partial genomic sequence analyses of the viral hemagglutinin and neuraminidase genes (data not shown).
Biocontainment facilities.
In vivo experiments with CA/04 were conducted in an Animal Biosafety Level 3 Enhanced containment facility and required use of personal protective equipment.
In vitro cell growth and manipulations.
MDCK cells were propagated in DMEM supplemented with L-alanyl-L-glutamine (GlutaMax, Invitrogen, Carlsbad, CA), penicillin, streptomycin, neomycin (Invitrogen), and γ-irradiated fetal bovine serum (HyClone, Pittsburgh, PA). All cells were negative by PCR for the presence of Mycoplasma DNA by using a Mycoplasma Detection kit (Takara Bio, Madison, WI). CA/04 was grown in 5% CO2 at 35 °C in MDCK in serum-free DMEM supplemented with antibiotics and mycoplasma- and extraneous virus-free trypsin (Worthington Biochemical Company, Lakewood, NJ) that had been treated with L-1-tosylamido-2-phenylethyl chloromethyl ketone. Because of its increased high specific activity, the treated trypsin was used at a final concentration of 1.0 μg/mL for MDCK cells. The Reed–Muench method was used to calculate TCID50 values.9
Ferrets and their prequalification for studies.
Studies used descented 3-mo-old neutered male and spayed female ferrets (approximately 0.5 to 0.9 kg; Triple F Farms, Sayre, PA). The animals were examined by a veterinarian upon receipt and over a quarantine and acclimation period of 5 d. None of the animals exhibited signs of epizootic catarrhal enteritis. Microscopy for coccidian cysts or helminth ova was performed on fecal specimens mixed with a sodium nitrate fecal flotation solution (Fecasol, EVSCO Pharmaceuticals, Buena, NJ) to detect infection with Eimeria, Isospora, or other enteric parasites. After ferrets were approved by the veterinarian for study inclusion, serum was collected from each animal, and an implantable programmable temperature transponder (model IPTT-300, Bio Medic Data Systems, Seaford, DE) was implanted in the scruff of the neck of each animal (6 d before study initiation date). The ferret sera were screened for antibodies to circulating influenza B and H1N1, H3N2, and CA/04 influenza A viruses by a hemagglutination inhibition assay.
Living conditions included 12:12-h light:dark cycles, an average relative humidity of 30%, and a room temperature of 64 to 84 °F (17.8 to 28.9 °C). The animals were fed pelleted ferret food (Triple F Farms), watered ad libitum, and housed and maintained under applicable laws and guidelines, with appropriate approvals from the Midwest Research Institute Animal Care and Use Committee.
Hemagglutination inhibition assays.
Ferret blood was collected from jugular veins, and sera was checked for antibodies against CA/04 and circulating seasonal influenza A and B viruses by using a standard hemagglutination inhibition assay11 using turkey red blood cells. Prior to performance of the hemagglutination inhibition assay, ferret sera were treated overnight with receptor destroying enzyme (Denka Seiken USA, Campbell, CA) at 37 °C to inactivate nonspecific hemagglutination inhibition assay activity, then heated at 56 °C for 60 min to inactivate remaining enzyme activity and complement proteins.
Intranasal inoculation studies.
Previously described procedures12 were used for intranasal instillation of 50 ferrets. Briefly, ferrets were anesthetized by intramuscular administration of ketamine HCl (25 mg/kg), xylazine (2 mg/kg), and atropine (0.05 mg/kg) and instilled with selected doses of viruses in isotonic PBS with 0.5% purified bovine serum albumin (to stabilize the virus) and antibiotics. Virus suspension (50 µL) was instilled into each nostril, and 5 ferrets of each sex were inoculated intranasally with 0, 107, 106, 105, or 104 TCID50 units of virus per ferret. The negative (no virus) control ferrets received doses of the diluent alone. The animals were separately housed in HEPA-filtered inlet and exhaust-ventilated isolation caging among 3 separate rooms, with representatives of each group randomized into each room. All animals underwent all procedures concurrently. All the animals were weighed once daily for the duration of the study. Body temperatures were recorded twice daily. A temperature increase of 1.4 °C or greater over baseline was considered significant.
