Abstract
Permanent jejunal fistulas enable easy, noninjurious, repeated and direct administration to and collection from the small intestines of conscious laboratory dogs. This study aimed at identifying potential alterations in the small intestinal morphology and function of this canine model after the surgery required to establish the fistulas. Assays of serum folate and cobalamin and 51Cr-EDTA permeability tests were performed before and 4 wk after experimental jejunoplasties in 14 laboratory beagle dogs. Serum folate concentrations (mean ± SD ) before (12.22 ± 1.80 µg/L) and after (14.14 ± 1.70 µg/L) jejunal surgery were within reference ranges for healthy dogs, although folate concentrations were higher after surgery. The cobalamin concentrations and the 6-h urinary excretion of 51Cr-EDTA before (573.50 ± 150.04 ng/L and 6.75 ± 1.56%, respectively) and after (496.71 ± 164.22 ng/L and 6.41 ± 1.10%) were normal for healthy dogs, and no significant differences between pre- and postsurgical values were detected. The findings of the present study indicate that the small intestinal vitamin absorption and permeability of laboratory beagle dogs with jejunal fistulas remains unimpaired.
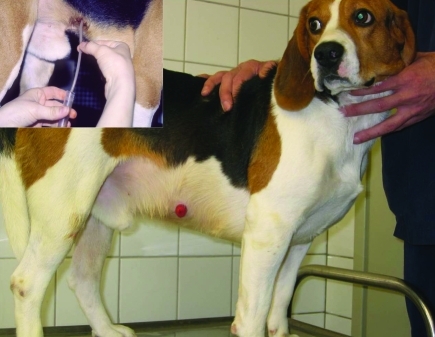
Several areas of preclinical biomedical research specifically require the use of laboratory animal models that enable the entrance, exploration of, and sampling from or within different parts of the gastrointestinal tract.15,20,25,27,28 In particular, laboratory dogs with permanent intestinal fistulas have proved useful in protocols requiring long-term intestinal access. Intestinal fistulas with ‘nipple’ valves connect the intestinal lumen with the dog's external surface in one lateral side of the abdominal wall (Figure 1). The major advantages of intestinal fistulas are that they enable easy, nonpainful, direct and frequent manipulation of the intestinal tract in a conscious dog without the need for chemical restraint and without causing discomfort to the animal.18, 30
Figure 1.
Intestinal sample collection from a conscious laboratory beagle dog with a jejunal fistula in the right flank. Adapted and reprinted with permission from reference 18.
Considerable surgical manipulation of the canine intestine is required to establish intussuscepted intestinal nipple valves in laboratory dogs. Assessment of fistulated dogs for postoperative complications including delayed gastrointestinal motility and changes in microflora have revealed no or only minimal effects due to fistulation.18 We hypothesized however that the magnitude of the surgical manipulations needed to create a dog with a jejunal fistula might give rise to further microabnormalities across the small intestine. Subtle anatomic and physiologic anomalies at the microstructural and biochemical levels potentially could confound research data generated from this dog model. If such a canine model is to be used in investigations involving physiologic, pharmacologic, and toxicologic studies, functional tests of the intestinal absorptive capacity and the integrity (that is, permeability) of the intestinal mucosa are warranted. However, to date and to the best of our knowledge, screenings of such potential abnormalities have not been done.
The folate and cobalamin test is a routine clinical test that provides information on the functional ability of the canine small intestine, and more specifically, on the absorptive efficiency of the jejunal and ileal enterocytes.5,6,29 The 51Cr-EDTA permeability test provides an assessment of physical injury to the intestinal mucosal lining and is particularly sensitive for detection of epithelial mucosal damage in dogs.4,16,17
The aim of the present study was to use serum folate and cobalamin absorption tests and the 51Cr-EDTA intestinal permeability urine test to evaluate subtle abnormalities in the function and morphologic integrity of the small intestine of this canine model before and after surgery to construct permanent jejunal fistulas in laboratory beagle dogs.
