Abstract
We report a simple fluidic system that can purify and concentrate diagnostic biomarkers through the capture and triggered release of stimuli-responsive polymer-antibody conjugates at porous membranes that are grafted with the same stimuli-responsive polymer. This technique is applied here to the capture and detection of a model streptavidin antigen and subsequently to clinical ranges of the malaria antigen Plasmodium falciparum histidine-rich protein 2 (PfHRP2) from spiked human plasma. The semi-telechelic end carboxyl groups of poly(N-isopropylacrylamide) (pNIPAAm) synthesized by reversible addition fragmentation chain transfer (RAFT) polymerization were modified with tetrafluorophenol to yield amine-reactive ester groups for conjugation to amine groups of anti-streptavidin and anti-PfHRP2 antibodies. Stimuli-responsive membranes were constructed from 1.2 μm pore-size, hydroxylated, nylon 6,6 filters (Loprodyne, from Pall Corporation). The surface hydroxyl groups on the filters were conjugated to a 2-ethylsulfanylthiocarbonylsulfanyl-2-methyl propionic acid (EMP) RAFT chain transfer agent and the surface-grafted pNIPAAm was obtained by subsequent polymerization. The number average molecular weight (Mn) and polydispersity indices (PDI) of the surface grafts were characterized and membranes with either 4100 and 8400 Mn pNIPAAm grafts showed greater than 80% anti-streptavidin capture efficiency. The 8400 molecular weight-graft membrane showed the highest release efficiency, and it was demonstrated that at 0.2 nM starting concentration the streptavidin could be concentrated approximately 40 fold by releasing into a small 50 μl volume. This concentrator system was applied to the capture and concentration of the Plasmodium falciparum HRP2 antigen and results showed that the PfHRP2 antigen could be processed and detected at clinically-relevant concentrations of this malaria biomarker.
Keywords: Reversible addition fragmentation chain transfer polymerization (RAFT), microfluidic, immunoassay, malaria, poly(N-isopropylacrylamide) (pNIPAAm)
INTRODUCTION
Biomarker testing can potentially help in the identification and epidemiological tracking of many diseases, as well as in their treatment monitoring. Achieving clinically-relevant immunoassay limits of detection for biomarkers has typically required more time-consuming methods and specialized equipment found in centralized and higher-resource laboratory facilities. Rapid diagnostic tests (RDTs) based on lateral flow, flow-through, or dipstick approaches have greatly aided diagnosis in low-resource settings but have detection sensitivities that can limit their applications. As an example, tests for malaria are suitable for detecting higher levels of antigen released by parasitized red blood cells, but are not adequate for detection of low levels of parasitemia common with patients from non-endemic regions with clinical disease(1–3) or in cases of subpatent parasitemia levels that can serve as transmission reservoirs (4, 5). Additionally, they are limited in use for speciation discrimination, which can be necessary for correct treatment choice and monitoring (6, 7). By improving detection limits and incorporating multiplex assays, rapid diagnostic technologies can move to the forefront of providing accurate diagnosis across the spectrum of medical needs, rather than simply providing faster results for conventional methods.
Microfluidic assays have features desirable in a rapid diagnostic test including smaller input samples, shorter diffusion and reaction times, lower reagent consumption, and containment of biohazard waste products (8–10). However, simply reformatting current immunoassays to fit in a microfluidic device setting may prove to be insufficient for overcoming intrinsic sensitivity and assay limitations. While microfluidic volumes reduce diffusion distance, capture at the channel surface is confounded by the dominance of the advective laminar flow regime (11). Absolute quantities of antigen fall as the sample size is reduced and preconditioning of a sample can lead to further dilution. There is still a need for simple, integratable reagent systems that can both purify and concentrate diagnostic targets in microfluidic or flow-through diagnostic technologies.
Our group has been investigating the use of “smart” polymer conjugates of biomolecules to address this need for rapid and efficient biomarker processing in microfluidic devices. In the past there have been two primary approaches that either utilized immunoconjugates that can be phase separated in solution and captured on surfaces, or that utilized stimuli-responsive surfaces for the capture of biomolecules or cells (12–20). More recently we have been investigating the joint application of the stimuli-responsive conjugates with stimuli-responsive microfluidic channel surfaces (16) Here we have grafted polymer brushes using the reversible-addition fragmentation chain transfer (RAFT) method, which allows control of properties through control of graft molecular weight and end-groups (21–23). Grafted porous membranes incorporating stimuli-sensitive polymers have been previously used in the separations field to perform separations on the basis of porosity, hydrophobicity, or surface access (24–33). These stimuli-responsive grafted membranes are particularly appealing when used jointly with stimuli-responsive antibody-polymer conjugates where the diffusion of the antigen and antibody in plasma and serum becomes the rate-limiting step (34–36).
Temperature-responsive micro- and nanoparticles have been previously isolated in microfluidic devices, but isolation was performed at microfluidic channel walls (16, 37–39). This requires migration of the particles away from the laminar flow stream. We present results showing that stimuli-responsive IgG and IgM antibody-pNIPAAm conjugates are efficiently captured and released on pNIPAAm-grafted nylon 6,6 membranes inserted into prefabricated microfluidic cards. The capture efficiency and sharpness of the immunocomplex sandwich release after capture were shown to be strongly enhanced by the surface-polymerized pNIPAAm on the membrane surface during constant microfluidic flow. This system was also shown to be applicable to processing of malaria antigen from spiked human plasma samples, resulting in detection limits comparable to ELISA for the malaria Plasmodium falciparum HRP2 antigen.