Nasal washes were collected at 1, 3, and 5 d after inoculation with CA/4 (infected animals) or vehicle only (sham-infected animals). Clinical signs including sneezing (before anesthesia), inappetence, dyspnea, and level of activity were assessed daily for the duration of the study (14 d). Inappetence was judged through visual observation of the food remaining in the feeder and spilled within the surrounding area. A scoring system (relative inactivity index)10 for evaluating the behavioral response of ferrets to influenza virus infection2,12 was used to assess the activity level as follows: 0, alert and playful; 1, alert but playful only when stimulated; 2, alert but not playful when stimulated; and 3, neither alert nor playful when stimulated. As part of the study design, the ferrets also were monitored daily for nasal and ocular discharge, neurologic dysfunction, and semisolid or liquid stools. Neurologic dysfunction for this study was defined as development of motor dysfunction (including paralysis or posterior paresis), convulsions, ataxia, seizures, and depression. In addition, ferrets with greater than 25% loss of body weight or with neurologic dysfunction were to be anesthetized by intramuscular administration of ketamine HCl (25 mg/kg), xylazine (2 mg/kg), and atropine (0.05 mg/kg) and euthanized sodium pentobarbital and phenytoin sodium (Beuthanasia D Special, Intervet/Schering-Plough, Millsboro, DE) or equivalent (Euthasol, Virbac, Fort Worth, TX) through the jugular vein.
Collection of nasal washes and virus titration.
Nasal washes were collected (one collection of each wash daily at 1, 3, and 5 d after inoculation with virus) after anesthesia with ketamine (25 mg/kg) essentially as described previously.12 Briefly, 500 µL sterile isotonic PBS containing 1% bovine serum albumin, and penicillin (100 U/mL), streptomycin (100 µg/mL), and gentamicin (50 µg/mL) was administered (250 µL/nostril) to induce sneezes in ketamine-anesthetized ferrets on days 1, 3, and 5 after inoculation with CA/04. Sneezes were collected in a culture dish and diluted to 1 mL with cold PBS containing antibiotics. A 100-µL aliquot of the diluted material was inoculated into a T25 flask containing MDCK cells and incubated to screen for the presence of CA/04 virus, and the remainder was stored at –80 °C. Samples positive for CA/04 by the screening test were titrated for 11 d in MDCK cells in 96-well microtiter plates.
Statistical analysis.
The growth rates of the dose groups were compared with that of the control by using a regression model for the daily body weight measurements with a random-animal effect; fixed effects for sex, dose, and day (continuous) along with the 2-way and 3-way interactions; and a first-order autoregressive covariance structure differing by sex. Maximum likelihood was used to estimate the model parameters. The estimated slope for each dose group was compared with the estimated slope for the control group to detect differences in growth rates. The change in body weight from day 0 to days 7 and 14 were analyzed by using ANOVA with factors for sex, dose, and the interaction between sex and dose. The least-squares estimates of the average change in bodyweight for each dose group were compared with the least-squares estimate of the average change in bodyweight for the control group.
The average temperature for the dose groups was compared with the control by using ANOVA for the daily temperature measurements with a random-animal effect, fixed effects for sex and dose along with the interaction, and a first-order autoregressive covariance structure. Maximum likelihood was used to estimate the model parameters. The estimated average temperature for each dose group was compared with the estimated average temperature for the control group to detect differences in body temperature. The change in temperature from baseline was analyzed by using ANOVA with factors for sex, dose, and day (discrete) with the 2-way and 3-way interactions and a compound symmetry covariance structure. Maximum likelihood was used to estimate the model parameters. For each day, the estimated average difference in temperature for each dose group was compared with the estimated slope for the control group to detect differences in growth rates.
All statistical computations were performed by using SAS version 9.2 (SAS Institute, Cary, NC). All P values less than 0.05 were considered statistically significant.
Results
Clinical observations.
No mortality occurred after intranasal challenge of ferrets with CA/04 at doses ranging from 104 to 107 TCID50. Minimal clinical signs were observed with varying, dose-independent incidence in both male and female ferrets from all CA/04-treated groups. These signs included decreased feed and water consumption, loose or green feces (or both), and rough coat. No animals infected with CA/04 displayed a relative inactivity score greater than 1 at any time during the study period, and none developed neurologic signs (data not shown). No clinical signs were seen in sham-infected ferrets.
Body temperature.
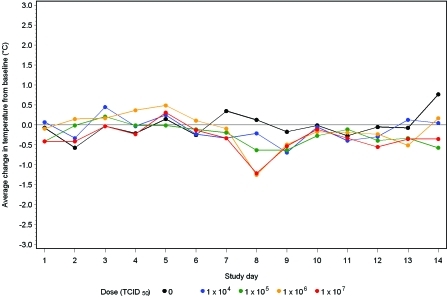
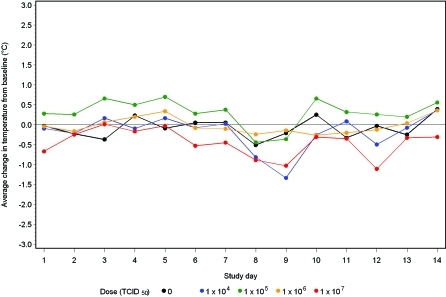
CA/04-infected male (Figure 1) and female (Figure 2) ferrets did not develop dose-dependent or -independent effects on body temperature.
