Abstract
Neuregulins (NRGs) are ligands of ErbB receptor tyrosine kinases. The NRG1-ErbB4 pathway has been shown to modulate hippocampal synaptic plasticity and network oscillations in the adult rodent brain. In order to identify cells that mediate these effects here we determine the expression pattern of ErbB4 in four functionally distinct classes of interneurons that represent the majority of all inhibitory neurons in the hippocampus. Based on data from 15 mice and 25,000 cells, we show that ErbB4 is expressed in cells that are positive for cholecystokinin (CCK, 54%), parvalbumin (PV, 42%), or neuronal nitric oxide synthase (nNOS, 39%) in a layer-specific and region-specific manner, whereas cells expressing somatostatin (SOM) are rarely immunoreactive for ErbB4 (1%). We next compared the numerical density (cells/mm3) and the distribution of interneurons between ErbB4−/− mice and wildtype controls. Based on data from 25 mice and 56,000 cells, we detected reductions of PV-positive and nNOS-positive cells in knockouts (−24% and −27%, respectively) but only a minor reduction of CCK-positive cells; no changes in SOM-positive cells were observed. The overall reduction of interneurons was verified by quantification of GAD67-immunoreactive cells (−24% in ErbB4−/− mice). The reduction of interneurons along the dorsoventral axis was more severe in intermediate and ventral portions than in the dorsal hippocampus, and regional reductions occurred in the CA1-3 regions and subiculum, whereas we found no significant changes in the dentate gyrus. The expression by different populations of interneurons suggests that ErbB4 can modulate several microcircuits within the hippocampus and mediate the previously reported effects of NRG1 on network oscillations and synaptic plasticity. The selective reduction of GABAergic cells in ErbB4−/− mice is consistent with the role of NRG-ErbB4 signaling in the generation and migration of interneurons during development, and with neuronal and behavioral functional deficits in adult ErbB4 knockouts.
Keywords: calcium binding protein, GABA, inhibition, neuregulin, schizophrenia
INTRODUCTION
ErbB4 is a receptor tyrosine kinase that is expressed by a variety of neurons throughout the brain. Neuregulin1-3 (NRG1-3) that exert their biological activity via an epidermal growth factor-like domain are major ligands for this receptor (Buonanno and Fischbach, 2001). High-level ErbB4 expression is widespread in embryonic tissues, and the important role of ErbB4 during development is further highlighted by the fact that ErbB4 knockout mice die on embryonic day 10.5 due to malformation of the heart (Gassmann et al., 1995). Mice that are genetically rescued from embryonic lethality by transgenic expression of human ErbB4 in the heart (ErbB4−/− HER4heart, here referred to as ErbB4−/−) show impairment of the mammary gland (Tidcombe et al., 2003). In the nervous system, lack of NRG1 causes hypomyelination of peripheral nerves, but its role in myelination of the brain is controversial (Roy et al., 2007; Brinkmann et al., 2008; Taveggia et al., 2008). NRG1 stimulates the proliferation of neural progenitor cells (Liu et al., 2005), and ErbB4 is expressed in germinal zones during development (Kornblum et al., 2000). Distinct populations of cortical GABAergic interneurons are generated in the medial, lateral and caudal eminences of the subpallidum during development (rev. (Flames and Marin, 2005)), and it has been shown that interneuron precursors from the medial and lateral ganglionic eminences express high levels of ErbB4 (Yau et al., 2003). A recent study suggests that NRG1 is chemoattractant for neurons that were generated in the medial ganglionic eminence and that NRG1-ErbB4 signaling guides tangentially migrating inhibitory interneurons from the subpallium to the developing cortex; consequently, the GABAergic innervation is impaired in adolescent ErbB4−/− mice (Flames et al., 2004). Cortical GABAergic cells express ErbB4 RNA (Lai and Lemke, 1991; Gerecke et al., 2001; Fox and Kornblum, 2005) and protein (Yau et al., 2003; Longart et al., 2007), and the highest levels within the brain are found in interneurons of the hippocampal formation.
The ErbB4 ligand NRG1 is expressed by hippocampo-septal projection neurons (Corfas et al., 1995) and by hippocampal pyramidal cells (Chen et al., 1994; Eilam et al., 1998), and is released in an activity-dependent fashion (Loeb et al., 2002; Ozaki et al., 2004). Both NRG1 and ErbB4 have been genetically and functionally associated with schizophrenia (Stefansson et al., 2002; Hahn et al., 2006; Hall et al., 2006; Li et al., 2006; Norton et al., 2006). Recent studies have provided evidence for a role of this signaling pathway in the power of network-dependent hippocampal gamma oscillations (Fisahn et al., 2009), in the regulation of cortical NMDA receptor surface expression (Gu et al., 2005), and in the modulation of long-term potentiation (LTP) at CA3-to-CA1 glutamatergic synapses (Huang et al., 2000; Kwon et al., 2005; Bjarnadottir et al., 2007; Pitcher et al., 2008). Of importance for understanding how NRG1 modulates LTP, and its proposed association with schizophrenia, we recently reported that locally applied NRG1 increases in vivo dopamine release in hippocamp CA1 and that its depotentiating effect on LTP is dopamine D4 receptor-dependent in acute hippocampal slices (Kwon et al., 2008). We suggested that the effects of NRG1 on dopamine release and depotentiation are likely to be mediated by local hippocampal interneurons, because we could not detect ErbB4-immunoreactivity on cells in the ventral tegmental area or on dopaminergic fibers in the hippocampus. ErbB4 immunoreactivity was also absent from pyramidal cells, but is abundant on many hippocampal interneurons.
Taken together, these studies suggest a developmental role for the NRG-ErbB4 pathway in the generation of interneurons, their migration, and differentiation in the neocortex and hippocampus. Moreover, this pathway is apparently directly involved in neuronal signal processing in the adult brain by modulating synaptic plasticity and oscillations (see above). Both aspects provide biological plausibility for an association of NRG-ErbB signaling with schizophrenia, since the number of interneurons is reduced in several cortical areas, and signal processing and oscillations are impaired in schizophrenia (reviewed in (Lewis and Hashimoto, 2007; Lisman et al., 2008)).
Despite the recent progress in elucidating the functions of NRG1-ErbB4 in the adult brain, it has proven difficult to delineate local circuit-based models that can sufficiently explain the different modulatory actions of this signaling pathway. The major obstacle towards this goal is that comprehensive data on the cellular and subcellular localization of ErbB4 are still missing, partly because widely used commercial antibodies show poor specificity. Given the manifold functional classes of hippocampal interneurons, information on the expression of ErbB4, or lack thereof, in distinct classes of interneurons is crucial for a better understanding of modulatory effects of NRGs on the hippocampal network and for the generation of adequate models. The current study was therefore designed to provide crucial data by identifying and quantifying the cellular expression of ErbB4 in four functionally different populations of interneurons that represent the majority of all inhibitory cells in the adult hippocampus (Kawaguchi and Kondo, 2002). These interneurons are identified by largely non-overlapping markers ((Jinno and Kosaka, 2002; Jinno and Kosaka, 2004); reviewed in (Somogyi and Klausberger, 2005; Jinno and Kosaka, 2006)). Cholecystokinin (CCK) and parvalbumin (PV) are preferably expressed in interneurons that target perisomatic regions of pyramidal cells and coordinate their activity during network oscillations (reviewed in (Mann and Paulsen, 2007)), somatostatin (SOM) is expressed in cells that provide feedback inhibition to distal portions of pyramidal cell dendrites in stratum lacunosum moleculare (Katona et al., 1999), and neuronal nitric oxide synthase (nNOS) is expressed in small interneurons that innervate pyramidal cells as well as other interneurons in apical layers of the hippocampus (Seress et al., 2005).
Given the manifold functions of GABAergic interneurons in the dynamic regulation of hippocampal network properties (McBain and Fisahn, 2001), the aim of the present study is twofold: First, to present a comprehensive and systematic analysis of ErbB4 expression in distinct subpopulations of interneurons. Second, to investigate whether the lack of ErbB4 during development and maturation interferes with inhibition in the adult hippocampus. We show, using highly specific and well-characterized mouse anti-N-ErbB4 (Chen et al., 1996) and novel rabbit anti-C-ErbB4 antibodies (Vullhorst et al., submitted), that ErbB4 is expressed in a substantial percentage of CCK-, PV-, and nNOS-immunoreactive neurons in an area-specific and layer-specific manner, whereas ErbB4 expression in SOM-immunoreactive cells is negligible. Moreover, we report a selective reduction of nNOS- and PV-positive cells in ErbB4−/− mice, whereas the numerical densities of CCK- and SOM-positive cells show no major changes. These findings suggest that NRG-ErbB4 signaling may play a major role in many hippocampal microcircuits that mediate different functional properties, such as synaptic plasticity and oscillatory network activity.
MATERIALS AND METHODS
Animals
Immunohistological experiments used adult mice (12-16 weeks); n=14 C57BL/6 wildtype mice (Jackson Laboratories, MA) and n=12 heart-rescued ErbB4−/− mice (Tidcombe et al., 2003) that were backcrossed for >15 generations into C57BL/6. Animals were raised under a 12h light/12h dark cycle with food and water provided ad libitum. All procedures were approved and followed the NIH Guidelines for the Care and Use of Laboratory Animals.