EXPERIMENTAL SECTION
Materials
2,2′-Azoisobutyronitirile (AIBN) was recrystallized from methanol. N-isopropylacrylamide (NIPAAm, Aldrich, 97%) was recrystallized twice from hexane and dried under vacuum prior to use. Anhydrous dimethylformamide (DMF), anhydrous dimethylsulfoxide (DMSO), dichloromethane, anhydrous ethyl ether, 2,3,5,6-tetrafluorophenol (TFP), diisopropylcarbodiimide (DIC), and dimethylaminopyridine (DMAP), were used as received. Prepared 10 mM phosphate-buffered saline (PBS) and phosphate buffered saline with 0.05% Tween-20 (PBS-T) dry reagents were purchased from Sigma and dissolved in distilled water. 3,3′,5,5′ tetramethylbenzidine, TMB, substrate was purchased from KPL. Monoclonal mouse anti-Plasmodium falciparum histidine-rich protein 2 IgM antibody, monoclonal mouse anti-Plasmodium falciparum HRP2 peroxidase-conjugated detection IgG antibody, and recombinant Plasmodium falciparum histidine-rich protein 2 (pfHRP2) antigen were purchased from Immunology Consultants Laboratory. Polyclonal IgG antibodies against streptavidin were purchased from Abcam. Loprodyne hydroxylated Nylon 6,6 membranes, 1.2 μm pore size, were from Pall. The chain transfer agent (CTA) S-ethyl-S′-(α,α′-dimethyl-α″-acetic acid)trithiocarbonate, EMP, was synthesized previously as described(40). Dialysis membranes were purchased from Spectrum laboratories. Prior to use, pooled human plasma (sodium EDTA, Valley Biomedical) was filtered using Whatman GD/X filters, to remove precipitate formed after thawing from the frozen aliquot, and stored at 4 ºC for up to 1 week.
Preparation of pNIPAAm-bound membranes
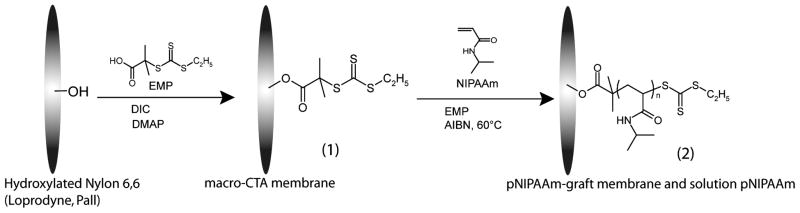
Membranes with graft pNIPAAm were prepared as shown in Scheme 1. The CTA 2-ethylsulfanylthiocarbonylsulfanyl-2-methyl propionic acid (EMP) (100mM), DIC (100mM), and DMAP (10mM) were combined in 10 ml anhydrous DMF and added to vacuum-dried Loprodyne membrane. The reaction was stirred for 48 hours at room temperature. Membranes were washed extensively in acetone and ethanol, dried by vacuum at room temperature and then stored under ambient conditions. Polymerization was mediated by chain transfer agent using the reversible addition-fragmentation chain transfer (RAFT) technique(41). Membrane with and without bound CTA were immersed in a solution polymerization vessel containing the following in DMF: EMP chain transfer agent (13.2 or 40 mmol,) N-isopropylacrylamide monomer (0.2 g/ml) and (2.7 or 8 mmol) AIBN. Following nitrogen purging, polymerization proceeded at 60ºC for 18–24 hours. Solution polymer was retained and analyzed and membranes were washed extensively with acetone and ethanol and soaked 48 hours at 4°C in several changes of distilled water to remove non-covalently adsorbed or entangled polymer.
Scheme 1.
Synthesis of pNIPAAm-polymerized membranes. Membranes were prepared by first coupling the EMP chain transfer agent via esterification to produce a macro-CTA membrane (1). Second, the membrane was immersed in a RAFT-mediated polymerization of NIPAAm with additional EMP and AIBN to yield solution homopolymer and covalently-linked surface polymer (2)
Cleavage of PNIPAAm from surface of membranes
1N sodium hydroxide solution (2 ml per cm2 of membrane) was added to membranes derived from polymerization reactions, and were heated to 70ºC for 1 hour. Solutions were neutralized with 1N hydrochloric acid, and each membrane was washed with 2 ml of distilled water. All solutions were then combined and dialyzed against several changes of distilled water for 72 hours, lyophilized, and stored dry until analysis. Polymers were dissolved in DMF and treated with immobilized TCEP for 1 hour prior to characterization by gel-permeation chromatography.
Synthesis of semi-telechelic (pNIPAAm) and pNIPAAm-TFP ester
The RAFT polymerization of N-isopropylacrylamide monomer included the following: 13.2 mmol EMP CTA, 0.2 g/ml NIPAAm, and 2.7 mmol AIBN. After nitrogen purging, the polymerization proceeded at 60ºC for 18 hours. The resulting polymer was precipitated from DMF twice in cold ethyl ether, filtered, dried under vacuum, and analyzed by GPC. PNIPAAm of number-average molecular weight (Mn) equal to 14,500, and polydispersity index (weight average molecular weight/number average molecular weight, or Mw/Mn) equal to 1.15 was used for preparation of tetrafluorophenyl (TFP) ester. The end carboxyl group was reacted with tetrafluorophenol (Scheme 2). The reagents PNIPAAm (1 g, 13.8 mM), DIC (42 mg, 67 mM), tetrafluorophenol (56 mg, 67 mM), and DMAP (4.1 mg, 6.7 mM) were combined in 5 ml anhydrous dichloromethane at room temperature for 24 hours. Activated polymer was then precipitated with cold anhydrous ethyl ether. Precipitates were immediately dried by vacuum and stored dry at −20ºC.
Scheme 2.
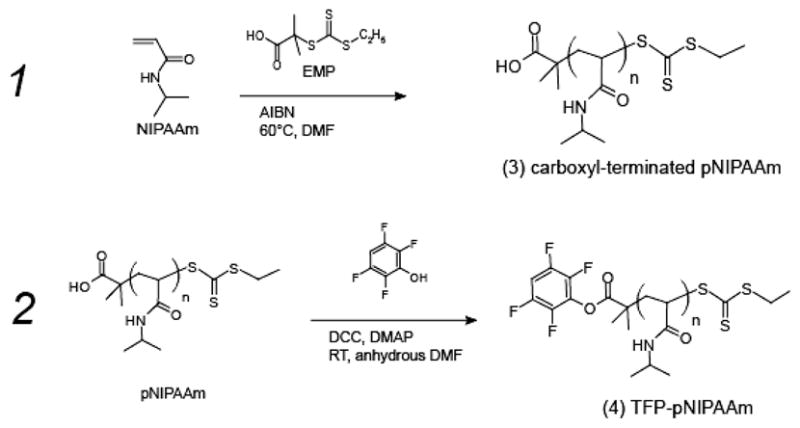
1. Synthesis of semi-telechelic pNIPAAm through RAFT polymerization results in carboxyl terminated pNIPAAm (3). 2. Tetrafluorophenol is coupled with the carboxyl end of pNIPAAm to result in TFP-pNIPAAm (4).