Figure 1.
Body temperature change in male ferrets after challenge with influenza virus A/CA/04/2009 (H1N1). Baseline is the average body temperature during study days −3 to 0.
Figure 2.
Body temperature change in female ferrets after challenge with influenza virus A/CA/04/2009 (H1N1). Baseline is the average body temperature during study days −3 to 0.
Body weight.
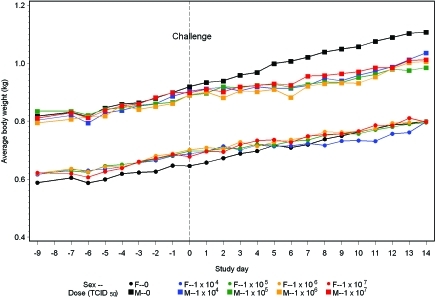
In both male and female ferrets, body weight did not decrease. However, as compared with uninfected ferrets, gains in body weight were significantly lower in the 104, 105, and 106 TCID50 dose groups during study days 0 to 7 and 0 to 14 and in the 107 TCID50 group for study days 0 to 14 (P < 0.05 for all comparisons; Table 1 and Figure 3).
Table 1.
Body weight measurements (kg) by dose (no. of infectious viral particles per ferret) and sex for study days 0, 7, and 14
| Body weight (kg) |
|||||||||||
| Male |
Female |
||||||||||
| Dose | Day 0 | Day 7 | % Increase day 0 to day 7 | Day 14 | % Increase day 0 to day 14 | Day 0 | Day 7 | % Increase day 0 to day 7 | Day 14 | % Increase day 0 to day 14 | |
| 0 | Mean | 0.919 | 1.021 | 11.1 | 1.108 | 20.5 | 0.645 | 0.719 | 11.4 | 0.800 | 24.0 |
| SD | 0.078 | 0.083 | 0.083 | 0.137 | 0.139 | 0.145 | |||||
| 1 × 104 | Mean | 0.903 | 0.923 | 2.2a | 1.036 | 14.7a | 0.687 | 0.723 | 5.2a | 0.798 | 16.1a |
| SD | 0.127 | 0.106 | 0.108 | 0.097 | 0.099 | 0.098 | |||||
| 1 × 105 | Mean | 0.889 | 0.927 | 4.3a | 0.985 | 10.7a | 0.697 | 0.735 | 5.4a | 0.795 | 14.1a |
| SD | 0.104 | 0.091 | 0.127 | 0.073 | 0.095 | 0.125 | |||||
| 1 × 106 | Mean | 0.891 | 0.921 | 3.4a | 1.006 | 12.9a | 0.701 | 0.745 | 6.2a | 0.799 | 14.0a |
| SD | 0.067 | 0.095 | 0.059 | 0.063 | 0.048 | 0.061 | |||||
| 1 × 107 | Mean | 0.898 | 0.956 | 6.4 | 1.013 | 12.7a | 0.678 | 0.748 | 10.4 | 0.797 | 17.7a |
| SD | 0.053 | 0.062 | 0.093 | 0.065 | 0.078 | 0.092 | |||||
Statistically significantly different (P < 0.05) from corresponding day 0 control based on F-test for ANOVA.
Figure 3.
Average body weight before and after challenge with influenza virus A/CA/04/2009 (H1N1).
Nasal wash titers.
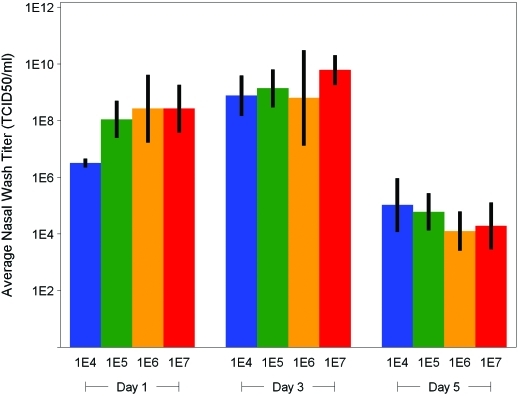
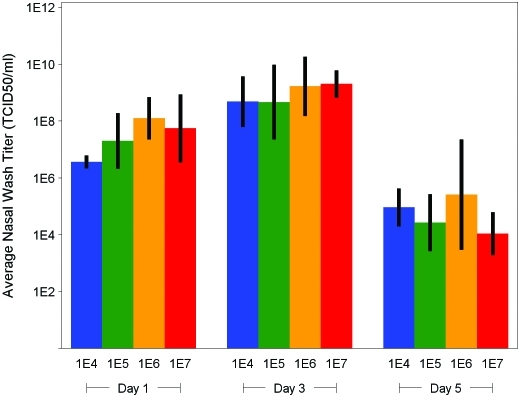
Nasal wash titer results indicated that CA/04 was replicating at all dose levels, with an increase in titer (over inoculum) observed at study day 1 in both male (Figure 4) and female (Figure 5) ferrets. Compared with day 1 values, day 3 virus titers were higher (P < 0.05) in all dose groups for both male and female ferrets and then fell significantly (P < 0.05) in all dose groups by study day 5 (Figures 4 and 5). Sham-infected animals were consistently negative for influenza virus over the testing period.