Immunohistology
Animals were anaesthetized with brief exposure to gaseous isoflurane and subsequent i.p. injections of Avertin (2,2,2 tribromoethanol, 250mg/kg) and perfused through the left cardiac ventricle with 15ml PBS (0.1M, pH 7.0, 21°C), followed by 60ml 4% paraformaldehyde in PBS (pH 7.4) over 15min. After dissection, brains were postfixed overnight at 4°C and cut horizontally on a vibratome (50μm). All hippocampal sections were subsequently collected on 12-well tissue-culture plates in PBS with 15mM sodium azide and stored at 4°C. This collection scheme generated 12 systematic random sets of 5-7 sections per well that covered the whole dorsoventral extent of the hippocampal formation. For each interneuron marker and each animal, one set of sections (600μm section interval) was processed for double-immunofluorescence, and two sets (300μm section interval) for immunohistochemistry, in 0.1M PBS pH7.4 as follows: 2×10min washes in 0.1M PBS; (only immunohistochemistry: 30min in PBS with 1% H2O2, followed by 3×10min washes); 90min blocking in 0.1M PBS containing 10% normal donkey serum, 0.25% Triton-X100; primary antibody incubation for 40h at 4°C in blocking solution; 3×10min washes; secondary antibody for 90min at room temperature in blocking solution; 3×10min washes. Immunofluorescence sections were mounted in Mowiol-DABCO and stored at 4°C. Immunohistochemical sections were stained with diaminobenzidine (DAB Black Kit, Zymed, Carlsbad, CA) for 5min at room temperature, followed by 3×10min washes, air-dried, dehydrated with ethanol, cleared with 3×10 min xylene, and mounted in Permount. As controls, sections without primary and/or secondary antibodies exhibited only low-intensity general background fluorescence. The specificity of the mouse monoclonal (epitope in extracellular domain) and rabbit polyclonal (epitope in intracellular domain) ErbB4 antibodies was additionally tested by double-immunofluorescence that resulted in almost perfect overlay (Fig.1). Application of both mouse and rabbit anti-ErbB4 antibodies on tissue of ErbB4−/− mice resulted in low-intensity general background staining. The rabbit polyclonal anti-ErbB4 antibody (Serum 5941) has undergone stringent tests for specificity in a variety of applications that include Western blotting, immunoprecipitation, and immunolabeling of cultured hippocampal neurons (Vullhorst et al., submitted).
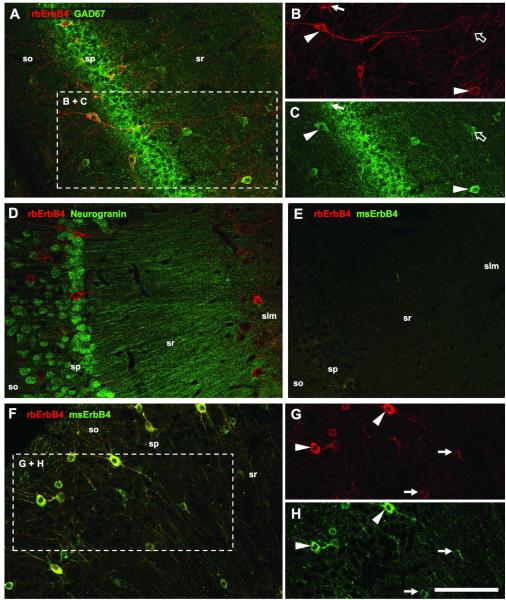
Figure 1. ErbB4 is expressed in hippocampal interneurons.
Double-immunofluorescence reveals that the majority of GAD67-immunoreactive cells strongly express ErbB4 (A-C, arrowheads). However, some cells show higher immunoreactivity for either ErbB4 (arrow) or GAD67 (open arrow). The cell morphology suggests that ErbB4 is exclusively expressed in interneurons. We verified this with immunofluorescence labeling of ErbB4 and neurogranin (D); the immunoreactive cell populations are non-overlapping. The specificity of the mouse (msErbB4) and rabbit (rbErbB4) antibodies against ErbB4 was tested on ErbB4−/− sections (E), and by double-immunofluorescence labeling of wildtype tissue (F-H). Most cells showed similar strong staining with both antibodies (arrowheads), and the immunofluorescence intensity was also similar in cells with lower ErbB4 expression (arrows). Abbreviations: (so) stratum oriens, (sp) str. pyramidale, (sr) str. radiatum, (slm) str. lacunosum moleculare. Scale bar = 100μm.
Primary antibodies: rabbit polyclonal anti-parvalbumin (1:3000; Swant, Bellinzona, Switzerland); rabbit polyclonal anti-neuronal nitric oxide synthase (1:4000; Zymed, San Francisco, CA); rabbit polyclonal anti-somatostatin (1:500) and mouse monoclonal anti-cholecystokinin (1:500; Digestive Diseases Research Center, UCLA, Los Angeles, CA); rabbit polyclonal anti-GAD67 (1:3000; Chemicon, Temecula, CA); rabbit polyclonal anti-ErbB4 (Serum 5941, 0.5μg/ml); mouse monoclonal anti-ErbB4 (AB-1; 1:300, NeoMarkers, Fremont, CA). Secondary antibodies: donkey anti-rabbit Cy3 (1:1000), donkey anti-rabbit HRP (1:1000) and donkey anti-mouse HRP (1:500; Jackson ImmunoResearch, West Grove, PA); donkey anti-mouse Alexa-488 (1:500; Molecular Probes, Eugene, OR). All secondary antibodies were highly pre-adsorbed to minimize cross-reactivity.
Microscopy and cell quantification
Immunofluorescence was analyzed using a confocal microscope (Zeiss 510 Meta) at 10×, 20× and 40×Oil magnification. Diaminobenzidine staining was visualized on a Nikon SMZ-U binocular microscope, equipped with a digital camera, at 5× magnification. Single images were assembled to image arrays that cover the entire hippocampal formation using the Photomerge function in Adobe Photoshop CS. The presented images are representative for the staining patterns, and were adjusted for overall brightness and contrast using Adobe Photoshop CS.
As evident from our images, the immunolabeling was very good on the surface of sections. However, the antibody penetration was insufficient even after prolonged incubations of up to five days; we therefore did not use an unbiased stereological approach. Coexpression of ErbB4 with markers for interneurons was determined by counting only neurons that were clearly, and independently, identifiable in both the red and green color channels, rather than solely on appearance of yellow pixels in the superimposed images. It is, therefore, unlikely that we overestimated the number of neurons that coexpress ErbB4 and one of the interneuron markers; our data rather indicate the lower boundary of the range of coexpression. The positive cells were identified, assigned to the appropriate area and layer, individually labeled with a graphic tool and counted separately in each of the two color channels. Thereafter, the two color channels were merged and double-labeled cells counted. For immunohistochemistry, 11-14 sections per animal that covered the entire dorsoventral extent of the hippocampus were each assigned to the appropriate of five dorsoventral levels and the data from 2-3 sections within each level were pooled. The areas (mm2) of layers within hippocampal subregions were quantified using Zeiss image analysis software (LSM5 Image Browser), and the numerical density (ND, cells/mm3) was calculated using the focal sampling range of confocal images, section thickness, number of analyzed sections, and section interval as variables. During all procedures the investigator was unaware of the genotype of individual mice.
Data Analysis and Statistics
For the four interneuron-markers, the number of animals per genotype was n=3 for double-immunofluorescence (except CCK: n=4), and n=4 for all five immunohistochemistry experiments, including GAD67. Possible differences between genotypes in total numerical density of hippocampal interneurons were analyzed with two-tailed unpaired Student’s t-test, and preceded by an F-test to verify equal variance. The distribution of interneurons across areas, layers, or dorsoventral levels was analyzed with two-way ANOVA and post-hoc Bonferroni tests. The significance level for all tests was set to p=0.05. All data are presented as means ± S.E.M.
RESULTS
ErbB4 is specifically immunolabeled in interneurons
We began by investigating the expression of ErbB4 in interneurons versus pyramidal cells because the cellular location of ErbB4 is a matter of ongoing debate (Lai and Lemke, 1991; Yau et al., 2003; Li et al., 2007; Longart et al., 2007; Mechawar et al., 2007), partly because of poor specificity of commercially available antibody preparations (Vullhorst et al., submitted). We used double-immunofluorescence for ErbB4 and the interneuron marker GAD67 (Fig.1A-C) and found that the majority of GAD67-immunoreactive somata coexpress ErbB4. In contrast, ErbB4 immunoreactivity was not detected on pyramidal cells. This result was verified by double-immunofluorescence labeling of ErbB4 and neurogranin (Fig.1D), which is a marker for principal cells but is not expressed in interneurons (Singec et al., 2004). The specificity of the two anti-ErbB4 antibodies that were raised in mouse or rabbit and that target epitopes in either the extracellular or the intracellular domain, respectively (see Materials and Methods), was tested by double-immunofluorescence. The application on tissue from ErbB4−/− mice resulted in low-level general background fluorescence (Fig.1E). We also performed double-immunofluorescence on wildtype tissue and found that the anti-ErbB4 antibodies consistently co-labeled somata and dendrites of interneurons (Fig.1F-H). These tests, together with a comprehensive analysis of the specificity of the rabbit anti-ErbB4 antibody in various applications (Vullhorst et al., submitted) validated the reliability of these reagents for the subsequent quantitative investigation of ErbB4 expression on different subpopulations of hippocampal interneurons.