Materials and Methods
Animals.
Male purebred laboratory beagle dogs (n = 14; Harlan-Winkelmann, Borchen, Germany) were used in the study. The dogs were acclimated for at least 1 mo before starting on the study. All the dogs were housed (Faculty of Veterinary Medicine, University of Helsinki, Finland) in indoor pens and spent about 4 h daily in outdoor runs. Dogs were exposed to both natural and artificial lighting (0700 to 1600). Feeding throughout the study consisted of commercial canned dog food (1.5 cans per dog; 400-g can, Pedigree, Fortivil, Waltham, Masterfoods, Helsinki, Finland) given twice daily. Water was freely available at all times. At the beginning of the study, the dogs were between 11 and 40 mo (mean, 18.50 mo) of age and ranged in body weight from 13.00 to 17.00 kg (mean, 15.02 kg). To determine the health status of the dogs, physical examinations were carried out, and blood samples were taken from each subject for hematologic and biochemical examinations. Before entering the study, the dogs were treated with fenbendazole (50 mg/kg PO for 3 consecutive days; Axilur, Intervet International, Boxmeer, The Netherlands), and fecal samples were collected afterward for endoparasite examination.
Ethics.
The dogs were cared for and used in the experiments in accordance with the prevailing Finnish and European legislation on the use and care of laboratory animals.9–12 The experimental protocol was approved by the local Animal Ethics Committee of the University of Helsinki, Finland.
Study design.
Specific tests of small intestinal absorption and permeability were carried out on each laboratory beagle dog 2 wk before and 4 wk after surgical construction of permanent intussuscepted jejunal nipple valves.
Construction of intussuscepted jejunal fistulas.
Permanent jejunal fistulas with nipple valves were constructed as described previously.26 The dogs were premedicated with a combination of medetomidine (20 µg/kg IM) and butorphanol (0.2 mg/kg; Torbugesic, Fort Dodge Animal Health, Southampton, NY), and anesthesia was induced with propofol (0.5 to 1 mg/kg IV; Rapinovet, Schering-Plough Animal Health, Farum, Denmark) and maintained with isoflurane in oxygen. After midline incision of the abdomen, a jejunal segment of approximately 25 cm was isolated and resected 170 cm distally from the pylorus. The free ends of the intestine were joined by an end-to-end anastomosis with appositional simple interrupted sutures of 4-0 polydioxanone (Ethicon, Johnson and Johnson, New Brunswick, NJ) suture material, and the isolated segment was used to create intussusceptions by grasping and pulling with Allis forceps approximately 12 cm from the proximal end. The distal end of the intussusception was sutured to the jejunum in an end-to-side anastomosis with appositional simple interrupted sutures. The proximal end was exteriorized through the right flank of each dog into the skin surface. The nipple valve was created by opening the distal end of the intestine and folding back, creating a nipple that subsequently was anchored to the skin and peritoneum by using interrupted polydioxanone sutures. The abdominal incision was closed in a routine manner. Analgesic agents were carprofen (4 mg/kg IV; Rimadyl, Pfizer, Vericore, Dundee, UK) and buprenorphine (0.02 mg/kg IV; Temgesic, Reckitt and Colman, Hull, UK), which were given a few minutes before surgical incision. In addition, buprenorphine (0.02 mg/kg SC) was given to each dog twice during the day after completion of the surgery, and carprofen (4 mg/kg SC) once daily for 2 d after completion of the surgery. Further analgesia was unnecessary because the dogs were in good clinical condition and showed no signs of pain or distress. No antibiotic medication was used perioperatively or during the study.
Testing small intestinal permeability and serum vitamin absorption.