Gel-permeation chromatography
Polymer samples from solution and surface-derived polymerizations were analyzed using gel permeation chromatography (GPC) to determine their number average molecular weight (Mn) and polydispersity index (PDI = weight average molecular weight/number average molecular weight), using Viscotek model VE2001 sampler and VE3580 refractive index (RI) detector. Samples were compared against poly(methylmethacrylate) standards to determine molecular weight.
Antibody Conjugation and Purification
1 mg of polyclonal mouse IgG anti-streptavidin or monoclonal mouse IgM anti-Plasmodium falciparum HRP2 was diluted in 10 mmol phosphate buffered saline (PBS), 100 mM sodium bicarbonate, pH 8.5 to a final concentration of 2 mg/ml. PNIPAAm-TFP ester was dissolved in anhydrous DMSO (0.2 mg/μl). 25 μl of the pNIPAAm-TFP in DMSO was added to the antibody solution and the reaction was mixed gently overnight at 4º C. Conjugates were purified from tetrafluorophenol and excess salts with a desalting column. Conjugated antibodies were separated by heating the reaction mixture to 40ºC to induce conjugate aggregation followed by centrifugation for 2 minutes at 13,000 rpm. The supernatant was removed and the polymer pellet was resolubilized in phosphate buffered saline, pH 7.4. Conjugates were dialyzed against 1× PBS using 100,000 MWCO dialysis membranes over several days to reduce the content of unreacted pNIPAAm. Polymer contents of the resulting solutions were measured by UV absorbance at 307 nm against polymer concentration standards. Protein contents were determined by absorbance at 280 nm with a derived correction factor for contribution of polymer content to absorbance at 280 nm. Antibody concentrations used in immunocomplex samples were based on protein content only since an exact molecular weight of the antibody conjugate was not established. Antibody conjugates were stored at 4ºC in phosphate-buffered saline, pH 7.4.
Microfluidic Card Assembly
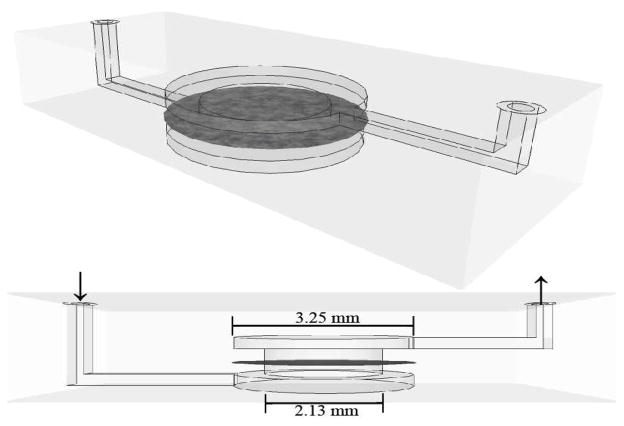
Punched pieces of membrane were positioned between two-part multilayer microchannels constructed of poly-ethylene terepthalate (PET), and poly-methyl methacrylate (PMMA) with an adhesive layer (excluding membrane region). Five layers contained stacked circular cut-outs to serve as a membrane well area, with inner layer diameters of 2.1 mm and outer layer diameters of 3.25 mm. Membrane pieces were positioned between two of the layers and sealed. The upper and lower membrane well layers were connected on opposing sides to rectangular channels of dimensions 9.2 mm length, 0.36 mm width, and 0.2 mm height. Fluid was introduced into the lower rectangular channel to flow up and through membrane and exit through the upper rectangular channel.
Immunocomplex Samples
100 nM of anti-streptavidin IgG polyclonal antibody conjugate (total pNIPAAm concentration of 0.58 mg/ml) was mixed with 10nM Alexa-488 labeled streptavidin. A negative control of free pNIPAAm at 0.58 mg/ml mixed with labeled streptavidin was included as a conjugate-free control. Samples were allowed to dwell 15 minutes prior to running through card. For concentration from a buffer-diluted solution, a 50 μl sample of 100 nM anti-streptavidin IgG antibody conjugate and 10nM Alexa 488-labeled streptavidin was diluted 50-fold in PBS-T to 2.5 ml.
Malaria Antigen Immunocomplex Samples
PfHRP2 antigen was spiked into >99% human plasma at concentrations of 8 – 200 ng/ml, and mixed with anti-PfHRP2 IgM pNIPAAm conjugate, and anti-PfHRP2 horseradish peroxidase-conjugated IgG in PBS. The final concentrations were 50% plasma, PfHRP2 antigen from 4 ng/ml (68 pM) to 100 ng/ml (1.7 nM), 14 μg/ml anti-pfHRP2-pNIPAAm conjugate (14.7 nM), and 0.4 μg/ml anti-pfHRP2 peroxidase-coupled detection antibody. Final polymer concentration was 25 μg/ml. Samples were allowed to dwell 15 minutes at room temperature prior to running.
Capture and Release in a Microfluidic Card
Capture conjugate solutions were introduced via syringe pump as a 50 μl sample plug leading wash buffer of PBS-tween (0.05%). Room-temperature samples were flowed to the membrane, wetting it, but samples did not penetrate through membrane prior to heating. For capture conditions, the entire card was heated on a solid block heater set to 40ºC. Temperature in the card was measured by a thermocouple placed through upper layer at one port. When the temperature of thermocouple reached 35ºC (under 30 seconds), flow through the membrane was initiated (25 μl per minute for 2 minutes, followed by 50 μl per minute for 4 minutes), with collection of exiting 50 μl fractions. For release, the card was cooled on a 10ºC cold block until thermocouple read 25ºC. Flow was resumed at 50 μl per minute, and 15 or 50 μl fractions collected. For concentration of a diluted sample, the entire 2.5 ml sample was flowed through the membrane during heating and released upon cooling into two 50 μl fractions.
Detection of Released anti-pfHRP2 Detection Antibody
Released pfHRP2 samples were pelleted using thermal precipitation, then resuspended into 100 μl 3,3′,5,53′ tetramethylbenzidine (TMB) substrate for color development. Reaction was stopped by 50 μl 1N HCl. Absorbance of sample was determined using a UV-VIS spectrophotometer plate reader at A450 nm- A650nm. Aliquots of a dilution series of detection antibody were combined with 100 μl of TMB substrate to create a standard curve of detection antibody concentration and control for color development relative to detection antibody concentration.
