Abstract
During the typical healing response to an implanted biomaterial, vascular-rich granulation tissue forms around the implant and later resolves into a relatively avascular, fibrous capsule. We have previously shown that a microvascular construct (MVC) consisting of isolated microvessel fragments suspended in a collagen I gel forms a persistent microcirculation in lieu of avascular scar when implanted. The current study evaluated the potential for microvascular constructs to maintain a vascularized tissue environment around an implanted biomaterial. An analysis of the peri-implant tissue around bare expanded polytetrafluoroethylene (ePTFE), ePTFE embedded within a microvascular construct, or ePTFE embedded within collagen alone revealed that the presence of the MVC, but not collagen alone, promoted vascular densities comparable to that of the granulation tissue formed around bare ePTFE. The vessels within the microvascular construct surrounding the ePTFE were perfusion competent, as determined by India ink perfusion casting, and extended into the interstices of the polymer. In contrast to bare ePTFE, the presence of the MVC or collagen alone significantly reduced the number of activated macrophages in association with ePTFE. Similar results were observed for ePTFE modified to increase cellularity and prevent the formation of an avascular scar. The microvascular construct may prove effective in forming vascularized tissue environments and limiting the number of activated macrophages around implanted polymers thereby leading to effective implant incorporation.
Keywords: granulation tissue, fibrous capsule, structural adaptation, microvascular networks
INTRODUCTION
Implanted biomaterials, such as expanded polytetrafluoroethylene (ePTFE), generate a foreign body response resulting in the eventual formation of an avascular fibrous capsule in association with the implant 1, 7, 10, 11, 13. Polymer-associated healing is initiated by activated inflammatory cells, such as macrophages, at the tissue:implant interface giving rise to an active granulation tissue, which if not manipulated, leads to the fibrous tissue. Concomitant with the progressive increase in fibrosity in the peri-implant region is a reduction in blood vessel density in the polymer-associated tissue. Because the fibrous capsule can limit the functionality of an implanted device, a common objective has been to prevent fibrosis and simultaneously maintain high vascularity in the peri-implant tissue 5, 17, 21.
A common strategy to disrupt fibrosis is to manipulate the tissue environment surrounding the implanted polymer either by modifying the material or incorporating disruptive factors 4, 14. For example, surface modification of a material by either covalent or non-covalent attachment of biomolecules (e.g. matrix molecules, cytokines, etc) prevents or delays the progression from granulation tissue to a fibrotic “scar” 8, 15. Similarly, angiogenic factors are used to induce vascularization in the peri-implant tissue 22. Commonly, these angiogenic factors are incorporated with the material to be slowly released upon implantation 9, 24. However, another approach has been to pre-vascularize an implant site so that the material is placed within a preformed vascular bed 2, 3. Regardless of the approaches taken, a successful outcome is still considered to involve maintenance of peri-implant vascularity.
We explored a new strategy for preventing fibrous capsule formation involving the placement of the material into a pre-vascularized interfacial tissue environment prior to implantation. Previously, we have shown that prevascularizing a collagen type I gel with isolated microvessel fragments derived from adipose tissue (called a microvascular construct) leads to the formation of a functional microcirculation within the gel when implanted 18. The microvessels placed within the construct persist, spontaneously inosculate with the host vasculature, are perfused with blood, and structurally remodel into a stereotypical, stable microvascular network 18. Consequently, we hypothesized that surrounding a biomaterial with a microvascular construct prior to implantation would create a sustainable vascularized tissue environment for the material without prior manipulation of the material or the implantation site.