To assess the serum concentrations of folate and cobalamin, a baseline blood sample was taken from each dog after an overnight fast. The blood was left at room temperature for at least 1 h and then centrifuged for 12 min at 2,100 × g to obtain serum. Sera were collected into tubes and frozen at −20 °C until subsequent use in a chemiluminescent assay (Immulite 1000 Immunoassay System, Siemens Healthcare Diagnostics, Deerfield, IL). For the 51Cr-EDTA permeability test, fresh single-test solutions comprising approximately 3.7 MBq (100 µCi) 51Cr-EDTA (Nycomed Amersham, Little Chalfont, Buckinghamshire, UK) dissolved in 50 mL distilled water were prepared in the early morning of the experiments. A counting standard from the test solution was retained in a 1-mL aliquot, which was further diluted (1:50) before radioactivity measurements. Immediately after the first blood sample was taken, the test dog was sedated with medetomidine (25 µg/kg IM; Domitor, Orion Pharma, Turku, Finland) and the urinary bladder emptied by catheterization. The 51Cr-EDTA test solution was administered intragastrically by using an orogastric tube, and the test solution was flushed with 50 mL water. Atipamezole (100 µg/kg IM; Antisedan, Orion Pharma) was injected to reverse the sedative effects of medetomidine, and the dog was placed in a metabolic cage for 6 h for urine collection. After the 6-h period, medetomidine (25 µg/kg IM) again was administered to the dog and the urine from the bladder collected by catheterization. The urine from the metabolic cage and urinary bladder were pooled and the total urine volume recorded for further test calculations. A 1-mL aliquot of urine was retained and used for the measurement of radioactivity. Gamma irradiation from the standard and urine aliquot were counted (1270 Rackgamma II, LKB-Wallac, Turku, Finland) concurrently for 10 min. The formula to calculate the amount of 51Cr-EDTA in urine as a percentage of the intragastrically administered test solution was as follows:
 |
Statistical analysis.
Statistical analysis was performed by using SPSS 11.0 software (SPSS, Chicago, IL). Data are expressed as mean ± 1 SD. The significance of differences in test results before and after surgery was determined by one-way ANOVA, and the Student–Newman–Keuls test was used for comparison of the means. A P value of less than 0.05 was regarded as statistically significant. The relationship between the results was assessed by using the Pearson correlation coefficient (r).
Results
Two weeks before surgery, all dogs were determined to be healthy on the basis of physical examinations and hematologic and serum biochemical analysis. Blood urea nitrogen and serum creatinine concentrations suggested normal glomerular function. Exocrine pancreatic function was considered normal according to measurements of serum trypsin-like immunoreactivity concentrations. Examinations for fecal endoparasitic ova were negative. Body weights of the dogs showed no significant (P > 0.05) differences before and after surgery.
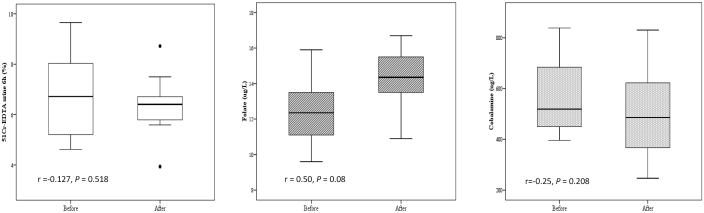
In the 14 beagle dogs of the present study before surgery (Table 1), serum concentrations (mean ± 1 SD [range]) of folate were 12.22 ± 1.80 µg/L (9.30 to 15.90 µg/L; reference range, 5.0 to 17.5 µg/L); serum cobalamin concentrations were 573.5 ± 150.04 ng/L (396 to 839 ng/L; reference range, 250 and 850 ng/L). Serum concentrations of folate and cobalamin after jejunal fistula surgery were 14.14 ± 1.70 µg/L (9.30 to 15.90 µg/L) and 496.71 ± 164.22 ng/L (247 to 831 ng/L), respectively. After intragastric administration of 51Cr-EDTA to nonoperated beagle dogs, the percentage (mean ± SD [range]) of 51Cr-EDTA in urine after 6 h was 6.75% ± 1.56% (4.62% to 9.65%). Four weeks after the surgery was performed, urinary excretion of 51Cr-EDTA after 6 h was 6.41% ± 1.10% (3.94% to 8.72%). Neither serum cobalamin nor 51Cr-EDTA urinary excretion differed before and after jejunal surgery. However, folate serum concentrations were significantly higher (P < 0.05) after surgery (Figure 2).