RESULTS and DISCUSSION
Construction of Stimuli-Responsive Membranes
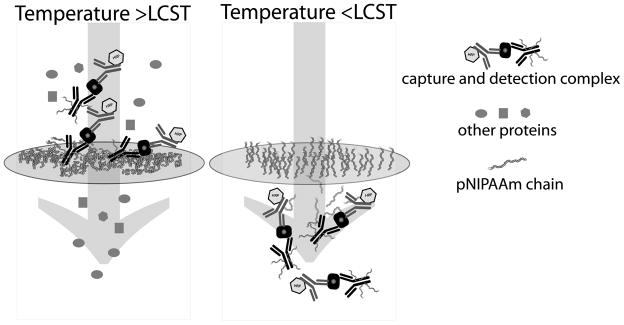
The microfluidic system consists of two stimuli-responsive components: (1) a porous, polymer-grafted membrane and (2) antibody conjugates (Figure 1). Above the critical solution temperature (LCST), the grafted polymers on the membrane surface and the antibody conjugate polymers in the flowing solution will both phase separate, leading to capture of the antibody conjugate immunocomplex at the membrane during flow. Below the LCST, the antibody conjugate immunocomplex is returned to the flow stream as the pNIPAAm chains become solvated. Membranes are inserted between layers of a microfluidic card constructed of poly-ethylene terepthalate (PET) and poly-methyl methacrylate (PMMA). (Figure 2)
Figure 1.
Schematic system design for immunocomplex capture and concentration under conditions of capture (>LCST) and release (<LCST). Covalently-modified pNIPAAm-antibody, bound antigen, and detection antibody are captured at membrane during polymer aggregation above the LCST, while unconjugated plasma components flow through. When the membrane region of the device is cooled, the polymer becomes hydrophilic and molecular conjugates are released back into the flow stream, carrying their cargo of antigen and detection antibody with them.
Figure 2.
Schematic of microfluidic channel used in capture and release experiments, shown in isometric projection (top) and side projection (bottom) views. Stacked layers of cut poly-ethylene terepthalate (PET) form the membrane well area, with inner layer diameters of 2.13 mm and outer layer diameters of 3.25 mm. Membrane pieces were positioned between two of the layers and sealed. The upper and lower membrane well layers are connected on opposing sides to rectangular channels of dimensions 9.2 mm length, 0.36 mm width, and 0.2 mm height. Fluid is introduced into the lower rectangular channel to flow up and through membrane and exit through the upper rectangular channel.
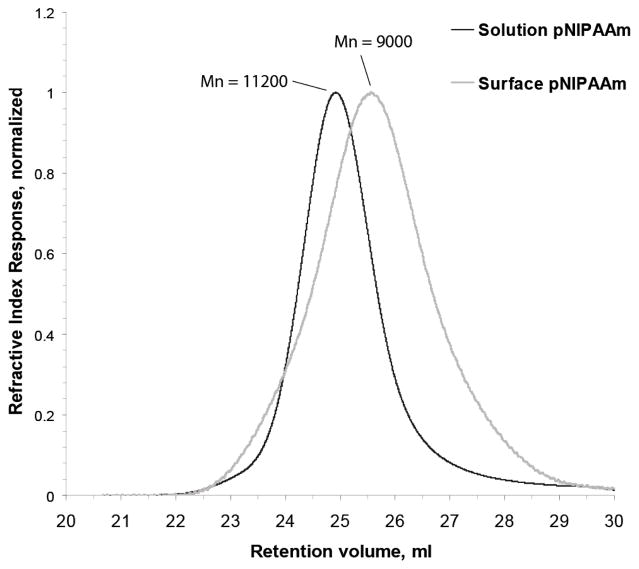
As shown in Scheme 1, pNIPAAm-graft membrane material was prepared in two steps. First, the surface hydroxyls on 1.2 μm pore-size Loprodyne membranes were converted to RAFT CTAs using the trithiocarbonate EMP. Second, surface grafts were synthesized by immersing the macro-CTA within a polymerization solution containing additional EMP using previously identified conditions (41). For Mn and PDI analysis, the ester bond between the CTA and membrane surface was hydrolyzed to cleave the grafted polymers from the membrane surface. Cleavage products derived from grafted membranes yielded milligram quantities of a soluble product per 3 cm2 membrane material. A negative control membrane with no immobilized CTA, included in the polymerization to control for adsorbed or entangled polymer versus a covalent graft, did not yield either a measureable product or a detectable peak as analyzed by gel-permeation chromatography (GPC). By varying solution EMP concentration, molecular weights of the surface and solution polymers could be tuned. Number average molecular weight (Mn) and polydispersity indices (PDI) were analyzed for the different polymerization products (Table 1). Figure 3 shows representative GPC traces of a cleavage product with its corresponding solution polymer product. The peak shape of the surface-derived product was uniform and absent of any higher molecular weight shoulder, indicating it was not likely caused by chain-chain coupling as has been previously reported in RAFT polymerization from surfaces (23).
Table 1.
Chain transfer agent concentration, molecular weight and polydispersity indices for polymerization products from surface grafting reactions used in this study. Solution and surface-derived polymers were analyzed by GPC. All polymerizations were performed in the presence of EMP-bound membranes, stirred at 60ºC for 18–24 hours in DMF with 0.2 g/ml NIPAAm monomer and AIBN initiator equal to 0.2 times the solution EMP concentration.
| Reaction | Reaction EMP, mM | Solution Mn (PDI) | Surface Mn (PDI) |
|---|---|---|---|
| 1 | 40 mM | 3500 (1.2) | 4300 (1.3) |
| 2 | 13.2 mM | 9500 (1.2) | 8400 (1.3) |
| 3 | 13.2 mM | 11200 (1.2) | 9000 (1.2) |
Figure 3.
Analysis of grafted poly(N-isopropylacrylamide) and solution polymerized poly(N-isopropylacrylamide) by gel-permeation chromatography. Normalized refractive index responses from the two syntheses are overlaid to show solution (black) and surface-cleaved (grey) polymer retention volumes, and are labeled with the calculated number-average molecular weight (Mn). Samples were derived from the same polymerization reaction containing EMP-bound 1.2 μm Nylon 6,6 membranes, monomer, initiator, and solution EMP.