MATERIALS AND METHODS
EPTFE discs implantation
All animal studies were performed with protocols approved by the University of Arizona IACUC and according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (#85-23 Rev. 1985). Surgeries were performed as previously described 16. Briefly, for each procedure the animals were anesthetized with an intraperitoneal injection of 400 mg/kg Avertin®. 6 mm diameter ePTFE discs, prepared as punches from standard (650 µm nominal wall thickness) 4 mm diameter tubular vascular grafts (IMPRA, Inc., Tempe, AZ) were implanted in either the right and left dorsal subcutaneous position in a random order with a total of two samples per animal and between 4–7 samples per group in CB-17/Icr SCID mice (Experimental Mouse Shared Resource, University of Arizona). Implants were removed after 28 days and placed in phosphate-buffered, 2% paraformaldehyde.
Microvascular construct preparation
The microvascular construct was prepared with isolated rat adipose-derived microvessel fragments embedded (15,000 – 20,000 fragments/ml) in type I rat tail collagen gel (3 mg/ml) as described before 18. Briefly, epididymal fat pads were harvested, finely minced with scissors, and digested at 37°C with vigorous shaking with equal volumes of collagenase (2 mg/ml) + DNAse I (1 mg/ml) for 7 min. The collagenase used (type I Worthington Biochemical Company, NJ) was lot tested to yield high numbers of fragments with intact morphologies. The digestate was washed twice with centrifugation using Dulbecco's cation free-PBS + 0.1% BSA to remove adipocytes and the suspended pellet was sequentially screened (nylon screens of 500 µm and 20 µm mesh size; Small Parts, Logansport, IN). Fragments captured on the 20 µm screen were collected, suspended at 20,000 fragments per ml of unpolymerized type I collagen and kept on ice until used. Collagen was prepared by mixing ice cold collagen (acidified, high-concentration collagen type I acid; BD Biosciences, Bedford, MA) with ice cold 4x DMEM to a final concentration of 3 mg/ml, adjusting the pH to approximately 7.4 with 1 M NaOH and keeping on ice to prevent polymerization. Immediately prior to implantation, ePTFE discs (unmodified or modified) were placed on edge in 100 µl of unpolymerized collagen gel with or without microvascular fragments and the constructs were polymerized for 30 min. at 37°C in a humidified incubator.
EPTFE modification
Tubular ePTFE was used without further processing (control) or following either denucleation or surface modification with extracellular matrix (ECM) prior to cutting into discs. Denucleation of ePTFE and adsorption of extracellular matrix molecules to ePTFE surfaces are modifications previously shown to improve tissue incorporation and increase peri-implant tissue vascularity11, 13. Inclusion of these proven modifications provided for a comparison to the prevascularized strategy as well as an examination of possible potentiating affects. Denucleation was accomplished by removing the air from the interstices of the material using successive 70% and 100% ethanol submersions over 20 min intervals. For ECM-modification, denucleated tubular ePTFE was placed in DCF-PBS for 1 hour followed by incubation with an ECM-rich medium conditioned for 48 hr. by human squamous epithelial cells (HaCaT; a generous gift from Dr. Norbert Fusenig, German Cancer Research Center) known to secrete laminin-5 and other extracellular matrix molecules 13.
Ink vascular casting
Visualization of the microvascular network associated with each implant was performed as previously described with modifications 6. India ink (no. 3232; Hunt Manufacturing) was dialyzed against 4X volumes of PBS overnight and filtered through a 20µm nylon mesh and stored at 4°C. For casting, the vasculature was flushed free of blood with heparinized (100U/ml) saline containing sodium nitroprusside (SNP, 10−5M) through a PE60 catheter into the left ventricle at a flow rate of 4 ml/min. Approximately 3 ml of india ink, warmed to 37°C and containing heparin and SNP (as above), was then perfused through the catheter at a flow rate of 2 ml/min. The implants were carefully removed and placed in phosphate buffered 2% paraformaldehyde for 24 hrs. Following fixation, the tissue was dehydrated in a series of ethanol dilutions and cleared in methyl salicylate for visualization with a stereomicroscope.