Table 1.
Results of small intestinal permeability and absorption tests in laboratory beagle dogs before and after surgery to construct permanent jejunal fistulas
| 51Cr-EDTA (normal, 0.0%–14.07%) | Folate (normal, 5.0–17.5 µg/L) | Cobalamin (normal, 250–850 ng/L) | ||||
| Dog no. | Before | After | Before | After | Before | After |
| 1 | 4.74 | 6.08 | 11.1 | 13.5 | 431 | 358 |
| 2 | 6.21 | 7.46 | 12.7 | 12.3 | 684 | 508 |
| 3 | 8.03 | 6.25 | 13.5 | 14.2 | 585 | 667 |
| 4 | 7.67 | 6.71 | 12.0 | 10.9 | 801 | 538 |
| 5 | 4.80 | 7.50 | 15.9 | 15.9 | 777 | 652 |
| 6 | 6.01 | 3.94 | 13.6 | 14.5 | 442 | 367 |
| 7 | 4.62 | 6.59 | 12.3 | 13.6 | 473 | 388 |
| 8 | 6.44 | 6.46 | 12.6 | 14.9 | 839 | 831 |
| 9 | 7.64 | 5.79 | 9.6 | 15.3 | 516 | 623 |
| 10 | 9.65 | 5.71 | 12.4 | 13.5 | 450 | 591 |
| 11 | 5.21 | 6.64 | 9.3 | 11.5 | 522 | 464 |
| 12 | 8.29 | 8.72 | 10.1 | 15.5 | 645 | 432 |
| 13 | 8.17 | 5.59 | 14.0 | 16.7 | 396 | 288 |
| 14 | 6.99 | 6.35 | 12.0 | 15.6 | 468 | 247 |
| Mean | 6.75 | 6.41 | 12.22 | 14.14 | 573.50 | 496.71 |
| 1 SD | 1.56 | 1.10 | 1.80 | 1.70 | 150.04 | 164.22 |
Figure 2.
Statistical analysis of the relationships between the levels of 51Cr-EDTA, folate, and cobalamin before and after surgery (n = 14). The line in the box represents the median (50%); the lower line represents the lower (25%) quartile, and the upper line represents the upper (75%) quartile. The limits of the upper and lower vertical lines indicate the highest and lowest data values, respectively. Asterisks indicate outliers. Lines within the boxes show the Pearson correlation (linear adjustment).
Discussion
The extent to which a biological structure in an animal model resembles the corresponding structure in the target species (for example, human or other animal) is defined as the ‘fidelity’ of the model. A high-fidelity model with a close resemblance to the target is considered an advantage when developing certain models. For these reasons, the assessment of small intestinal integrity and function in laboratory dogs undergoing surgical operations to create permanent jejunal fistulas is important, given the potential effect of the fistulation on the physiologic response of the dogs during certain research studies, particularly in pharmacology and toxicology. The fistula and associated experimental surgery can be postulated to affect the reliability and validity of data generated from this in vivo model, particularly in studies involving the absorption and permeation of test compounds through the small intestine.19 At present, whether jejunal fistulas influence the small intestinal microstructure and absorption of this particular canine model is unknown.