Covalent Antibody Conjugates
Esterification of the carboxyl end of EMP of pNIPAAm with tetrafluorophenyl groups resulted in an amine-reactive active ester polymer (Scheme 2). Capture antibodies were reacted with the active ester polymer and the resulting product was purified from unreacted antibody through precipitation. After thermal precipitation of the reaction mixture, the pellet contained between 20 and 30% of the reacted antibody, while mock reactions with carboxyl-terminated pNIPAAm in identical quantity yielded less than 1% of the starting quantity of antibody. Reduction in polymer content after dialysis was confirmed by the absorbance at 307 nm relative to the 280 nm peak, permitting quantification of both protein and polymer concentration when used in conjunction with a derived correction factor for contribution to the absorbance at 280 nm by the trithiocarbonate moiety. However, while dialysis reduced the total polymer content by over 80%, it was not complete enough based on polymer-alone dialysis (data not shown) to permit quantification of polymer per protein calculations.
Microfluidic Separation Efficiencies of Model Anti-streptavidin Immunocomplex Conjugate
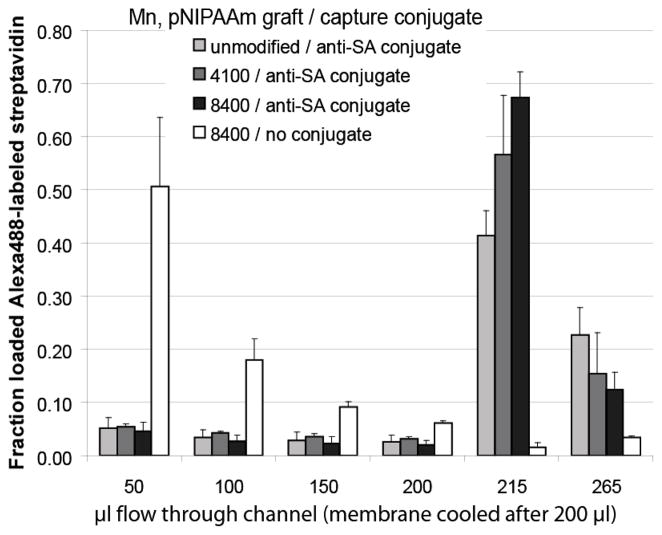
Fluorescently-labeled streptavidin was captured with anti-streptavidin pNIPAAm antibody conjugates at membranes in microfluidic channels under flow of 50 μl per minute (Figure 4). Anti-streptavidin conjugates of pNIPAAm bound fluorescently-labeled streptavidin at the membrane under heated conditions while streptavidin in a physical mixture with pNIPAAm flowed through the membrane and exited the channel as indicated by fluorescence of the flow-through solutions. Membranes (1.2 μm pore) that were either unmodified or modified with 4100 and 8400 molecular weight pNIPAAm all showed greater than 80% capture efficiency. The 8400 molecular weight-graft membrane showed the highest release efficiency, with greater than 65% of loaded streptavidin in the first 15 μl, and an overall release of approximately 80%. The immunocomplex was released from unmodified membranes much more slowly and less efficiently than the pNIPAAm-grafted membranes, with only about 40% released in the first 15 μl. Results seen by Ebara, et al., also showed slower release of pNIPAAm-streptavidin complexes from unmodified PDMS versus pNIPAAm-graft PDMS (38). While it was initially hypothesized that the capture of conjugates with the pNIPAAm-grafted membrane would be higher than with the unmodified membrane, general hydrophobic effects and hydrogen bonding to the free amide and hydroxyl groups of the unmodified surface could explain the similar capture efficiencies. Nylon 6,6 has only a moderate affinity for dehydrated pNIPAAm, suggesting that the surface hydroxylation may play a role in the affinity (13, 14). Collapse of pNIPAAm brushes on a surface is dependent on both density and molecular weight, with lower densities and smaller molecular weights exhibiting a noticeably less-sharp or absent transition above the LCST (21, 22). To our knowledge, no studies have been published on how such parameters affect the resolubilization of end-grafted chains. The 4100 molecular weight grafted membranes’ release of the anti-streptavidin immunocomplex was not significantly less than that of the higher molecular weight graft membrane. However, in light of overall trend observed, larger molecular weight graft-membranes were chosen for future work.
Figure 4.
Anti-streptavidin antibody conjugate-bound Alexa 488-labeled streptavidin (SA) captured at membranes during heated flow (50 μl per minute) and released with cooled flow (50 μl per minute). Each 50 μl sample contained 10 nM streptavidin with either no antibody conjugate (white), or 100 nM anti-streptavidin pNIPAAm conjugate (grey to black). Membranes tested were unmodified Loprodyne (light grey), 4,100 Mn-graft (medium grey), or 8,400 Mn-graft (black and white) pNIPAAm-polymerized Loprodyne. Fluorescence of recovered flow fractions was measured using a plate reader fluorimeter against a standard curve.
Capture and Enrichment of Model Antigen Using the Microfluidic Concentrator
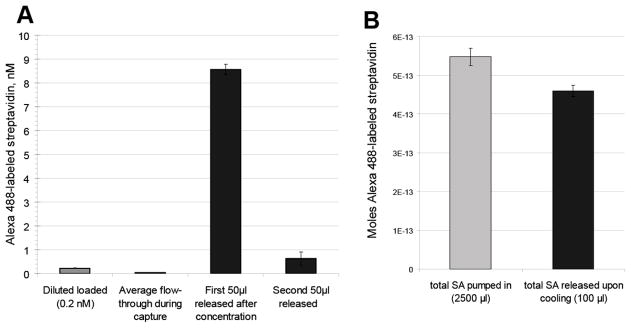
To further test the ability of this system to concentrate antigen at the membrane surface, 100 nM anti-streptavidin conjugate and 10 nM Alexa 488-labeled streptavidin mixture were diluted 50-fold in PBS-T and flowed through heated membranes. The released antibody conjugate and antigen were concentrated approximately 40 times, from 0.2 nM to 8.5 nM in the first 50 μl released (Figure 5). The release efficiency was 84% (total released/ total loaded). Evidence of concentration of the direct antibody conjugate is also seen in the reduced flow-through concentration, indicating retention during capture conditions. The capture of the diluted solution is not expected to be mediated by particle size exclusion by the membrane pores (1.2 μm) since pNIPAAm at the concentration of the diluted solution (11.6 μg/ml) would not be expected to form particles above the nominal pore size of the membranes during the time of the capture phase, even in the absence of the antibody conjugate which would likely favor smaller particles pNIPAAm in solution (42). This also highlights a benefit over separation by a thermal precipitation in that much more dilute concentrations of antibody conjugate, that would not be able to be separated by thermal precipitation, can be efficiently separated using a membrane capture system. The mechanism of capture may be different in that the affinity between the membrane and the polymer is responsible for the capture, whereas it is a critical particle size that is responsible for precipitation in a thermal centrifugation. For the purposes of enrichment and purification of a sample, it is advantageous to reduce the need for adding additional polymer since it could change the solubility and viscosity of the final purified species.
