Abstract
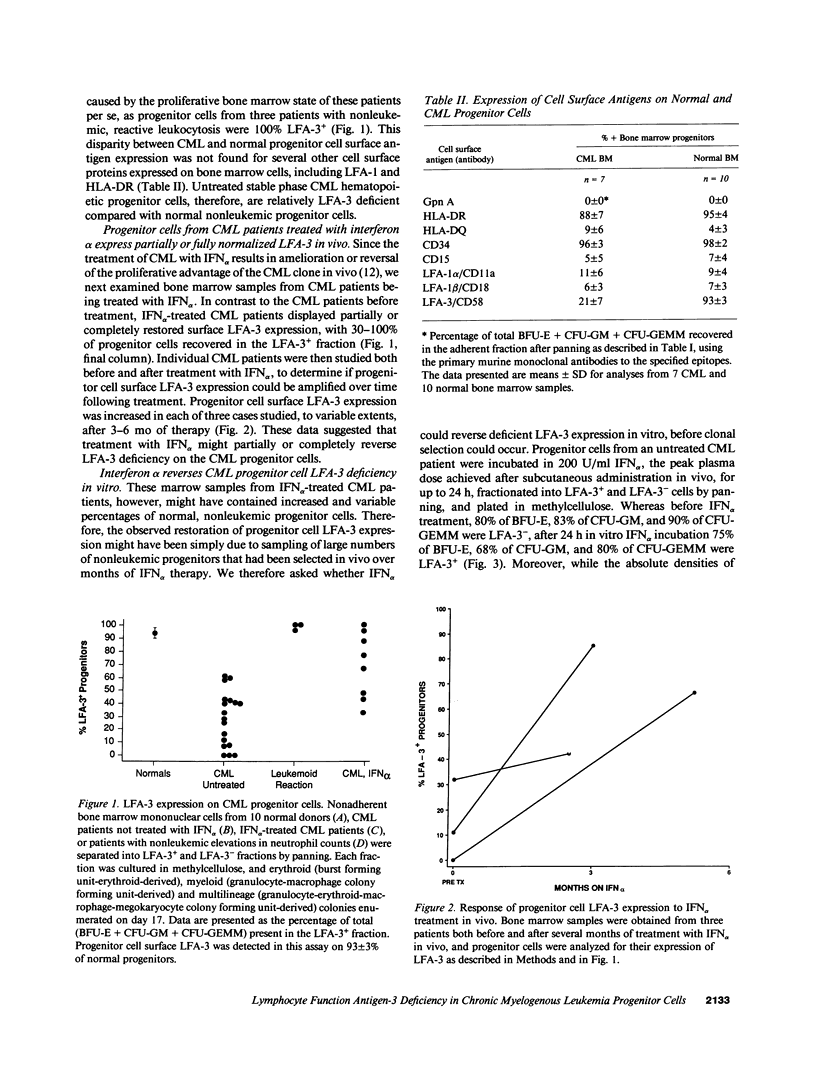
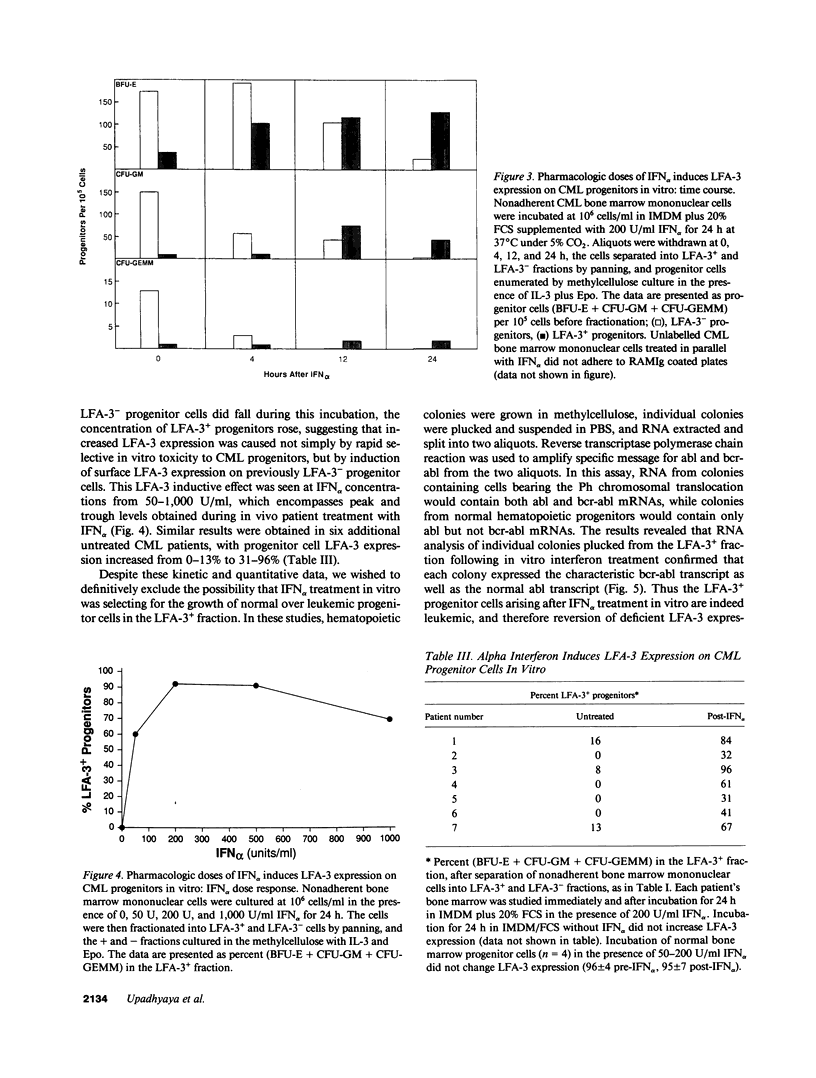
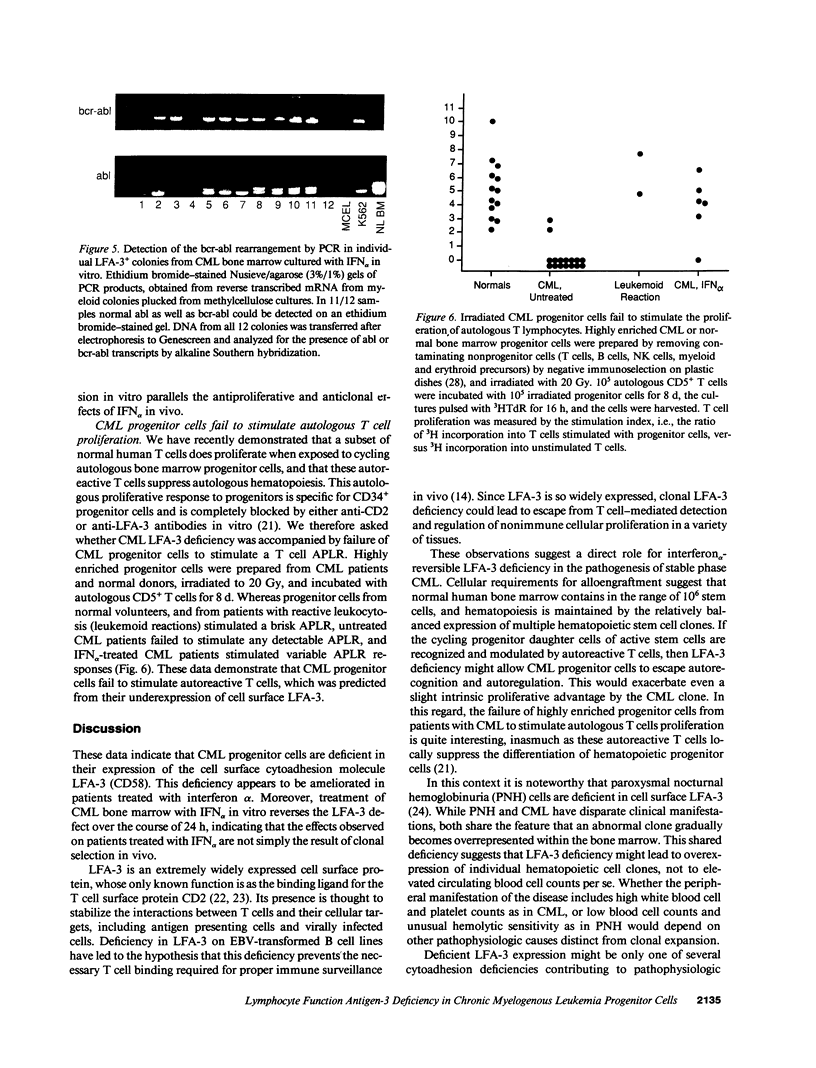
Hematopoietic cells from the malignant clone in chronic myelogenous leukemia (CML) maintain and expand a proliferative advantage over normal hematopoietic cells within the bone marrow. This advantage is often ameliorated or reversed in vivo by IFN alpha. Based upon earlier studies suggesting decreased adhesiveness of CML progenitor cells, we asked whether CML progenitor cells are deficient in their expression of the cytoadhesion molecule lymphocyte function antigen-3 (LFA-3, CD58) which is normally expressed on hematopoietic progenitors. Progenitor cells from untreated CML patients showed greatly reduced or absent LFA-3 expression, whereas progenitors from patients treated with IFN alpha in vivo or in vitro expressed surface LFA-3 at more normal levels. LFA-3-deficient CML progenitor cells were unable to stimulate normal regulatory proliferative responses in autologous T cells. We hypothesize that IFN alpha-sensitive LFA-3 deficiency reflects a cell surface cytoadhesion defect which may help explain adhesive abnormalities of CML progenitor cells in vitro and clonal proliferation in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards A., Rubin C. M., Westbrook C. A., Paskind M., Baltimore D. The first intron in the human c-abl gene is at least 200 kilobases long and is a target for translocations in chronic myelogenous leukemia. Mol Cell Biol. 1987 Sep;7(9):3231–3236. doi: 10.1128/mcb.7.