The present study examined and compared the small intestinal integrity and absorptive capacity of 2 vitamins in 14 laboratory beagle dogs before and after experimental surgery to construct intussuscepted jejunal fistulas. Intestinal function can be assessed by measuring the absorptive efficiency of small-intestinal enterocytes. The folate and cobalamin assay is based on serum measurements of these 2 molecules and has proved of considerable value in assisting the identification and characterization of small intestinal enteropathies in dogs, such as malabsorption syndromes.2,3,6,14,22,29 Both folate and cobalamin are absorbed in the canine small intestine by active processes. Whereas folate absorption takes place in the proximal jejunum by specific carriers, cobalamin is absorbed by receptor-mediated endocytosis in the ileum. Therefore, small intestinal dysfunction can lead to impairment of carrier-mediated absorption in the proximal and distal canine small intestine and is evidenced by decreased serum concentrations of folate and cobalamin vitamins.2,3,6,14,22,29 Our hypothesis was that surgically induced damage to the cellular membranes of the enterocytes as a consequence of jejunoplasties can lead to decreased intrinsic absorptive capacity of the small intestine of fistulated dogs. According to our findings from the current study, the serum concentrations of both folate and cobalamin after surgery in laboratory beagle dogs were within the range of reference values for healthy dogs, suggesting that the absorptive capacity of the small intestine remained normal. Nonetheless, significantly higher folate concentrations were detected in serum after surgery. This increase in serum folate after surgery can be explained by a shift in the microflora toward lactic acid bacteria, similar to the situation that occurs in the small intestine of dogs undergoing surgical construction of intestinal fistulas, because this group of bacteria is primarily responsible for synthesizing folate in the intestine.18,23
Similarly to the serum concentrations of folate and cobalamin, results from the 51Cr-EDTA permeability tests were normal before and after surgery in each of the 14 laboratory beagle dogs of the present study. Tests of small intestinal permeability are sensitive screening methods for detecting subtle abnormalities and have proven to be valuable diagnostic tools in humans and other animal species, including dogs.1,4,7,8,13,21,26 Changes in intestinal permeability are mediated largely by damage to the tight junctions between adjacent enterocytes and have shown strong correlation with jejunal biopsy results. 51Cr-EDTA meets the criteria for an ideal permeability probe and is considered the ‘gold standard’ marker. An abnormally high percentage recovery from urine or serum 6 h after an ingested dose of 51Cr-EDTA suggests increased small intestinal permeability, which is indicative of small intestinal mucosal damage.1,4,7,8,13,21,26 In our beagle dogs after surgery, we regarded the urinary excretion of 51Cr-EDTA at 6 h after intragastric administration as normal and comparable to that seen in the same dogs prior the surgical procedure, suggesting that small intestinal integrity remained unchanged after jejunal nipple valve implantation. These findings are in agreement with similar studies in humans, in which indexes of intestinal permeability remained constant before and after partial intestinal resections in patients with severe Crohn disease.24
In addition, results of the current study are supported by a previous report on the intestinal function of laboratory dogs with permanent intestinal fistulas.18 In the cited study, surgical construction of permanent intussuscepted nipple valves appeared not to or only minimally influence the normal functioning of the small intestine. During the course of the study, bowel motility remained unaffected, according to examinations using barium-impregnated polyethylene spheres. Moreover, small intestinal bacterial overgrowth did not emerge after construction of the permanent intestinal fistulas, given that the counts of total aerobic bacteria in samples taken from the small intestine or feces did not increase after surgery and because clinical signs associated with bacterial overgrowth of the small intestine, including diarrhea, flatulence, and borborygmus, did not occur. A minor shift in the quality of the small intestinal microflora in terms of lactic acid bacteria occurred after surgery in that study.14 The findings on small intestinal bacterial overgrowth and lactic acid bacterial counts are consistent with our data presented here on the normal serum values of folate and cobalamin and the increased concentration of folate after surgery.