ErbB4 expression in CCK-positive cells
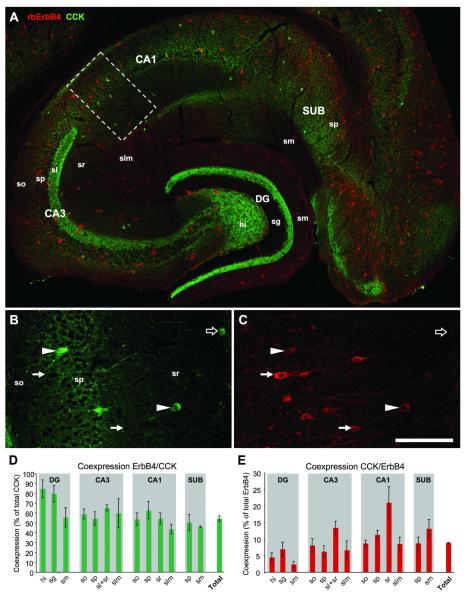
We started the analysis by double-immunofluorescence labeling of ErbB4 and CCK, and quantified the percentages of coexpression in cells of both populations. ErbB4 is expressed in a large number of interneurons that cover all areas and all layers of the hippocampal formation whereas the number of CCK-immunoreactive cells is much lower (Fig.2A). Strong axonal CCK-expression is evident in the inner third of stratum moleculare of the dentate gyrus (DG) and in stratum lucidum of CA3. While our study focuses on immunoreactive somata, it is worth mentioning that CCK-immunoreactivity in stratum moleculare of DG has consistently been observed with different antibodies in rats and mice (Jinno and Kosaka, 2003; Matyas et al., 2004; Hefft and Jonas, 2005). In contrast, the labeling of stratum lucidum occurs only in mice (Jinno and Kosaka, 2003; Hefft and Jonas, 2005) and is most intense with the mouse anti-CCK antibody that we have used. High power microscopic analysis has revealed that CCK-immunoreactivity is locally concentrated within mossy fiber terminals (Jinno and Kosaka, 2003). Our single channel images show coexpressing cells in strata pyramidale and radiatum of CA1, as well as cells being immunoreactive for only one marker (Fig.2B,C). We found that, throughout the entire hippocampus (n=4), on average 54±3% of CCK-positive cells coexpress ErbB4 (“Total” in Fig.2D). The amount of coexpression showed little difference between hippocampal areas and layers with the exception of DG where coexpression in CCK cells was above 80% in both granule cell layer and hilus. The average coexpression of CCK in ErbB4-positive cells was 8.8±0.2% (Fig.2E). The regional variability was much higher, ranging from 2.3±0.9% in stratum moleculare of DG to 21±5% in stratum radiatum of CA1.
Figure 2. The majority of CCK cells coexpress ErbB4.
A compound double-immunofluorescence image shows the distribution of a low number of CCK-positive somata and numerous ErbB4-positive somata in all regions of the hippocampal formation (A). Note the dense axonal staining for CCK in DG (inner third of sm) and CA3 (sl). Single channel images from CA1 (B-C; location indicated by dashed rectangle in A) show coexpression (arrowheads) and expression of either CCK (open arrow) or ErbB4 (arrow). The punctae surrounding immunonegative somata in sp are basket terminals (B). Quantification (n=4) of coexpession in CCK cells (D) and ErbB4 cells (E); “Total” is the percentage of coexpression across the entire hippocampus. Abbreviations: (so) stratum oriens, (sp) str. pyramidale, (sr) str. radiatum, (slm) str. lacunosum moleculare, (sm) str. moleculare, (hi) hilus, (sg) str. granulosum, (DG) dentate gyrus, (CA1-3) cornu ammonis areas 1-3, (SUB) subiculum. Scale bar = 290μm (A), 100μm (B,C).
ErbB4 expression in PV-positive cells
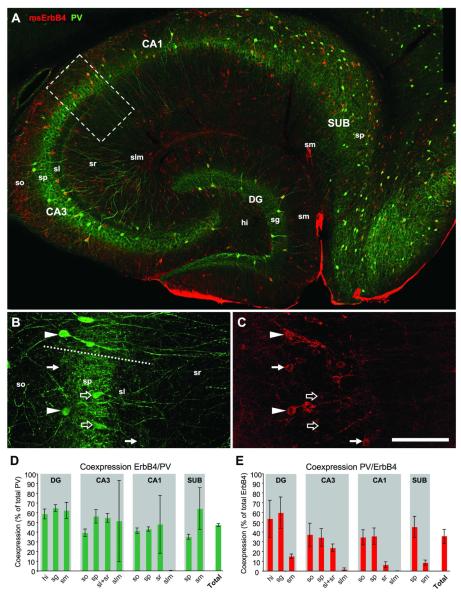
We next investigated PV-immunoreactive neurons, which are mostly located in the principal cell layers of all hippocampal areas, and in stratum oriens of CA1-3 and hilus of DG (Fig.3A). Both coexpressing cells and cells single-labeled for either PV or ErbB4 are evident in stratum pyramidale (Fig.3B,C). On average 42±2% of all hippocampal PV cells coexpressed ErbB4 (n=3), and there was little variation between areas and layers (Fig.3D). The average percentage of PV-coexpressing ErbB4 cells across the entire hippocampus was 35±8% (Fig.3E). In contrast to PV cells, the regional amount of coexpressing ErbB4 interneurons varied substantially from almost 60% in the granule cell layer of DG to 1.5% in stratum lacunosum moleculare of CA3. However, this is simply due to the distribution pattern of PV cells, which are almost absent from upper stratum radiatum and stratum lacunosum moleculare. Taken together, our results show similar patterns of substantial ErbB4-coexpression in both populations of basket cells, PV- and CCK-positive cells, suggesting that activation of the NRG1-ErbB4 pathway is likely to influence both pathways that provide perisomatic inhibition within the hippocampal network.
Figure 3. ErbB4 is expressed by half of PV-immunoreactive neurons.
PV-positive somata are almost exclusively located within and below principal cell layers and a high number of cells coexpress ErbB4 as indicated by yellow pixels (A). Consistent with the functional role of PV cells in providing perisomatic inhibition, the principal cell layers (sp, sg) show the highest overall staining intensity. Single channel images (B,C) from the border of CA1-3 (dashed line in B) show coexpression (arrowheads) and expression of either PV (open arrows) or ErbB4 (arrows). Quantification (n=3) of coexpession in PV cells (D) and ErbB4 cells (E); “Total” is the percentage of coexpression across the entire hippocampus. Note that the large error bars in (D) are due to the very low number of PV cells in the upper layers sr, sm and slm, resulting also in low percentages of coexpression in these layers in (E). Abbreviations: see Fig.2. Scale bar = 290μm (A), 100μm (B,C).
Lack of ErbB4 expression in SOM-positive cells
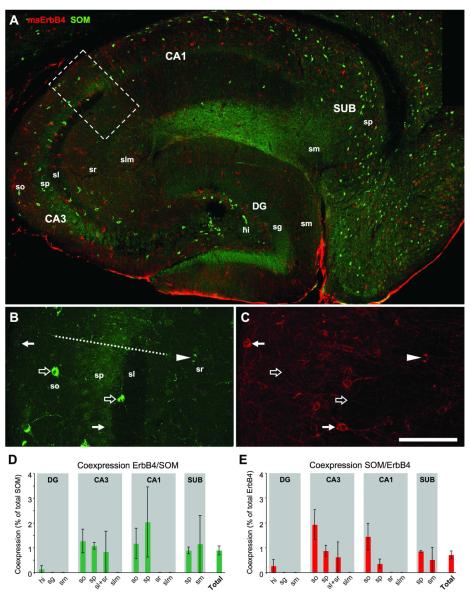
SOM-immunoreactive cells are mostly located in the hilus and in strata oriens and pyramidale, but sparsely in apical layers. In CA1, they are mostly confined close to the alveus, whereas in CA3 they are also frequently found in stratum pyramidale and the lower half of stratum radiatum. SOM cells in hippocampus are mostly O-LM cells and the innervation of stratum lacunosum moleculare is clearly visible in CA1 (Fig.4A). Single channel images reveal that most SOM-positive cells do not coexpress ErbB4 (Fig.4B,C). This result is verified by the quantification of coexpression (n=3), showing that less than 1% of the populations of SOM cells and ErbB4 cells coexpress both markers (Fig.4D,F).
Figure 4. SOM cells do not express ErbB4.
(A) The distribution of SOM-immunoreactive somata is similar to PV cells; they are mostly confined to layers so, sp, and hi. In CA3 close to DG, however, SOM cells are also frequently found in sr. Note the dense fiber plexus in slm of CA1 that is the terminal field of O-LM cell projections. Single channel images (B-C) show that most somata are immunoreactive for either SOM (open arrows) or ErbB4 (arrows); however, one cell in sr coexpresses both markers (arrowhead). The quantitative analysis (n=3) reveals that the maximum coexpression is 2% in both SOM cells (D) and ErbB4 cells (E). The overall frequency of coexpression in the entire hippocampus is below 1% for both cell populations (“Total” in D-E). Abbreviations: see Fig.2. Scale bar = 290μm (A), 100μm (B,C).