Histology and immunohistochemistry
Explants fixed in phosphate buffered 2% paraformaldehyde were dehydrated, embedded in paraffin and sectioned (6 µm thick) for evaluation. Each section contained the entire implant. Tissue structure was determined with standard hematoxylin and eosin staining. Vessel elements were identified using the Griffonia simplicifolia-1 lectin (biotinylated lectin-GS-1; 1:250; Vector Laboratories, Burlingame, Ca) and activated macrophages were identified using an antibody against the F4/80 160 kD glycoprotein antigen (biotinylated-monoclonal, 1:100 Serotec, Inc., Raleigh, NC). A peroxidase conjugated streptavidin kit with 3, 3’ diaminobenzidine (DAB) (Dako Inc., Carpinteria, Ca) was used as the reporter system for both evaluations. Methyl green staining was used as a counter-stain. All measurements were made along the entire tissue-polymer interface on both sides of the polymer excluding the two image fields closest to the polymer edge (to avoid potential edge effects).
Vessel and macrophage density
Digital images of randomly selected fields (54 µm × 162 µm) immediately adjacent to and along the polymer were obtained with a transmission light microscope fitted with a 40x water-immersion objective. Vessel density was calculated as the number of discreet vessel (GS-1-positive) structures counted per field. A structure was counted as a vessel if: 1) there was a positive GS1 stain and 2) there was an identifiable lumen. Activated macrophage density was determined by counting the total number of F4/80-positive structures per field located within a 54 µm-wide region immediately adjacent to the polymer or within a 54 µm-wide region just into the polymer. Vessel density is expressed as the mean number of vessels/mm2 ± s.e.m for each group. Macrophage density for each implant group was expressed as mean number of F4/80 positive cells/mm2 ± s.e.m.