Figure 4.
Nasal wash titers in male ferrets challenged with influenza virus A/CA/04/2009 (H1N1). Error bars represent the 95% confidence interval for the mean. Mean and confidence bounds are based on a lognormal distribution of virus titer.
Figure 5.
Nasal wash titers in female ferrets challenged with influenza virus A/CA/04/2009 (H1N1). Error bars represent the 95% confidence interval for the mean. Mean and confidence bounds are based on a lognormal distribution of virus titer.
Discussion
Supporting our 3 hypotheses for this study, 1) virus levels in inoculated ferrets were high early during infection (days 1 and 3) and fell by 5 d after infection; 2) relative to that in nonchallenged animals, body weight was affected during the course of infection; and 3) only mild clinical signs and no neurologic signs were observed. The results of this study are consistent with those of recent report describing older ferrets,7 which showed similar virus peaks, effect on body weight, and mild clinical signs. A notable difference is that in the present study, ferrets developed only a decrease in body weight gain, whereas in the previous study with older ferrets,7 animals actually lost weight.
In light of the equivocal observations on body weight gain and nasal wash titer in all dose groups, intranasal inoculation with as low a dose as 104 infectious virus particles per ferret could be considered sufficient for challenge in subsequent vaccine efficacy studies. In addition, because the ferret model does not result in lethality, endpoints for inclusion in an H1N1 vaccine efficacy and challenge study can include body weight gain and nasal wash titer.
Acknowledgments
We thank Cheryl J Nevins, William A Sosna, Douglas J Brant, Amanda D Yates, Linda Poindexter, and Brandi Wilson for technical assistance during the study and Courtney Bokenkroger for providing statistical consultation. This work was supported by the Defense Advanced Research Projects Agency (DARPA), contract no. W911NF-09-C-0114.
The views, opinions, and findings contained in this article are those of the authors and should not be interpreted as representing the official views or policies, either expressed or implied, of the Defense Advanced Research Projects Agency or the Department of Defense.
References
- 1.Centers for Disease Control and Prevention (CDC) 2009. Swine influenza A (H1N1) infection in two children--Southern California, March-April 2009.MMWR Morb Mortal Wkly Rep 58:400–402 [PubMed] [Google Scholar]
- 2.Govorkova EA, Rehg JE, Krauss S, Yen HL, Guan Y, Peiris M, Nguyen DT, Hanh TH, Puthavathana P, Hoang TL, Buranathai C, Lim W, Webster RG, Hoffmann E. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J Virol 79:2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton SB, Daniels DE, Sosna WA, Jeppesen ER, Owells JM, Halpern MD, McCurdy KS, Rayner JO, Lednicky JA. 2010. Gas-permeable ethylene bags for the small-scale cultivation of highly pathogenic avian influenza H5N1 and other viruses in embryonated chicken eggs. Virol J 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herlocher ML, Elias S, Truscon R, Harrison S, Mindell D, Simon C, Monto AS. 2001. Ferrets as a transmission model for influenza: sequence changes in HA1 of type A (H3N2) virus. J Infect Dis 184:542–546 [DOI] [PubMed] [Google Scholar]
- 5.Herlocher ML, Truscon R, Elias S, Yen HL, Roberts NA, Ohmit SE, Monto AS. 2004. Influenza viruses resistant to the antiviral drug oseltamivir: transmission studies in ferrets. J Infect Dis 190:1627–1630 [DOI] [PubMed] [Google Scholar]
- 6.Maher JA, DeStefano J. 2004. The ferret: an animal model to study influenza virus. Lab Anim (NY) 33:50–53 [DOI] [PubMed] [Google Scholar]
- 7.Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. 2009. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HH, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol 79:11788–11800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed LJ, Muench H. 1938. A simple method for estimating 50% endpoints. Am J Hyg 27:493–497 [Google Scholar]
- 10.Reuman PD, Keely S, Schiff GM. 1989. Assessment of signs of influenza illness in the ferret model. J Virol Methods 24:27–34 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. [Internet] Manual on animal influenza diagnosis and surveillance. [Cited 10 Mar 2010]. Available at: http://www.who.int/vaccine_research/diseases/influenza/WHO_manual_on_animal-diagnosis_and_surveillance_2002_5.pdf
- 12.Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol 76:4420–4429 [DOI] [PMC free article] [PubMed] [Google Scholar]