ErbB4 expression in nNOS-positive cells
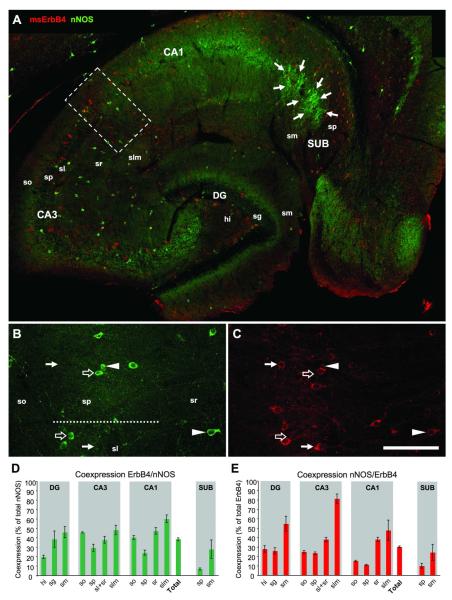
nNOS-immunoreactive somata are located in all hippocampal areas and layers. Axonal staining is evident in the inner third of stratum moleculare in DG and in all layers of CA1 except of stratum lacunosum moleculare (Fig.5A). In DG and CA1-2, nNOS appears to be exclusively expressed in interneurons; in CA1, however, nNOS-immunoreactivity is clearly detectable in pyramidal cells, but it is weaker than in interneurons. A cluster of pyramidal cells in the subiculum (SUB) that project to CA1 pyramidal cells (Seress et al., 2002) is strongly immunoreactive. Higher magnification imaging shows that not all nNOS-positive cells coexpress ErbB4 (Fig.5B, C), in contrast to an earlier study that reported 100% coexpression in nNOS cells of the hippocampus of adult rats (Yau et al., 2003). Quantification (n=3) reveals that 39±2% of nNOS-positive cells in DG and CA1-3 coexpress ErbB4 (Fig.5D). The lowest amounts of coexpression were found in principal cell layers and hilus (20±2% in hilus), whereas the highest coexpression occurred in the apical molecular layers (60±4% in CA1). Due to the additional expression of nNOS in numerous pyramidal cells that are always immunonegative for ErbB4, the pyramidal layer of SUB shows coexpression in only 6.8±1.1% of all nNOS-positive cells. Since our analysis focuses on interneurons we excluded SUB from calculating the percentage of coexpressing cells across the hippocampus. “Total” coexpression is 29±2% in all nNOS-positive cells (Fig.5D), and coexpression within the population of all ErbB4-positive cells is 30±1% across all layers of DG and CA1-3 (Fig.5E). The lowest percentage of coexpressing ErbB4-positive cells was found in principal cell layers (11.3±0.7% in sp of CA1) and the highest coexpression in upper molecular layers (81±5% in slm of CA3).
Figure 5. One third of nNOS-positive interneurons express ErbB4.
nNOS-immunoreactive somata are located in all hippocampal areas, with the highest densities in hi, and in sp of CA1-3 and SUB (A). Immunoreactivity for nNOS is evident on neurites in the inner third of sm in DG, and in all layers of CA1. The highest density of nNOS-ErbB4 coexpressing cells is in slm of CA3. Note the cluster of strongly immunoreactive pyramidal cells in SUB (arrows in A) that are consistently negative for ErbB4. Single channel imaging in CA1-3 (B-C) shows cells that express either nNOS (open arrows) or ErbB4 (arrows), as well as double-immunoreactive somata (arrowheads). Regional analysis of coexpression (n=3) in all nNOS-positive cells (D) and all ErbB4-positive cells (E). Please note that “Total” represents only coexpression in DG and CA1-3; in order to focus on interneurons SUB was excluded to avoid bias towards the high number of nNOS-positive/ErbB4-negative pyramidal cells in sp of SUB. Abbreviations: see Fig.2. Scale bar = 290μm (A), 100μm (B,C).
Summary of ErbB4 expression in interneurons
Figure 6A summarizes our data on the distribution of ErbB4-positive cells and the coexpression with markers for the four subpopulations of hippocampal interneurons. The analysis of regional coexpression indicates that the four populations of interneurons account for most of ErbB4-positive cells, even with our conservative method of defining coexpression that likely represents the lower limit of the actual range (see Materials and Methods). There are, however, pronounced regional differences in the percentage of unidentified ErbB4-immunoreactive cells, ranging from less than 8% in stratum granulosum of DG to almost 54% in stratum moleculare of SUB. We find the highest numerical densities (ND, cells/mm3) of ErbB4-immunoreactive cells consistently in principal cell layers of all hippocampal areas and in the hilus, whereas molecular layers show distinctly lower NDs. Across all regions of the hippocampus, the ND is 1414±114 in wildtype mice (n=9). We also analyzed the distribution of ErbB4-immunoreactive somata along the dorsoventral extent of the hippocampus (Figure 6B). Consistent with numerous reports of a dorsoventrally increasing GABAergic cell density (reviewed in (Moser and Moser, 1998; Jinno and Kosaka, 2006)) we found that the ND of ErbB4-positive cells is 1104±68 in the most dorsal part, 1576±135 in the intermediate part, and 1591±171 in the ventral hippocampus. The dorsoventral gradient is significant (n=9, 5 dorsoventral levels, one-way ANOVA, F=7.106, p=0.0003), which is attributable to the first level being significantly lower than levels 3-5 (all p<0.01, Bonferroni post-hoc tests).
Figure 6. Distribution and identity of hippocampal ErbB4-immunoreactive cells.
The four classes of interneurons account for most of hippocampal ErbB4-immunoreactive cells (A). Numbers below the graphs give the numerical density (cells/mm3) of ErbB4-positive neurons ± S.E.M. for each layer in each area. The four interneuron-markers were sufficient to identify the majority of hippocampal ErbB4-positive cells, covering more than 50% in 12 of 13 regions, and more than 70% in 6 regions. The legend shows the number of coexpressing cells and the total cell count for each interneuron marker in immunofluorescence experiments from wildtype animals; note that the number of animals that contributed to the total cell count is different for the markers (CCK: n=4; PV, SOM, nNOS: n=3; ErbB4: n=9). ErbB4-immunoreactive cells show a gradient of increasing cell density (+50%) along the dorsoventral axis (B). Abbreviations: see Fig.2.
Analysis of interneuron density in adult wildtype versus ErbB4−/− mice
It has been indicated that the GABAergic system may be compromised in the hippocampus of adolescent (P20) ErbB4−/− mice (Flames et al., 2004), but it is unknown whether this impairment is general or might be different between interneuron subtypes. We hypothesized that the interneuron populations that exhibit substantial levels of coexpression with ErbB4, namely CCK, PV and nNOS, would be more susceptible to lack of ErbB4 than SOM-positive cells. Therefore, we next investigated this question with immunohistochemical staining and brightfield microscopy because this procedure allowed us to easily quantify large amounts of neurons, and determined the ND of immunoreactive cells in four subareas (DG, CA3, CA1, SUB) at five different levels of the dorsoventral axis of the hippocampus of C57BL6 and ErbB4−/− mice (n=4 each).
Area-specific reduction of nNOS-immunoreactive cells in ErbB4−/− mice
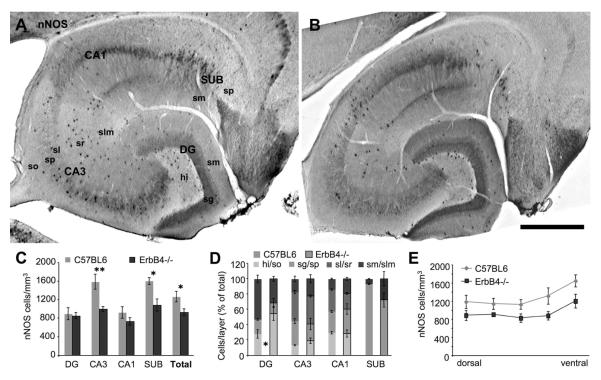
Our analysis reveals that the ND of nNOS-positive cells is severely reduced across the hippocampal regions of ErbB4−/− mice (Fig.7C; n=4 each, 4 regions, 2-way ANOVA, F=18.76, p=0.0002). This reduction is attributable to CA3 (−37%, p<0.01) and SUB (−32%, p<0.05; Bonferroni post-hoc test), the areas with the highest ND in wildtype animals. The total reduction in the entire hippocampus is 27% (n=4 each, 2-tailed t-test, p=0.049). The distribution of nNOS-positive cells across layers is changed in DG (n=3 each, 3 layers, 2-way ANOVA, F=9.853, p=0.003) and SUB (F=10.01, p=0.013) but not in any other area in ErbB4−/− mice (Fig.7D). Finally, the ND is significantly decreased across the dorsoventral axis of the hippocampus (n=4 each, 5 dorsoventral levels, 2-way ANOVA, F=19.46, p=0.0001), but post-hoc analysis shows that this is not attributable to any of the five levels (Fig.7E). The results indicate a severe reduction of the ND of nNOS-positive cells in CA3 and SUB that occurs along the entire dorsoventral extent of the hippocampus.
Figure 7. nNOS-expressing interneurons are substantially reduced in ErbB4−/− mice.
Representative images of immunohistochemically labeled nNOS-expressing cells shows their occurrence in all regions of wildtype hippocampus (A), and indicate a reduction of the cell density in CA3 and SUB of ErbB4 knockouts (B); note also the almost complete absence of staining in lateral entorhinal cortex (upper right corner in B). Quantification (n=4) confirms a significant reduction of nNOS cells in areas CA3 and SUB (C), as well as in the entire hippocampus (“Total”). The distribution across layers (D) is altered in DG and SUB; the change in DG is due to an increased percentage of cells located in hi but is not attributable to any specific layer in SUB. The ND of nNOS cells is equally reduced along the entire dorsoventral axis (E). Scale bar = 500μm.