Figure 5.
Concentration and enrichment of pNIPAAm-anti-streptavidin immunocomplex. Samples containing 100 nM anti-streptavidin antibody pNIPAAm conjugate and 10 nM Alexa 488-labeled streptavidin (SA) were diluted 50-fold into PBS-T. The entire diluted sample was flowed (100 μl per minute) through a heated membrane (pNIPAAm-modified membrane, 8400 Mn) and flow-through fractions collected (grey). After the membrane was cooled to 25ºC, 50 μl per minute flow was resumed and two 50 μl release fractions were recovered (black). A) Concentration (nM) per 50μl fraction was determined by measurement of fluorescence against a standard curve. B) Right: total moles streptavidin loaded (light grey) and released (black).
Purification and Concentration of Malaria Antigen Sandwich Complex from Spiked Human Plasma
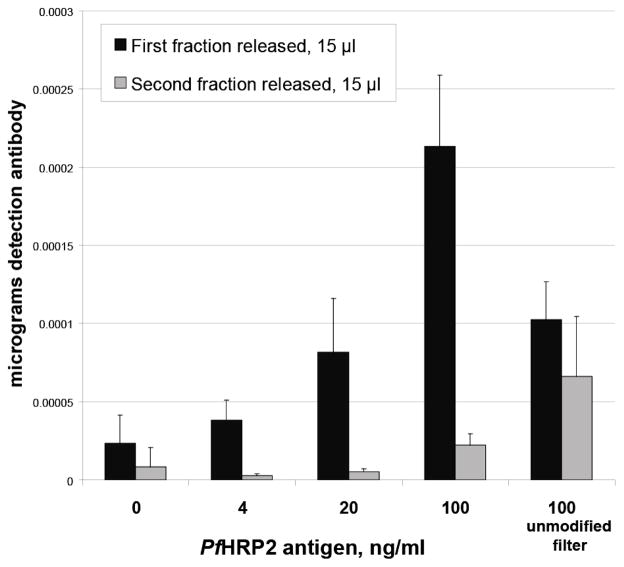
The quantity of released detection antibody was shown to be dependent on the Plasmodium falciparum HRP2 antigen concentration in the sample (Figure 6). The detection of 20 ng/ml to 100 ng/ml of PfHRP2 antigen from a 50 μl sample is within the relevant range of antigen for patient samples and comparable to the ranges detectable by standard ELISA of plasma samples (36). The time for the completed membrane assay was under 10 minutes of run time, much shorter than required for a standard ELISA, which typically consumes about 4.5 hours. While the concentration of antibody-pNIPAAm conjugate used in these experiments was higher than would typically be used in a standard ELISA, this quantity could likely be reduced to a concentration similar to that used in ELISA, given the high binding affinity of the IgM antibody. The presence of pNIPAAm membrane modification resulted again in sharper release kinetics as compared to the unmodified filter membrane. Due to the concentrating capabilities of the method in this work, we expect that it may be possible to increase detection of low levels of antigen from larger plasma volumes, levels which are typically undetectable by rapid diagnostic methods and challenging as well to detect by ELISA. Data for rapid tests in terms of ng/ml concentrations of PfHRP2 antigen are not currently available. This prevents us from making any direct comparison to existing rapid assay technologies. Most literature surrounding rapid tests is expressed in parasitemia levels, which cannot be consistently correlated with HRP2 concentration since parasite stage and infection time would impact this number in addition to the parasite density (43). Future work is planned to test the performance of this system against existing malaria rapid diagnostic tests. Other candidate antigens, such as pan-Plasmodium lactate dehydrogenase and aldolase, may also be added for a multiplexed assay (6).
Figure 6.
Released quantity of full-stack anti-PfHRP2 immunocomplex was dependent on antigen concentration and membrane modification. Samples containing anti-PfHRP2 antibody conjugate (14 μg/ml, 15 nM), PfHRP2 antigen in plasma (0 – 100 ng/ml range), and 0.4 μg/ml anti-PfHRP2 detection antibody were captured, washed with 400 μl PBS-T, and released into 15 μl fractions from pNIPAAm-modified membrane (Mn = 9000) or unmodified Loprodyne filter membrane. Total micrograms of peroxidase-conjugated anti-PfHRP2 detection antibody, per precipitated release fraction, was determined by measurement of TMB substrate color development against a standard curve.
CONCLUSIONS
A system of stimuli-sensitive antibody conjugates and porous grafted membranes was developed for the purpose of separating and concentrating immunocomplexes within a microfluidic channel. A TFP-ester of pNIPAAm provides a facile method for conjugating pNIPAAm to amine groups on an antibody to yield phase-transition reagents suitable for capture and release of antigen. The RAFT-mediated pNIPAAm modification of a porous membrane surface results in a sharp immunocomplex release profile. We have shown this method to be capable of high percentage capture and release of two antigens, and the ability to concentrate the antigen from dilute solutions. Full stack detection immunocomplexes identical to those used in ELISA for detection of PfHRP2 malaria antigen were separated from 50% human plasma samples. This technology has a wide range of applications for enrichment of target from samples, but can also be applied to sample preconditioning to remove specific unwanted elements from complex mixtures, such as IgG from IgM-based assays, without further diluting the sample. We have demonstrated concentration of antigen and full-stack immunocomplexes from complex human plasma, providing opportunities to concentrate dilute biomarkers and to expand detection ranges in microfluidic immunoassays.
Acknowledgments
The authors gratefully acknowledge funding from the Bill and Melinda Gates Foundation for Grand Challenges in Global Health, and the National Institutes of Health (Grant EB000252).