Statistics
All statistical tests were performed using the SigmaStat software (SPSS Inc., Chicago, Illinois). Comparisons within groups were performed using a one-way ANOVA with Tukey post-hoc test for multiple comparisons. Differences were considered statistically significant at p < 0.05.
RESULTS
Collagen gels or microvascular constructs alter peri-implant tissue formation
Similar to previously described observations12, 13, 23, bare ePTFE implants, regardless of whether or not the polymer was modified, were surrounded by a matrix-rich, cellular layer of tissue (Fig. 1). As expected, the tissue surrounding the HaCaT-modified ePTFE implant and within the polymer spaces appeared hyper-cellular13. In contrast, the tissue immediately adjacent to the ePTFE implants placed in either collagen gels or microvascular constructs (MVC) consisted of a loose matrix layer with relatively fewer cells (Fig. 1). Additionally, in contrast to bare ePTFE implants, few cells had penetrated into the ePTFE interstices when the collagen gels or MVCs were present (Fig. 1).
Figure 1.
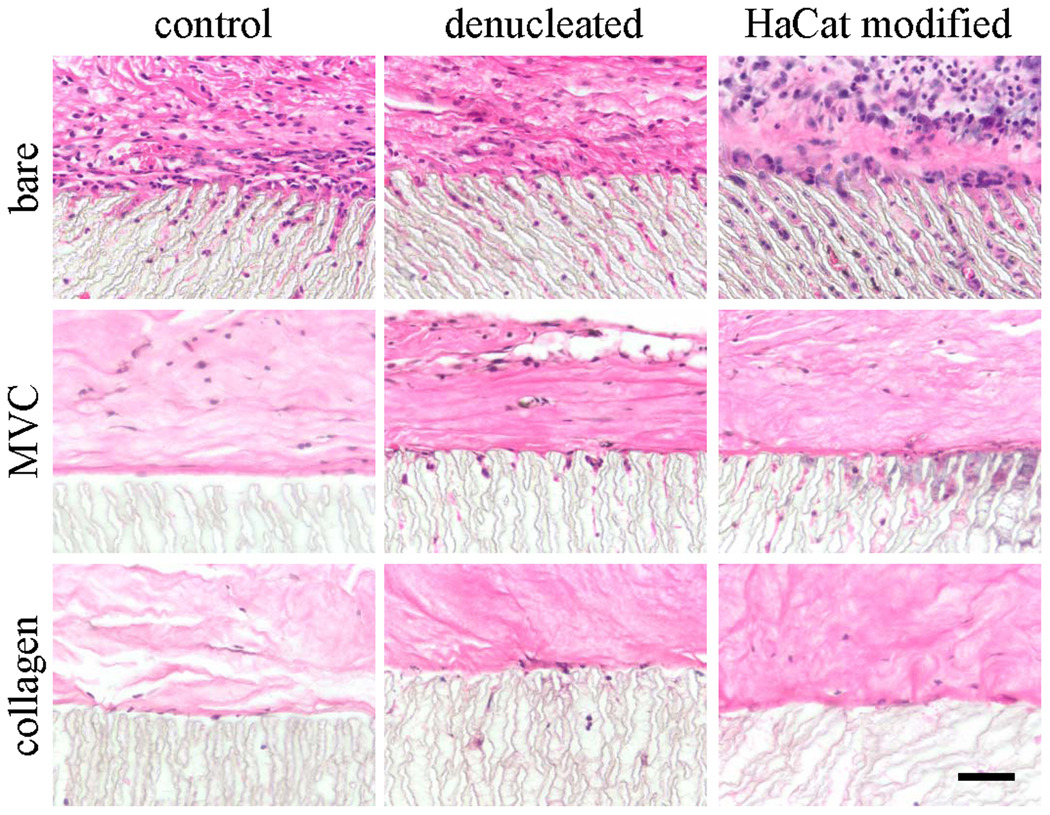
Light micrographs of hematoxylin and eosin-stained paraffin sections of ePTFE discs implanted for 28 days as is (bare) or embedded in a microvascular construct (MVC) or an avascular collagen gel (collagen). EPTFE discs were used without modification (control), denucleated, or modified with a secreted extracellular matrix preparation (HaCaT modified). Scale bar = 50 microns.