Homogeneous reduction of PV-immunoreactive cells in ErbB4−/− mice
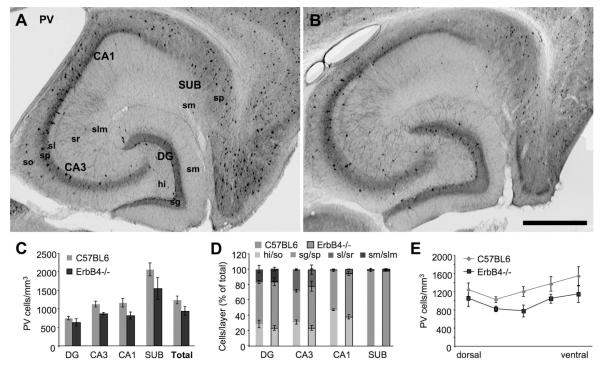
Our analysis showed a significant reduction of PV-immunoreactive cells in the hippocampal formation of ErbB4−/− compared to C57BL6 mice (n=4 each, 4 regions, 2-way ANOVA, F=9.492, p=0.005). Despite a consistent trend in all four regions (Fig.8C), the reduction was not attributable to any specific region according to the Bonferroni post-hoc test. We did not detect any changes in the distribution of PV cells across hippocampal layers (Fig.8D). However, we found a significant reduction along the dorsoventral axis of the hippocampus (n=4 each, 5 dorsoventral levels, 2-way ANOVA, F=11.76, p=0.002). Post-hoc analysis revealed that this was not due to significant changes in any of the five levels (Fig.8E). These results indicate a uniform reduction of PV-immunoreactive somata in ErbB4−/− mice (−24%) across the entire 3-dimensional extent of the hippocampus.
Figure 8. PV-immunoreactive somata are reduced in ErbB4−/− mice.
In comparison to wildtype hippocampus (A) the density and distribution pattern of PV-positive interneurons is changed (B). The images are representative in that they show a large area in CA1 and proximal SUB that is almost devoid of PV cells; this pattern of local depletion is typical, rather than widespread general reduction of cell density. The ND (n=4) is significantly reduced in hippocampus of ErbB4−/− mice (C) without changing the distribution of cells across layers (D). We also detected a significant reduction of PV cells along the dorsoventral axis (E). Scale bar = 500μm.
Minor reduction of CCK-immunoreactive cells in ErbB4−/− mice
The comparison of CCK-positive somata shows a uniform but non-significant trend towards a reduction in all four areas of the hippocampus of ErbB4−/− mice (Fig.9C; n=4 each, 4 regions, 2-way ANOVA, F=2.075, p=0.163). The distribution of immunoreactive cells across layers is also not significantly altered (Fig.9D), with the exception of SUB where a higher percentage of cells is located in stratum moleculare of ErbB4−/− mice (n=4 each, 2 layers, 2-way ANOVA, F=40.70, p<0.0001). Post-hoc analysis showed that the percentages of CCK cells are significantly altered in both strata pyramidale and moleculare of SUB (p<0.01, Bonferroni test). Analysis of the ND along the dorsoventral axis of the hippocampus revealed a significant reduction in ErbB4−/− mice (Fig.9E; n=4 each, 5 dorsoventral levels, 2-way ANOVA, F=5.553, p=0.025) albeit this was not attributable to a significant change in any of the five dorsoventral levels (Bonferroni post-hoc test). These results suggest that lack of ErbB4 has only minor effects on the population of hippocampal CCK-positive basket cells.
Figure 9. Minor reduction of CCK-positive cells in ErbB4−/− mice.
Immunohistological staining of hippocampus is similar between wildtype (A) and ErbB4−/− mice (B). The ND (n=4) of CCK-immunoreactive somata is consistently, but not significantly, lower in all areas of ErbB4−/−/ mice (C). The distribution of cells is not different between genotypes, with the exception of SUB where less cells are located in sp of knockouts (D). The ND of CCK cells is significantly higher in wildtype animals across the dorsoventral axis; however, this is not attributable to significant differences at any specific level. Scale bar = 500μm.
No change in the number of SOM-immunoreactive cells in ErbB4−/− mice
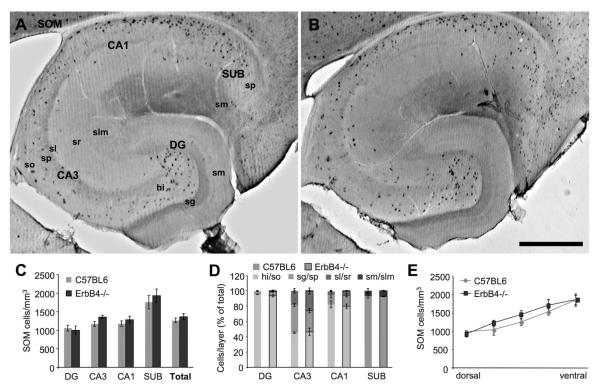
SOM-immunoreactive somata are most abundant in SUB (1.8 ×103 cells/mm3) and show a lower density of 1.0-1.2 ×103 cells/mm3 in all other hippocampal areas (Fig.10C). The ND of wildtype animals is not significantly different from ErbB4−/− mice (n=4 each, 4 subregions, 2-way ANOVA, F=1.589, p=0.220) and we did not detect any changes in the distribution across hippocampal layers (Fig.10D). Consistently, the ND across the dorsoventral axis is also not significantly different between genotypes (Fig.10E; n=4 each, 5 dorsoventral levels, 2-way ANOVA, F=2.403, p=0.132). We conclude that the ND of SOM-positive cells is not altered in the hippocampus of ErbB4−/− mice.
Figure 10. No difference in the number of SOM-positive interneurons.
Immunohistochemistry of SOM cells shows no obvious differences between wildtype (A) and knockout mice (B). Quantitative analysis (n=4) confirms the lack of changes in all hippocampal areas (C), for the distribution of somata across layers (D), and the ND along the dorsoventral axis (E). Scale bar = 500μm.
GAD67-immunoreactive cells are reduced in ErbB4−/− mice
Given the significant reduction of immunoreactive somata in three of the four populations of interneurons we asked whether there might be an actual loss of GABAergic cells in ErbB4−/− mice or rather a down-regulation of the marker proteins, because at least PV-expression is well known to be activity-dependent and highly variable (Scotti et al., 1997; Suzukawa et al., 1999). We therefore went on to compare the ND of GAD67-immunoreactive cells in the hippocampus of WT and ErbB4−/− mice. The images from wildtype and ErbB4−/− mice show a high density of GAD67-positive somata in most hippocampal regions with some exceptions, such as the DG hilus (Fig.11A,B). This staining pattern indicates that interneurons in the hilus express only low levels of GAD67; similarly, lack of either GAD67 or GABA-immunoreactivity have been reported before in several types of interneurons (Aika et al., 1994; Jinno et al., 1998). Therefore, our quantitative analysis shows that the ND in wildtype animals ranges from 1.50 ± 0.13 ×103 in DG to 2.80 ± 0.11 ×103 in SUB, with an average of 2.14 ± 0.07 ×103 across all subareas, which is in line with other data from mice (Aika et al., 1994), but lower than the sum of the NDs of CCK, PV, SOM, and nNOS cells.
Figure 11. Reduction of GAD67-positive cells in ErbB4−/− mice.
Analysis of GAD67 immunoreactivity in wildtype (A) and knockout mice (B) reveals significant reductions (n=4) in all hippocampal areas except DG, and in the entire hippocampus (C). The reduction occurs along the dorsoventral extent of the hippocampus, with the exception of the most dorsal level (D). Scale bar = 500μm.
We found that GAD67-positive cells are significantly reduced throughout the hippocampal subareas in ErbB4−/− mice (n=4 each, 4 subregions, 2-way ANOVA, F=46.11, p<0.0001). Post-hoc analysis revealed that the result is attributable to significant reductions in CA3, CA1, and SUB (CA3: −21%, p<0.05; CA1: −26%, p<0.01; SUB: −34%, p<0.001; Bonferroni test). The reduction throughout the entire hippocampus (“Total” in Fig. 11C) was 24% (n=4 each, p=0.0021, t-test). Immunoreactive somata were also significantly reduced along the dorsoventral axis (n=4 each, 5 dorsoventral levels, 2-way ANOVA, F=50.61, p<0.0001). The reduction is substantial in medial and ventral regions but was not significant in the most dorsal level of the hippocampus (Fig. 11D; Level 2: 29%, p<0.05; level 3: 37%, p<0.001; level 4: 26%, p<0.01; level 5: 26%, p<0.01; Bonferroni test). The reduction in GAD67-immunoreactive somata is consistent with our results on the diminished cell density of nNOS- and PV-immunopositive interneurons and indicates a loss of GABAergic cells rather than the down-regulation of nNOS and PV expression in interneurons of ErbB4−/− mice.
DISCUSSION
Here we report for the first time the precise cellular localization of ErbB4 in distinct classes of interneurons of wildtype mice, and the subtype-specific regional reduction of interneurons in adult ErbB4−/− mice. These data are crucial for a better understanding of modulatory effects of NRGs on the hippocampal network and for the generation of adequate models. Since ErbB4−/− mice are increasingly used as a tool to elucidate the significance of the NRG-ErbB4 signaling for hippocampal functions, the characterization of differences between wildtype and ErbB4−/− mice is important for the interpretation of electrophysiological and neurochemical experiments, because it may allow to differentiate effects that are attributable to the acute absence of ErbB4 on a given cell from effects being due to long-term adaptive changes of entire neural circuits that include this cell.