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombel L., Eaves C., Kalousek D., Gupta C., Eaves A. Long-term marrow culture of cells from patients with acute myelogenous leukemia. Selection in favor of normal phenotypes in some but not all cases. J Clin Invest. 1985 Mar;75(3):961–969. doi: 10.1172/JCI111797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Cooper M. R., McCall C. E. Absence of measurable leukocyte alkaline phosphatase activity from leukocytes of patients with chronic granulocytic leukemia. Clin Chem. 1970 Sep;16(9):798–799. [PubMed] [Google Scholar]
- Delattre O., Olschwang S., Law D. J., Melot T., Remvikos Y., Salmon R. J., Sastre X., Validire P., Feinberg A. P., Thomas G. Multiple genetic alterations in distal and proximal colorectal cancer. Lancet. 1989 Aug 12;2(8659):353–356. doi: 10.1016/s0140-6736(89)90537-0. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Selvaraj P., Mattaliano R. J., Springer T. A. Anchoring mechanisms for LFA-3 cell adhesion glycoprotein at membrane surface. 1987 Oct 29-Nov 4Nature. 329(6142):846–848. doi: 10.1038/329846a0. [DOI] [PubMed] [Google Scholar]
- Emerson S. G., Antin J. H. Bone marrow progenitor cells induce a regulatory autologous proliferative T lymphocyte response. J Immunol. 1989 Feb 1;142(3):766–772. [PubMed] [Google Scholar]
- Emerson S. G., Sieff C. A., Wang E. A., Wong G. G., Clark S. C., Nathan D. G. Purification of fetal hematopoietic progenitors and demonstration of recombinant multipotential colony-stimulating activity. J Clin Invest. 1985 Sep;76(3):1286–1290. doi: 10.1172/JCI112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. Y., Dowding C. R., Riley G. P., Goldman J. M., Greaves M. F. Altered adhesive interactions with marrow stroma of haematopoietic progenitor cells in chronic myeloid leukaemia. Nature. 