In summary, experimental jejunoplasties in laboratory beagle dogs were not associated with decreased absorption of folate and cobalamin or with higher urinary excretion of 51Cr-EDTA 4 wk after surgery. Therefore, neither the permeability nor the function of the small intestine were impaired due to the procedure. However, a longer-term follow-up of small intestinal function and permeability is warranted, given possible concerns regarding long-standing or chronic disease in the small intestine of fistulated dogs, resulting in depletion of body stores and thereby subnormal serum concentrations of both vitamins. Further studies also may be justified to rule out other ultrastructural morphologic anomalies that are not readily detected by the tests we used in this study.
Acknowledgments
We thank the Finnish Veterinary Foundation and Helvi Knuuttila Foundation for financial support. This study was conducted at the former facilities of the Experimental Unit, Faculty of Veterinary Medicine, University of Helsinki, Finland. We thank Dr Maria Carmen Collado for providing statistical consultation, Mr Seppo Lasanen for excellent technical assistance during experiments, and Dr Roy Siddall for reviewing this manuscript.
References
- 1.Akinbami FO, Brown GA, McNeish AS. 1989. Intestinal permeability as a measure of small intestinal mucosal integrity: correlation with jejunal biopsy. Afr J Med Med Sci 18:187–192 [PubMed] [Google Scholar]
- 2.Batt RM, Bush BM, Peters TJ. 1983. Subcellular biochemical studies of a naturally occurring enteropathy in the dog resembling chronic tropical sprue in human beings. Am J Vet Res 44:1492–1496 [PubMed] [Google Scholar]
- 3.Batt RM, Carter MW, Peters TJ. 1984. Biochemical changes in the jejunal mucosa of dogs with a naturally occurring enteropathy associated with bacterial overgrowth. Gut 25:816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batt RM, Hall EJ, McLean L, Simpson KW. 1992. Small intestinal bacterial overgrowth and enhanced intestinal permeability in healthy beagles. Am J Vet Res 53:1935–1940 [PubMed] [Google Scholar]
- 5.Batt RM, McLean L, Rutgers HC, Hall EJ. 1991. Validation of a radioassay for the determination of serum folate and cobalamin concentrations in dogs. J Small Anim Pract 32:221–224 [Google Scholar]
- 6.Batt RM, Morgan JO. 1982. Role of serum folate and vitamin B12 concentrations in the differentiation of small intestinal abnormalities in the dog. Res Vet Sci 32:17–22 [PubMed] [Google Scholar]
- 7.Bjarnason I, MacPherson A, Hollander D. 1995. Intestinal permeability: an overview. Gastroenterology 108:1566–1581 [DOI] [PubMed] [Google Scholar]
- 8.Bjarnason I, Peters T, Veall N. 1984. 51Cr-EDTA test for intestinal permeability. Lancet 2:523. [DOI] [PubMed] [Google Scholar]
- 9.Council of Europe 1986. European Convention for the protection of vertebrate animals used for experimental and other scientific purposes. European Treaty Series – No. 123, 3 pp Strasbourg (France): Council of Europe [Google Scholar]
- 10.Council of the European Communities 1986. Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Off J Eur Comm L358:1–29 [Google Scholar]
- 11.Finnish Government, Ministry of Agriculture and Forestry 1985. Asetus koe-eläintoiminnasta 1076/85. [Decree on the use of animals for experimental purposes]. Helsinki (Finland): Ministry of Agriculture and Forestry [Google Scholar]
- 12.Finnish Government, Ministry of Agriculture and Forestry 1996. Eläinsuojelulaki 274/96, § 31 ja -asetus 396/96. [Animal Welfare Act 274/96, § 31 and Animal Welfare Decree 396/96]. Helsinki (Finland): Ministry of Agriculture and Forestry [Google Scholar]