Microvascular constructs are associated with a moderate peri-implant vessel density but reduced macrophage density
Microvessels with identifiable lumens were present within a ~ 54 µm-thick tissue layer immediately adjacent to the ePTFE in all experimental treatments (Fig. 2). However, vessel density in the implants with collagen gels was significantly lower than the other treatments, regardless of the modification (Fig. 4). Similarly, activated macrophages (F4/80-positive cells) were present around and within the polymer of all of the implants (Figs. 3 and 4). However, co-implantation of either collagen gel or microvascular construct with the ePTFE significantly reduced the number of activated macrophages within the ePTFE spaces but not in the surrounding tissue (Fig. 4).
Figure 2.
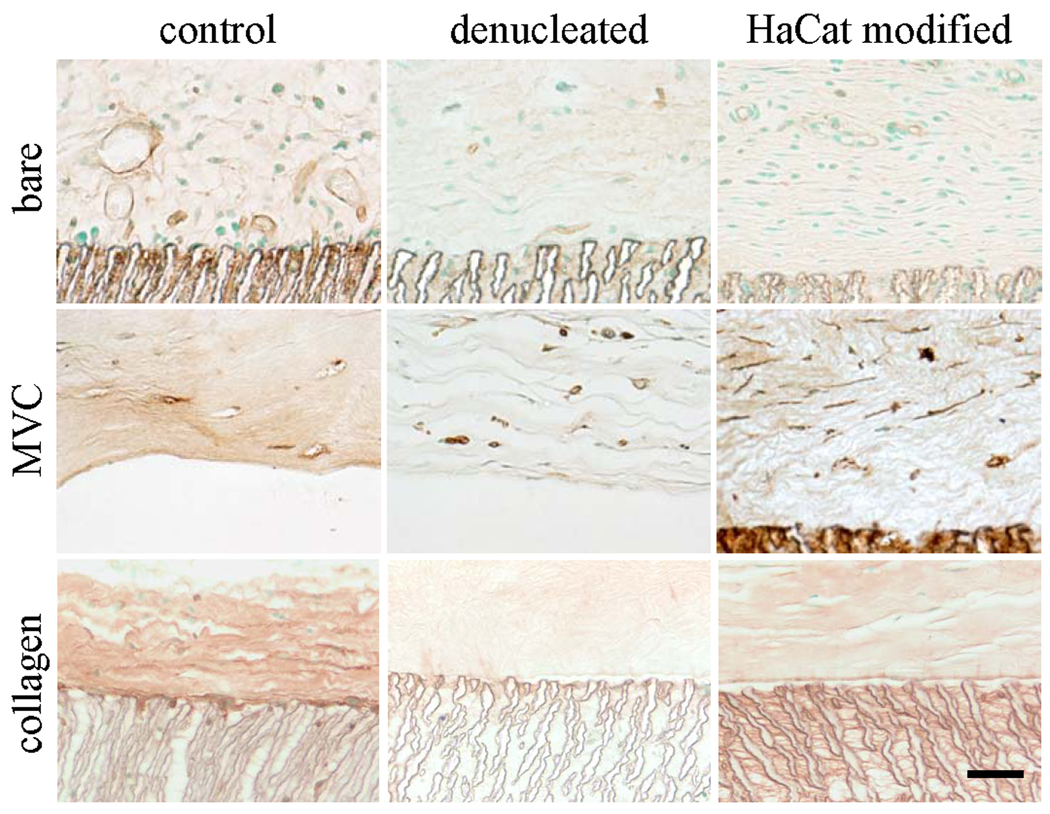
Light micrographs of GS-1-stained sections to identify blood vessels. EPTFE discs were implanted for 28 days as is (bare) or embedded in a microvascular construct (MVC) or an avascular collagen gel (collagen). EPTFE discs were used without modification (control), denucleated, or modified with a secreted extracellular matrix preparation (HaCaT modified). Scale bar = 50 microns.
Figure 4.
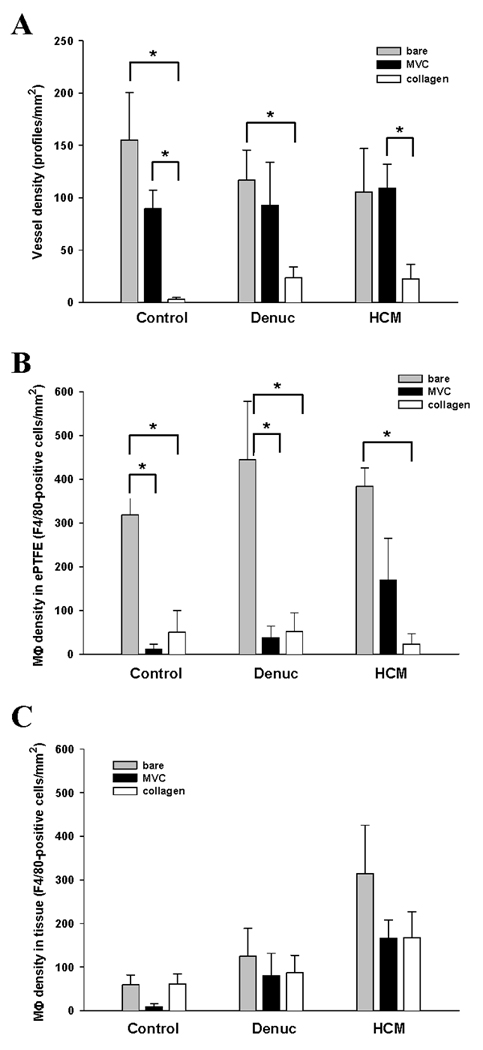
Vessel densities (A) were measured by counting the GS1-positive profiles located within the first 100 microns of peri-implant tissue in paraffin sections (n ≥ 3 different implants). Similarly, activated macrophage densities were determined by counting F4/80-positive cells within the interstices of the ePTFE (B) and the peri-implant tissue (C) in paraffin sections (n ≥ 3 different implants). Values represent mean ± s.e.m; * p <0.05.