LITERATURE CITED
- 1.Playford EG, Walker J. Evaluation of the ICT malaria P.f/P.v and the OptiMal rapid diagnostic tests for malaria in febrile returned travellers. Journal of Clinical Microbiology. 2002;40:4166–4171. doi: 10.1128/JCM.40.11.4166-4171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Den Broek I, Hill O, Gordillo F, Angarita B, Hamade P, Counihan H, Guthmann JP. EVALUATION OF THREE RAPID TESTS FOR DIAGNOSIS OF P. FALCIPARUM AND P. VIVAX MALARIA IN COLOMBIA. American Journal of Tropical Medicine and Hygiene. 2006;75:1209–1215. [PubMed] [Google Scholar]
- 3.Ratsimbasoa A, Fanazava L, Radrianjafy R, Ramilijaona J, Rafanomezantsoa H, Menard D. Short Report: Evaluation of Two New Immunochromatographic Assays for Diagnosis of Malaria. American Journal of Tropical Medicine and Hygiene. 2008;79:670–672. [PubMed] [Google Scholar]
- 4.Hopkins H, Bebell L, Kambale W, Dokomajilar C, Rosenthal PJ, Dorsey G. Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. Journal of Infectious Diseases. 2008;197:510–518. doi: 10.1086/526502. [DOI] [PubMed] [Google Scholar]
- 5.Fogg C, Twesigye R, Batwala V, Piola P, Nabasumba C, Kiguli J, Mutebi F, Hook C, Guillerm M, Moody A, Guthmann JP. Assessment of three new parasite lactate dehydrogenase (pan-pLDH) tests for diagnosis of uncomplicated malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102:25–31. doi: 10.1016/j.trstmh.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Moody A. Rapid diagnostic tests for malaria parasites. Clinical Microbiology Reviews. 2002;15:66. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray CK, Gasser RA, Magill AJ, Miller RS. Update on rapid diagnostic testing for malaria. Clinical Microbiology Reviews. 2008;21:97. doi: 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigl BH, Yager P. Microfluidics - Microfluidic diffusion-based separation and detection. Science. 1999;283:346–347. [Google Scholar]
- 9.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Microfluidic diagnostic technologies for global public health. Nature. 2006;442:412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 10.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annual Review of Biomedical Engineering. 2008;10:107–144. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 11.Kamholz AE, Yager P. Theoretical analysis of molecular diffusion in pressure-driven laminar flow in microfluidic channels. Biophysical Journal. 2001;80:155–160. doi: 10.1016/S0006-3495(01)76003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auditorehargreaves K, Houghton RL, Monji N, Priest JH, Hoffman AS, Nowinski RC. PHASE-SEPARATION IMMUNOASSAYS. Clinical Chemistry. 1987;33:1509–1516. [PubMed] [Google Scholar]
- 13.Monji N, Cole CA, Tam M, Goldstein L, Nowinski RC. APPLICATION OF A THERMALLY-REVERSIBLE POLYMER-ANTIBODY CONJUGATE IN A NOVEL MEMBRANE-BASED IMMUNOASSAY. Biochemical and Biophysical Research Communications. 1990;172:652–660. doi: 10.1016/0006-291x(90)90724-2. [DOI] [PubMed] [Google Scholar]
- 14.Monji N, Cole CA, Hoffman AS. ACTIVATED, N-SUBSTITUTED ACRYLAMIDE POLYMERS FOR ANTIBODY COUPLING - APPLICATION TO A NOVEL MEMBRANE-BASED IMMUNOASSAY. Journal of Biomaterials Science-Polymer Edition. 1994;5:407–420. doi: 10.1163/156856294x00112. [DOI] [PubMed] [Google Scholar]
- 15.Takei YG, Matsukata M, Aoki T, Sanui K, Ogata N, Kikuchi A, Sakurai Y, Okano T. TEMPERATURE-RESPONSIVE BIOCONJUGATES .3. ANTIBODY POLY(N-ISOPROPYLACRYLAMIDE) CONJUGATES FOR TEMPERATURE-MODULATED PRECIPITATIONS AND AFFINITY BIOSEPARATIONS. Bioconjugate Chemistry. 1994;5:577–582. doi: 10.1021/bc00030a013. [DOI] [PubMed] [Google Scholar]
- 16.Malmstadt N, Hoffman AS, Stayton PS. “Smart” mobile affinity matrix for microfluidic immunoassays. Lab on a Chip. 2004;4:412–415. doi: 10.1039/b315394k. [DOI] [PubMed] [Google Scholar]
- 17.Monji N, Hoffman AS. A NOVEL IMMUNOASSAY SYSTEM AND BIOSEPARATION PROCESS BASED ON THERMAL PHASE SEPARATING POLYMERS. Applied Biochemistry and Biotechnology. 1987;14:107–120. doi: 10.1007/BF02798429. [DOI] [PubMed] [Google Scholar]
- 18.Fong RB, Ding ZL, Hoffman AS, Stayton PS. Affinity separation using an Fv antibody fragment-“smart” polymer conjugate. Biotechnology and Bioengineering. 2002;79:271–276. doi: 10.1002/bit.10315. [DOI] [PubMed] [Google Scholar]
- 19.Lin P, Zheng H, Yang HH, Yang W, Zhang CG, Xu JG. Enzyme-linked fluorescence immunoassay for human IgG by using a pH-sensitive phase separating polymer as carrier. Chemical Journal of Chinese Universities-Chinese. 2003;24:1198–1200. [Google Scholar]
- 20.Hoffman AS, Stayton PS. Conjugates of stimuli-responsive polymers and proteins. Progress in Polymer Science. 2007;32:922–932. [Google Scholar]
- 21.Zhu X, Yan C, Winnik FM, Leckband D. End-grafted low-molecular-weight PNIPAM does not collapse above the LCST. Langmuir. 2007;23:162–169. doi: 10.1021/la061577i. [DOI] [PubMed] [Google Scholar]
- 22.Plunkett KN, Zhu X, Moore JS, Leckband DE. PNIPAM Chain Collapse Depends on the Molecular Weight and Grafting Density. Langmuir. 2006;22:4259–4266. doi: 10.1021/la0531502. [DOI] [PubMed] [Google Scholar]
- 23.Tsujii Y, Ejaz M, Sato K, Goto A, Fukuda T. Mechanism and kinetics of RAFT-mediated graft polymerization of styrene on a solid surface. 1. Experimental evidence of surface radical migration. Macromolecules. 2001;34:8872–8878. [Google Scholar]