We report that CCK-immunoreactive basket cells express ErbB4 in the hippocampal formation (54% of total). We also found that 42% of PV cells, 1% of SOM cells, and 39% of nNOS cells express ErbB4 in adult C57BL6 mice, whereas a previous study reported coexpression of 82%, 12%, and 100%, respectively, in Wistar rats (Yau et al., 2003). In addition to possible species differences between mice and rats several other reasons may account for the difference. While adult animals were used in both studies, the age of the rats is not known. However, the major difference is the sample size obtained by Yau et al. who derived their data from a much smaller number of cells. Finally, the substantially higher amount of coexpression in hippocampal SOM cells (Yau et al. 12% vs. 1% here) might be partially attributable to the fact that both the ErbB4 and the SOM antibodies in the earlier study were rabbit IgGs that were sequentially visualized by tyramide signal amplification and conventional immunofluorescence labeling, respectively. While generally valid, this approach can result in false positive identification of coexpression under adverse circumstances and requires stringent controls.
The other major finding we report is the reduction of inhibitory interneurons in adult ErbB4−/− mice. We show that the reduction of interneurons is not general, but rather is region-specific and preferentially affects populations that coexpress ErbB4 in a high percentage of cells. This result is in line with recent data from young (P20) ErbB4−/− mice (Flames et al., 2004); it is also consistent with high levels of ErbB4 expression on migrating and developing GABAergic cells (Yau et al., 2003; Ghashghaei et al., 2006) and the role of NRG1-ErbB4 signaling in promoting cell proliferation, migration, and survival (Erlich et al., 2001; Flames et al., 2004; Liu et al., 2005; Di Segni et al., 2006). As we will discuss below, the selective reduction of certain subtypes of interneurons will likely result in specific deficits of signal processing in the adult hippocampus that are different from a general impairment of hippocampal network inhibition (see Fig.12).
Figure 12. Localization and function of NRGs and ErbB4 within the hippocampal circuitry.
(A) Simplified diagram of afferent/efferent connections of, and internal microcircuits within, area CA1. Only the most frequent cell types are shown for clarity. The perisomatic region of pyramidal cells (PCs; black and grey) is innervated by basket terminals from PV (red) and CCK (orange) cells. Both PV and CCK cells receive excitatory feedforward input from CA3 PCs via Schaffer collaterals (sc; light blue), and feedback input from CA1 PCs (black lines). CCK cells are also targeted by cholinergic axons from the medial septum (ACh; light green), and CCK is expressed also in sc-associated interneurons that innervate apical dendrites of PCs. PV cells are strongly interconnected and additionally electrically coupled via dendritic gap junctions, and they innervate most PCs within their vicinity; this arrangement synchronizes the entire network during fast oscillations. CCK cells, in contrast, innervate fewer PC targets and thus coordinate activity within smaller neuronal ensembles during theta band activity. Reciprocal connections between PV and CCK cells can shift the activity between these two complementary pathways. Either basket cell type shows somatodendritic expression of ErbB4 (green dots), which can presumably be activated by NRG from all three afferent fiber systems (sc, ACh, PC recurrents; dashed axons). The left side shows SOM-positive/ErbB4-negative O-LM cells (brown) that provide feedback inhibition to apical dendrites of CA1 PCs. O-LM cells also target nNOS/ErbB4-coexpressing cells (pink) in slm. Both nNOS cells and dendrites of PCs are innervated by glutamatergic lateral perforant path fibers (lpp; blue) from the entorhinal cortex that are NRG-positive. This connectivity enables nNOS interneurons, depending on the present level of activity in CA1, to regulate the corticohippocampal excitation of PCs via shunting currents in slm PC dendrites. nNOS cells presumably also innervate other interneurons (IN; grey); whether IN express ErbB4 is not known. Therefore, NRG-ErbB4 signaling is positioned to modulate many major microcircuits within CA1, with the notable exception of the PC-SOM feedback pathway. (B) Comparison of CA1 microcircuits in wildtype and ErbB4−/− mice. nNOS and PV cells provide feedforward inhibition to pyramidal cells from EC and CA3 driven by the excitatory lpp and sc pathways, respectively, that also directly innervate PCs (wildtype, left side). The severe reduction of nNOS and PV cells in ErbB4−/− mice (right side) increases the excitation-to-inhibition ratio in PCs, leading to increased firing of PCs and simultaneously to a decrease in oscillatory power due to decreased temporal coordination of individual neuron activity, as indicated by the traces below PCs. In addition, the overall reduction of inhibition increases the likelihood to generate epileptiform activity. Abbreviations: see Figure 2; acetylcholine (ACh), entorhinal cortex (EC), lateral perforant path (lpp), Schaffer collateral (sc).
ErbB4 expression in basket and axo-axonic cells
CCK-positive cells show the highest percentage of ErbB4 coexpression (54%) among the four types of interneurons in the present study, and 42% of PV-immunoreactive cells are positive for ErbB4. CCK or PV are expressed in interneurons that innervate intermediate and distal dendrites, but in contrast to other markers also in interneurons that provide powerful inhibition to perisomatic regions of their target cells. Recent studies suggest that PV- and CCK-positive cells are engaged in parallel pathways that provide inhibition to perisomatic targets in a complementary way: Fast-spiking PV cells heavily innervate each other, are electrically coupled by gap-junctions (Baude et al., 2007), and, due to their innervation of more than 1500 pyramidal neurons each (Sik et al., 1995), are thought to synchronize the activity within the entire network during hippocampal oscillations, and especially during gamma oscillations (Klausberger et al., 2005). In contrast, CCK-expressing basket cells integrate synaptic stimuli over a much longer period and fire at lower frequencies than PV-positive cells (Glickfeld and Scanziani, 2006). They have been suggested to coordinate the firing of smaller neuronal assemblies within the hippocampal network during theta oscillations (Klausberger et al., 2005). Exogenously applied CCK increases inhibition from PV-positive cells onto pyramidal cells, while reducing GABA release from CCK-positive basket cells via retrograde activation of the presynaptic CB1 receptor (Foldy et al., 2007), indicating again the complementary organization of perisomatic inhibition. Importantly, our results suggest that NRG1-ErbB4 signaling has the potential to strongly influence the population-firing pattern of hippocampal neurons through both pathways, with likely different effects on network function.
Reduction of perisomatic inhibition in ErbB4−/− mice
CCK cells show the highest percentage of ErbB4 coexpression of all populations of hippocampal interneurons, but disruption of the NRG1-ErbB4 signaling pathway surprisingly has only minor effects on their number, in contrast to PV-immunoreactive cells that are substantially reduced throughout the entire hippocampus. This result indicates that the relation between ErbB4 expression in adult wildtype animals and a reduction of interneurons in ErbB4 knockouts could be more complicated than previously thought (Flames et al., 2004). Cortical interneurons that are generated in the medial ganglionic eminence during development, such as PV cells and the majority of SOM-positive cells, express high levels of ErbB4 during migration and are chemoattracted by NRG1 (Flames et al., 2004). Unfortunately, little is known about the origin of CCK-positive cells compared to other populations of interneurons such as SOM, PV or nNOS-expressing cells (Xu et al., 2004; Flames and Marin, 2005). A recent study by Rakic and coworkers indicates, however, that CCK cells derive from the caudal ganglionic eminence (Morozov et al., 2009), and this finding is supported by indirect evidence (Lopez-Bendito et al., 2004; Vitalis et al., 2007). CCK cells may therefore depend less on developmental ErbB4 signaling than was shown for cells that arise from the medial ganglionic eminence.
Taken together, the lack of major changes in the number and distribution of CCK-positive cells in ErbB4 knockouts suggests that are unlikely to substantially contribute to the increased generation of epileptiform activity in ErbB4−/− mice (A.B. and A. Fisahn, unpublished data). In contrast, the significant reduction of fast-spiking hippocampal PV-positive cells is entirely consistent with both an increased susceptibility to epileptiform activity, due to diminished perisomatic inhibition, and with a significant reduction of the power of kainate-induced gamma-oscillations in ErbB4 knockouts, due to reduced temporal coordination of pyramidal cell activity ((Fisahn et al., 2009); see also Fig.12B). The reduction of PV-immunoreactive cells is also reminiscent of similar findings in cortical areas from post-mortem studies of schizophrenic patients (Zhang et al., 2002; Lewis et al., 2005; Torrey et al., 2005). While ErbB4−/− mice, like all other single knockouts, are most likely not a suitable model for schizophrenia, these results support the notion that changes or impairments of NRG-ErbB4 signaling during development may have biological relevance for some aspects of the etiology underlying schizophrenia.
nNOS-positive cells: expression of ErbB4 and reduction in ErbB4−/− mice
We found that 39% of nNOS-positive cells in DG and CA1-3 express ErbB4, suggesting that NRGs can exert a considerable effect on these interneurons. Despite recent progress in characterizing nNOS-immunoreactive interneurons (Jinno and Kosaka, 2002) the amount of data on the source of their synaptic input and their postsynaptic targets continues to be limited. These interneurons likely provide feedforward-inhibition primarily onto the apical dendrites of principal cells in DG and CA3 (Seress et al., 2005). Other nNOS cells, defined by coexpression of neuropeptide Y and the α1 subunit of the GABAA receptor, were recently identified as slow-spiking interneurons that provide feedback inhibition to pyramidal cells (Fuentealba et al., 2008). Indirect evidence, such as small cell size and localization in slm and sr, suggests that some of the nNOS cells in CA1-3 may belong to the group of interneuron-selective type-2 cells (Freund and Buzsaki, 1996; Freund and Gulyas, 1997; Somogyi and Klausberger, 2005). Taken together, ErbB4 expression in a substantial percentage of nNOS cells opens the possibility that NRGs could modulate both feedforward and feedback inhibitory loops that converge onto hippocampal pyramidal neurons. However, due to the limited amount of available data, it is currently impossible to hypothesize what impact the reduction of 27% of nNOS cells might have on network properties in ErbB4−/− mice.