- 13.Frias R, Sankari S, Westermarck E. 2004. 51Cr-EDTA absorption blood test: an easy method for assessing small intestinal permeability in dogs. J Vet Intern Med 18:156–159 [DOI] [PubMed] [Google Scholar]
- 14.Fyfe JC, Giger U, Hall CA, Jezyk PF, Klumpp SA, Levine JS, Patterson DF. 1991. Inherited selective intestinal cobalamin malabsorption and cobalamin deficiency in dogs. Pediatr Res 29:24–31 [DOI] [PubMed] [Google Scholar]
- 15.Gurnsey MP, Johns DC. 1986. An improved ileal cannula for adult cockerels. Res Vet Sci 41:283–284 [PubMed] [Google Scholar]
- 16.Hall EJ, Batt RM. 1990. Enhanced intestinal permeability to 51Cr-labeled EDTA in dogs with small intestinal disease. J Am Vet Med Assoc 196:91–95 [PubMed] [Google Scholar]
- 17.Hall EJ, Batt RM, Brown A. 1989. Assessment of canine intestinal permeability, using 51Cr-labeled EDTA. Am J Vet Res 50:2069–2074 [PubMed] [Google Scholar]
- 18.Harmoinen JA, Mättö JM, Rinkinen ML, Wilsson-Rahmberg M, Westermarck E. 2001. Permanent jejunal fistula: promising method for obtaining small intestinal chyme without disturbing intestinal function. Comp Med 51:252–256 [PubMed] [Google Scholar]
- 19.Hau J. 2004. Animal models. : Hau J, Van Hoosier GL. Handbook of laboratory animal science. Boca Raton (FL): CRC Press [Google Scholar]
- 20.Hill RC, Ellison GW, Burrows CF, Bauer JE, Carbia B. 1996. Ileal cannulation and associated complications in dogs. Lab Anim Sci 46:77–80 [PubMed] [Google Scholar]
- 21.Hollander D. 1999. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep 1:410–416 [DOI] [PubMed] [Google Scholar]
- 22.Horadagoda NU, Batt RM. 1985. Lysosomal localisation of cobalamin during absorption by the ileum of the dog. Biochim Biophys Acta 838:206–210 [DOI] [PubMed] [Google Scholar]
- 23.LeBlanc JG, Savoy de Giori G, Smid EJ, Hugenholtz J, Sesma F. Folate production by lactic acid bacteria and other food-grade microorganisms. : Mendez-Vilas A. Microbiology book series: communicating current research and educational topics and trends in applied microbiology. Badajoz (Spain): Formatex [Google Scholar]
- 24.Jiang XH, Li N, Li JS. 2003. Intestinal permeability in patients after surgical trauma and effect of enteral nutrition versus parenteral nutrition. World J Gastroenterol 9:1878–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RS, Yee TK, Michielsen CE. 1971. A modified Thomas cannula for gastric and intestinal fistulas. J Appl Physiol 30:427–428 [DOI] [PubMed] [Google Scholar]
- 26.Marks SL, Williams DA. 1998. Time course of gastrointestinal tract permeability to 51Cr-labeled EDTA in healthy dogs. Am J Vet Res 59:1113–1115 [PubMed] [Google Scholar]
- 27.St Jean G, Rings D, Schmall L, Hull B, Hoffsis G. 1989. Comparison of 3 intestinal cannulas used to obtain repeated biopsy of the jejunal mucosa in the newborn calf. Cornell Vet 79:283–293 [PubMed] [Google Scholar]
- 28.Stotzer PO, Brandberg A, Kilander AF. 1998. Diagnosis of small intestinal bacterial overgrowth in clinical praxis: a comparison of the culture of small bowel aspirate, duodenal biopsies, and gastric aspirate. Hepatogastroenterology 45:1018–1022 [PubMed] [Google Scholar]
- 29.Suchodolski JS, Steiner JM. 2003. Laboratory assessment of gastrointestinal function. Clin Tech Small Anim Pract 18:203–210 [DOI] [PubMed] [Google Scholar]
- 30.Wilsson-Rahmberg M, Jonsson O. 1997. Method for long-term intestinal access in the dog. Lab Anim 31:231–240 [DOI] [PubMed] [Google Scholar]