1987 Jul 23;328(6128):342–344. doi: 10.1038/328342a0. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Roth M. S., Antin J. H., Bingham E. L., Ginsburg D. Detection of Philadelphia chromosome-positive cells by the polymerase chain reaction following bone marrow transplant for chronic myelogenous leukemia. Blood. 1989 Aug 1;74(2):882–885. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P., Dustin M. L., Silber R., Low M. G., Springer T. A. Deficiency of lymphocyte function-associated antigen 3 (LFA-3) in paroxysmal nocturnal hemoglobinuria. Functional correlates and evidence for a phosphatidylinositol membrane anchor. J Exp Med. 1987 Oct 1;166(4):1011–1025. doi: 10.1084/jem.166.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpaz M., Kantarjian H. M., McCredie K., Trujillo J. M., Keating M. J., Gutterman J. U. Hematologic remission and cytogenetic improvement induced by recombinant human interferon alpha A in chronic myelogenous leukemia. N Engl J Med. 1986 Apr 24;314(17):1065–1069. doi: 10.1056/NEJM198604243141701. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Wallner B. P., Frey A. Z., Tizard R., Mattaliano R. J., Hession C., Sanders M. E., Dustin M. L., Springer T. A. Primary structure of lymphocyte function-associated antigen 3 (LFA-3). The ligand of the T lymphocyte CD2 glycoprotein. J Exp Med. 1987 Oct 1;166(4):923–932. doi: 10.1084/jem.166.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang-Peng J., Canellos G. P., Carbone P. P., Tjio J. H. Clinical implications of cytogenetic variants in chronic myelocytic leukemia (CML). Blood. 1968 Nov;32(5):755–766. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]