SOM-positive interneurons: an inhibitory pathway independent of NRG-signaling?
O-LM cells provide local feedback-inhibition that is driven by glutamatergic input from pyramidal cells, and they target also interneurons in slm (Katona et al., 1999). SOM-expressing O-LM cells are thought to exhibit inhibitory control over lateral perforant path afferents (Yanovsky et al., 1997), and a recent study suggests that O-LM cells may be able to shift the balance between feedforward and feedback inhibition of pyramidal cells (Elfant et al., 2008). The lack of substantial ErbB4-expression in both SOM cells and pyramidal cells indicates that the bisynaptic O-LM-pyramidal neuron feedback loop might be the only pathway within the hippocampal network that could work independently from any direct NRG effect. Despite the fact that SOM cells are generated in the medial ganglionic eminence and presumably express substantial levels of ErbB4 during migration and maturation, their number is not affected in adult hippocampus of ErbB4−/− mice, indicating again that embryonal ErbB4 expression does not sufficiently predict whether or not interneurons in the adult hippocampus will be affected by the disruption of NRG-ErbB4 signaling.
Functional implications of ErbB4 distribution in hippocampal interneurons
Our analysis shows that several functionally distinct populations of hippocampal interneurons express ErbB4, with the notable exception of SOM-positive cells. Figure 12 summarizes these data, showing the location of ErbB4 within local circuits, and the afferent fiber systems that provide external drive to CA1. Furthermore, Figure 12 includes likely sources of NRGs, namely the entorhinal cortex via the lateral perforant path (Pinkas-Kramarski et al., 1994), CA3 Schaffer collaterals (Eilam et al., 1998), and cholinergic axons from the medial septum (Corfas et al., 1995). For increased clarity we focus on CA1 circuitry; however, it is worth mentioning that NRG-expression has also been reported in granule cells of DG (Eilam et al., 1998), in addition to release from the medial perforant path (Roysommuti et al., 2003). It should also be noted that NRG2 and NRG3 mRNA levels are higher in the brain as compared to NRG1, both postnatally (Anton et al., 2004) and in adulthood (Longart et al., 2004), and NRG2 expression in vitro has been reported not in axons but in dendrites (Longart et al., 2004). Figure 12 is therefore meant to primarily summarize our present results rather than provide a comprehensive overview of all possible NRG-ErbB signaling pathways. Of importance for the interpretation of functional roles of ErbB4, we report only partial overlay with any of the four interneuron markers. This result is not surprising because none of these markers is sufficient to delineate a homogeneous population of interneurons. In fact, using different parameters such as firing properties, morphology, connectivity, and coexpression of several marker proteins, dozens of subpopulations of interneurons have been suggested (Markram et al., 2004; Somogyi and Klausberger, 2005; Ascoli et al., 2008).
The high degree of coexpression in three of the four populations of interneurons suggests that NRGs could modulate different microcircuits within the hippocampal network (Fig.12A). Since the release of NRGs and the successful activation of ErbB receptors seem to depend on synaptic activity, it seems likely that only a subset of these microcircuits is activated at any given time, depending on the actual functional state of the network. For example, we have recently shown that NRG1 dramatically increases the power of gamma oscillations in CA3 of acute hippocampal slice preparations (Fisahn et al., 2009). Since network synchronization is crucial in order to generate and maintain high frequency oscillations, PV-positive basket cells have been suggested to play a critical role (Bartos et al., 2007; Fuchs et al., 2007), and we show that almost half of PV-positive cells express ErbB4. These results suggest that NRG1-ErbB4 signaling during gamma oscillations increases the recruitment of PV-positive fast-firing basket cells that synchronize CA3 pyramidal cells. Moreover, PV-positive cells are reduced in ErbB4−/− mice, and we have reported that the power of kainate-induced gamma oscillations is reduced by 60% in ErbB4−/− mice (Fisahn et al., 2009). It is worth noticing, however, that additional types of interneurons, such as bistratified and trilaminar cells, are also known to contribute to gamma oscillations (Gloveli et al., 2005), suggesting that different local circuits might coexist that could modulate the generation of gamma oscillations under specific circumstances (Middleton et al., 2008). A similar model could be useful to explain our finding that NRG1 selectively increases the power of kainate-induced, but not carbachol-induced, hippocampal gamma oscillations (Fisahn et al., 2009).
We also provide a simplified model of possible functional differences between wildtype and ErbB4−/− mice (Fig.12B). The reduction of PV-positive cells throughout the entire hippocampus will likely result in hyperactivity of principal cells. SOM-positive O-LM cells provide an inhibitory feedback loop onto CA1 pyramidal cells that is thought to prevent hyperactivity within the circuit. The increased occurrence of epileptiform activity in sections from ErbB4−/− hippocampus suggests, however, that this feedback loop is insufficient to overcome the reduced inhibition provided by PV-positive and nNOS-positive interneurons. It therefore seems plausible that Schaffer collaterals could deliver increased excitatory drive onto CA1 pyramidal cells and Schaffer collateral-associated interneurons, such as CCK- and PV-positive cells. The decrease in PV-positive basket cells suggests, however, that the excitation-to-inhibition ratio will be increased and that the coordination of pyramidal cell firing will be impaired, resulting in temporally disorganized activity and, consequently, reduced oscillatory power in the gamma frequency band (see above). On the other hand, the reduction of nNOS-positive interneurons that receive excitatory input from the entorhinal cortex via the lateral perforant path is likely to weaken signal processing in the hippocampus and reduce the coordination between both brain areas, resulting in functional impairment of spatial orientation and memory tasks.
In conclusion, our results suggest that NRGs can affect several inhibitory microcircuits in the hippocampus via ErbB4. It is known that the expression and release of NRGs are regulated by neuronal activity, and recent reports indicate that the effects of NRG1 on network oscillation and synaptic plasticity are also activity-dependent. It is, however, as of yet unclear how NRG-ErbB4 signaling modulates the activity of individual cells and whether or not these cells will react in a uniform manner or, again, dependent on their subtype and present state of activity. Importantly, our results showing the interneuron type-specific reduction of GABAergic cells in ErbB4−/− mice suggest that any differences between genotypes in response to experimental challenges should be interpreted with caution because they cannot exclusively be attributed to the acute lack of ErbB4 on individual cells.
ACKNOWLEDGEMENTS
Confocal microscopy imaging was performed at the Microscopy & Imaging Core of NICHD with the assistance of Dr. Vincent Schram and Chip Dye. The authors thank Dr. Detlef Vullhorst for generating the ErbB4 antibody, Dr. Irina Karavanova and Daniel Abebe for animal mating and genotyping, Constantin P. Firme for valuable technical assistance, and Dr. Leqin Yan for performing an exploratory screen on ErbB4 expression in interneurons. We also like to thank Dr. Chris McBain (Laboratory of Cellular and Synaptic Neurophysiology, NICHD) for critical reading of the manuscript. This research was supported by the Intramural Research Program of the National Institutes of Health, NICHD.