- 24.Friebe A, Ulbricht M. Controlled pore functionalization of poly(ethylene terephthalate) track-etched membranes via surface-initiated atom transfer radical polymerization. Langmuir. 2007;23:10316–10322. doi: 10.1021/la7016962. [DOI] [PubMed] [Google Scholar]
- 25.Geismann C, Yaroshchuk A, Ulbricht M. Permeability and electrokinetic characterization of poly(ethylene terephthalate) capillary pore membranes with grafted temperature-responsive polymers. Langmuir. 2007;23:76–83. doi: 10.1021/la0603774. [DOI] [PubMed] [Google Scholar]
- 26.Wu GG, Li YP, Han M, Liu XX. Novel thermo-sensitive membranes prepared by rapid bulk photo-grafting polymerization of N,N-diethylacrylamide onto the microfiltration membranes Nylon. Journal of Membrane Science. 2006;283:13–20. [Google Scholar]
- 27.Liang L, Shi MK, Viswanathan VV, Peurrung LM, Young JS. Temperature-sensitive polypropylene membranes prepared by plasma polymerization. Journal of Membrane Science. 2000;177:97–108. [Google Scholar]
- 28.Singh N, Wang J, Ulbricht M, Wickramasinghe SR, Husson SM. Surface-initiated atom transfer radical polymerization: A new method for preparation of polymeric membrane adsorbers. Journal of Membrane Science. 2008;309:64–72. [Google Scholar]
- 29.Ulbricht M. Advanced functional polymer membranes. Polymer. 2006;47:2217–2262. [Google Scholar]
- 30.Yusof AHM, Ulbricht M. Polypropylene-based membrane adsorbers via photo-initiated graft copolymerization: Optimizing separation performance by preparation conditions. Journal of Membrane Science. 2008;311:294–305. [Google Scholar]
- 31.Nagase K, Kobayashi J, Kikuchi AI, Akiyama Y, Kanazawa H, Okano T. Effects of graft densities and chain lengths on separation of bioactive compounds by nanolayered thermoresponsive polymer brush surfaces. Langmuir. 2008;24:511–517. doi: 10.1021/la701839s. [DOI] [PubMed] [Google Scholar]
- 32.Ying L, Yu WH, Kang ET, Neoh KG. Functional and surface-active membranes from poly(vinylidene fluoride)-graft-poly(acrylic acid) prepared via RAFT-mediated graft copolymerization. Langmuir. 2004;20:6032–6040. doi: 10.1021/la049383v. [DOI] [PubMed] [Google Scholar]
- 33.Nagase K, Kobayashi J, Kikuchi A, Akiyama Y, Annaka M, Kanazawa H, Okano T. Influence of graft interface polarity on hydration/dehydration of grafted thermoresponsive polymer brushes and steroid separation using all-aqueous chromatography. Langmuir. 2008;24:10981–10987. doi: 10.1021/la801949w. [DOI] [PubMed] [Google Scholar]
- 34.Zorrilla S, Hink MA, Visser A, Lillo MP. Translational and rotational motions of proteins in a protein crowded environment. Biophysical Chemistry. 2007;125:298–305. doi: 10.1016/j.bpc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman SB, Minton AP. MACROMOLECULAR CROWDING - BIOCHEMICAL, BIOPHYSICAL, AND PHYSIOLOGICAL CONSEQUENCES. Annual Review of Biophysics and Biomolecular Structure. 1993;22:27–65. doi: 10.1146/annurev.bb.22.060193.000331. [DOI] [PubMed] [Google Scholar]
- 36.Kifude CM, Rajasekariah HG, Sullivan DJ, Jr, Stewart VA, Angov E, Martin SK, Diggs CL, Waitumbi JN. Characterization of Enzyme Linked Immunosorbent Assay for Plasmodium falciparum Histidine-Rich Protein 2 in Blood, Plasma and Serum. Clinical and Vaccine Immunology. 2008 doi: 10.1128/CVI.00385-07. CVI.00385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malmstadt N, Yager P, Hoffman AS, Stayton PS. A smart microfluidic affinity chromatography matrix composed of poly(N-isopropylacrylamide)-coated beads. Analytical Chemistry. 2003;75:2943–2949. doi: 10.1021/ac034274r. [DOI] [PubMed] [Google Scholar]
- 38.Ebara M, Hoffman JM, Hoffman AS, Stayton PS. Switchable surface traps for injectable bead-based chromatography in PDMS microfluidic channels. Lab on a Chip. 2006;6:843–848. doi: 10.1039/b515128g. [DOI] [PubMed] [Google Scholar]
- 39.Lai JJ, Hoffman JM, Ebara M, Hoffman AS, Estournes C, Wattiaux A, Stayton PS. Dual magnetic-/temperature-responsive nanoparticles for microfluidic separations and assays. Langmuir. 2007;23:7385–7391. doi: 10.1021/la062527g. [DOI] [PubMed] [Google Scholar]
- 40.Convertine AJ, Lokitz BS, Vasileva Y, Myrick LJ, Scales CW, Lowe AB, McCormick CL. Direct Synthesis of Thermally Responsive DMA/NIPAM Diblock and DMA/NIPAM/DMA Triblock Copolymers via Aqueous, Room Temperature RAFT Polymerization. Macromolecules. 2006;39:1724–1730. [Google Scholar]
- 41.Schilli C, Lanzendorfer MG, Muller AHE. Benzyl and cumyl dithiocarbamates as chain transfer agent in the RAFT polymerization of N-isopropylacrylamide. In situ FT-NIR and MALDI-TOF MS investigation. Macromolecules. 2002;35:6819–6827. [Google Scholar]
- 42.Kulkarni S, Schilli C, Muller AHE, Hoffman AS, Stayton PS. Reversible meso-scale smart polymer-protein particles of controlled sizes. Bioconjugate Chemistry. 2004;15:747–753. doi: 10.1021/bc034215k. [DOI] [PubMed] [Google Scholar]
- 43.Desakorn V, Dondorp AM, Silamut K, Pongtavornpinyo W, Sahassananda D, Chotivanich K, Pitisuttithum P, Smithyman AM, Day NPJ, White NJ. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99:517–524. doi: 10.1016/j.trstmh.2004.11.014. [DOI] [PubMed] [Google Scholar]