REFERENCES
- Aika Y, Ren JQ, Kosaka K, Kosaka T. Quantitative analysis of GABA-like-immunoreactive and parvalbumin-containing neurons in the CA1 region of the rat hippocampus using a stereological method, the disector. Exp Brain Res. 1994;99:267–276. doi: 10.1007/BF00239593. [DOI] [PubMed] [Google Scholar]
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Baude A, Bleasdale C, Dalezios Y, Somogyi P, Klausberger T. Immunoreactivity for the GABAA receptor alpha1 subunit, somatostatin and Connexin36 distinguishes axoaxonic, basket, and bistratified interneurons of the rat hippocampus. Cereb Cortex. 2007;17:2094–2107. doi: 10.1093/cercor/bhl117. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, Novak TJ, Stefansson K, Gurney ME, Andresson T. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, Radyushkin K, Goebbels S, Fischer TM, Franklin RJ, Lai C, Ehrenreich H, Birchmeier C, Schwab MH, Nave KA. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Chen MS, Bermingham-McDonogh O, Danehy FT, Jr., Nolan C, Scherer SS, Lucas J, Gwynne D, Marchionni MA. Expression of multiple neuregulin transcripts in postnatal rat brains. J Comp Neurol. 1994;349:389–400. doi: 10.1002/cne.903490306. [DOI] [PubMed] [Google Scholar]
- Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, Leitner O, Ratzkin BJ, Bacus SS, Yarden Y. An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem. 1996;271:7620–7629. [PubMed] [Google Scholar]
- Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- Di Segni A, Farin K, Pinkas-Kramarski R. ErbB4 activation inhibits MPP+-induced cell death in PC12-ErbB4 cells: involvement of PI3K and Erk signaling. J Mol Neurosci. 2006;29:257–267. doi: 10.1385/JMN:29:3:257. [DOI] [PubMed] [Google Scholar]
- Eilam R, Pinkas-Kramarski R, Ratzkin BJ, Segal M, Yarden Y. Activity-dependent regulation of Neu differentiation factor/neuregulin expression in rat brain. Proc Natl Acad Sci U S A. 1998;95:1888–1893. doi: 10.1073/pnas.95.4.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfant D, Pal BZ, Emptage N, Capogna M. Specific inhibitory synapses shift the balance from feedforward to feedback inhibition of hippocampal CA1 pyramidal cells. Eur J Neurosci. 2008;27:104–113. doi: 10.1111/j.1460-9568.2007.06001.x. [DOI] [PubMed] [Google Scholar]
- Erlich S, Goldshmit Y, Lupowitz Z, Pinkas-Kramarski R. ErbB-4 activation inhibits apoptosis in PC12 cells. Neuroscience. 2001;107:353–362. doi: 10.1016/s0306-4522(01)00350-5. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 Modulates Hippocampal Gamma Oscillations: Implications for Schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Flames N, Marin O. Developmental mechanisms underlying the generation of cortical interneuron diversity. Neuron. 2005;46:377–381. doi: 10.1016/j.neuron.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Foldy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10:1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Kornblum HI. Developmental profile of ErbB receptors in murine central nervous system: implications for functional interactions. J Neurosci Res. 2005;79:584–597. doi: 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyas AI. Inhibitory control of GABAergic interneurons in the hippocampus. Can J Physiol Pharmacol. 1997;75:479–487. [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Begum R, Capogna M, Jinno S, Marton LF, Csicsvari J, Thomson A, Somogyi P, Klausberger T. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Weber J, Pevny L, Schmid R, Schwab MH, Lloyd KC, Eisenstat DD, Lai C, Anton ES. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci U S A. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562:131–147. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, Thomson PA, Porteous DJ, Cunningham-Owens DG, Johnstone EC, Lawrie SM. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci. 2006;9:1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Jinno S, Aika Y, Fukuda T, Kosaka T. Quantitative analysis of GABAergic neurons in the mouse hippocampus, with optical disector using confocal laser scanning microscope. Brain Res. 1998;814:55–70. doi: 10.1016/s0006-8993(98)01075-0. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Patterns of expression of calcium binding proteins and neuronal nitric oxide synthase in different populations of hippocampal GABAergic neurons in mice. J Comp Neurol. 2002;449:1–25. doi: 10.1002/cne.10251. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Heterogeneous expression of the cholecystokinin-like immunoreactivity in the mouse hippocampus, with special reference to the dorsoventral difference. Neuroscience. 2003;122:869–884. doi: 10.1016/j.neuroscience.2003.08.039. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Patterns of colocalization of neuronal nitric oxide synthase and somatostatin-like immunoreactivity in the mouse hippocampus: quantitative analysis with optical disector. Neuroscience. 2004;124:797–808. doi: 10.1016/j.neuroscience.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Cellular architecture of the mouse hippocampus: a quantitative aspect of chemically defined GABAergic neurons with stereology. Neurosci Res. 2006;56:229–245. doi: 10.1016/j.neures.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Katona I, Acsady L, Freund TF. Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience. 1999;88:37–55. doi: 10.1016/s0306-4522(98)00302-9. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O’Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum HI, Yanni DS, Easterday MC, Seroogy KB. Expression of the EGF receptor family members ErbB2, ErbB3, and ErbB4 in germinal zones of the developing brain and in neurosphere cultures containing CNS stem cells. Dev Neurosci. 2000;22:16–24. doi: 10.1159/000017423. [DOI] [PubMed] [Google Scholar]
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Paredes D, Gonzalez CM, Neddens J, Hernandez L, Vullhorst D, Buonanno A. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci U S A. 2008;105:15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T. Deciphering the disease process of schizophrenia: the contribution of cortical gaba neurons. Int Rev Neurobiol. 2007;78:109–131. doi: 10.1016/S0074-7742(06)78004-7. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The Neuregulin-1 Receptor ErbB4 Controls Glutamatergic Synapse Maturation and Plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15:1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ford BD, Mann MA, Fischbach GD. Neuregulin-1 increases the proliferation of neuronal progenitors from embryonic neural stem cells. Dev Biol. 2005;283:437–445. doi: 10.1016/j.ydbio.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Loeb JA, Hmadcha A, Fischbach GD, Land SJ, Zakarian VL. Neuregulin expression at neuromuscular synapses is modulated by synaptic activity and neurotrophic factors. J Neurosci. 2002;22:2206–2214. doi: 10.1523/JNEUROSCI.22-06-02206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longart M, Chatani-Hinze M, Gonzalez CM, Vullhorst D, Buonanno A. Regulation of ErbB-4 endocytosis by neuregulin in GABAergic hippocampal interneurons. Brain Res Bull. 2007;73:210–219. doi: 10.1016/j.brainresbull.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longart M, Liu Y, Karavanova I, Buonanno A. Neuregulin-2 is developmentally regulated and targeted to dendrites of central neurons. J Comp Neurol. 2004;472:156–172. doi: 10.1002/cne.20016. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sturgess K, Erdelyi F, Szabo G, Molnar Z, Paulsen O. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb Cortex. 2004;14:1122–1133. doi: 10.1093/cercor/bhh072. [DOI] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Matyas F, Freund TF, Gulyas AI. Immunocytochemically defined interneuron populations in the hippocampus of mouse strains used in transgenic technology. Hippocampus. 2004;14:460–481. doi: 10.1002/hipo.10191. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Lacoste B, Yu WF, Srivastava LK, Quirion R. Developmental profile of neuregulin receptor ErbB4 in postnatal rat cerebral cortex and hippocampus. Neuroscience. 2007;148:126–139. doi: 10.1016/j.neuroscience.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Middleton S, Jalics J, Kispersky T, Lebeau FE, Roopun AK, Kopell NJ, Whittington MA, Cunningham MO. NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc Natl Acad Sci U S A. 2008;105:18572–18577. doi: 10.1073/pnas.0809302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov YM, Torii M, Rakic P. Origin, early commitment, migratory routes, and destination of cannabinoid type 1 receptor-containing interneurons. Cereb Cortex. 2009;19(Suppl 1):i78–89. doi: 10.1093/cercor/bhp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, Spurlock G, Kirov G, Buckland P, Waddington JL, Gill M, Corvin AP, Owen MJ, O’Donovan MC. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Itoh K, Miyakawa Y, Kishida H, Hashikawa T. Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J Neurochem. 2004;91:176–188. doi: 10.1111/j.1471-4159.2004.02719.x. [DOI] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Eilam R, Spiegler O, Lavi S, Liu N, Chang D, Wen D, Schwartz M, Yarden Y. Brain neurons and glial cells express Neu differentiation factor/heregulin: a survival factor for astrocytes. Proc Natl Acad Sci U S A. 1994;91:9387–9391. doi: 10.1073/pnas.91.20.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher GM, Beggs S, Woo RS, Mei L, Salter MW. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. Neuroreport. 2008;19:139–143. doi: 10.1097/WNR.0b013e3282f3da10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, Benoit-Marand M, Chen C, Moore H, O’Donnell P, Brunner D, Corfas G. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci U S A. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Carroll SL, Wyss JM. Neuregulin-1beta modulates in vivo entorhinal-hippocampal synaptic transmission in adult rats. Neuroscience. 2003;121:779–785. doi: 10.1016/s0306-4522(03)00503-7. [DOI] [PubMed] [Google Scholar]
- Scotti AL, Bollag O, Kalt G, Nitsch C. Loss of perikaryal parvalbumin immunoreactivity from surviving GABAergic neurons in the CA1 field of epileptic gerbils. Hippocampus. 1997;7:524–535. doi: 10.1002/(SICI)1098-1063(1997)7:5<524::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Seress L, Abraham H, Hajnal A, Lin H, Totterdell S. NOS-positive local circuit neurons are exclusively axo-dendritic cells both in the neo- and archi-cortex of the rat brain. Brain Res. 2005;1056:183–190. doi: 10.1016/j.brainres.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Seress L, Abraham H, Lin H, Totterdell S. Nitric oxide-containing pyramidal neurons of the subiculum innervate the CA1 area. Exp Brain Res. 2002;147:38–44. doi: 10.1007/s00221-002-1242-2. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsaki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singec I, Knoth R, Ditter M, Volk B, Frotscher M. Neurogranin is expressed by principal cells but not interneurons in the rodent and monkey neocortex and hippocampus. J Comp Neurol. 2004;479:30–42. doi: 10.1002/cne.20302. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa J, Omori K, Okugawa G, Fujiseki Y, Heizmann CW, Inagaki C. Long-lasting c-fos and NGF mRNA expressions and loss of perikaryal parvalbumin immunoreactivity in the development of epileptogenesis after ethacrynic acid-induced seizure. Brain Res. 1999;834:89–102. doi: 10.1016/s0006-8993(99)01554-1. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci U S A. 2003;100:8281–8286. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. Eur J Neurosci. 2007;26:331–344. doi: 10.1111/j.1460-9568.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, Buonanno A. The neuregulin receptor ErbB4 is selectively expressed in GABAergic interneurons in the adult hippocampus. submitted. submitted.
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky Y, Sergeeva OA, Freund TF, Haas HL. Activation of interneurons at the stratum oriens/alveus border suppresses excitatory transmission to apical dendrites in the CA1 area of the mouse hippocampus. Neuroscience. 1997;77:87–96. doi: 10.1016/s0306-4522(96)00461-7. [DOI] [PubMed] [Google Scholar]
- Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sun J, Reynolds GP. A selective reduction in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia patients. Chin Med J (Engl) 2002;115:819–823. [PubMed] [Google Scholar]