Figure 3.
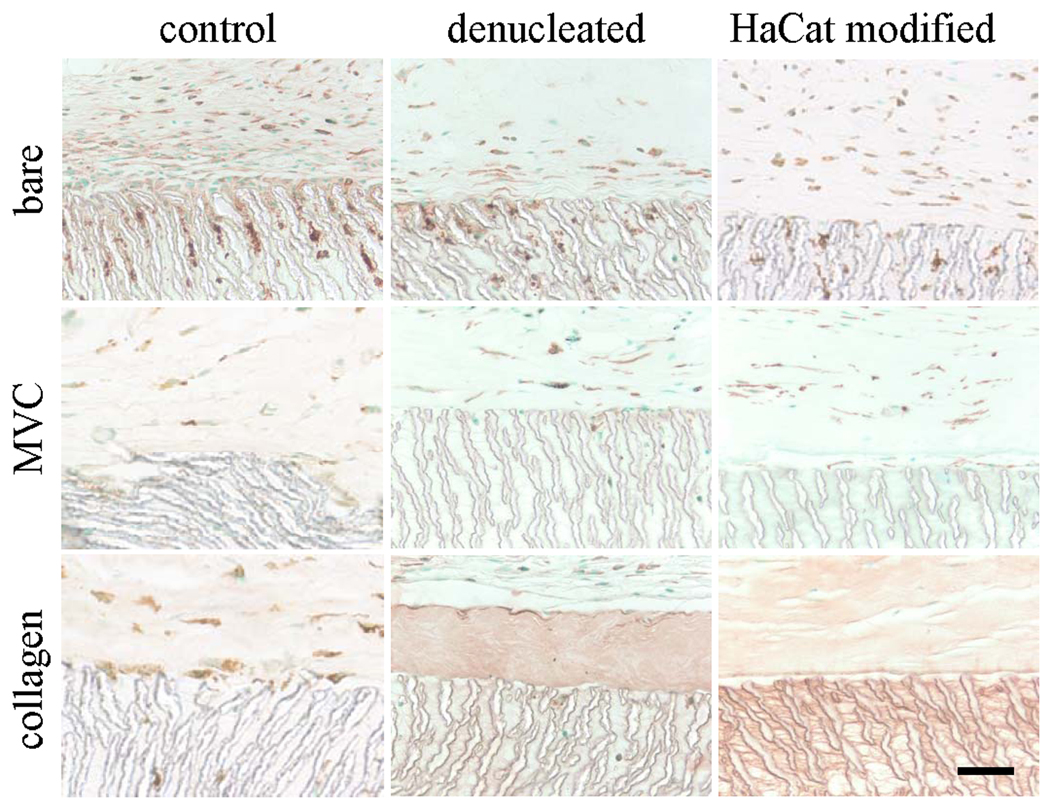
Light micrographs of paraffin sections immunostained for F4/80 to identify activated macrophages. EPTFE discs were implanted for 28 days as is (bare) or embedded in a microvascular construct (MVC) or an avascular collagen gel (collagen). EPTFE discs were used without modification (control), denucleated, or modified with a secreted extracellular matrix preparation (HaCaT modified). Scale bar = 50 microns.
The microvessels associated with the implants are perfusion competent
Vascular ink casting confirmed that the vessels associated with the implants were perfusion-competent 28 days after implantation (Fig. 5). Patent vessel networks were present in peri-implant tissue of bare and MVC-associated ePTFE implants (Fig. 5 A – E). In contrast, ink-filled vessels were observed in very limited regions in ePTFE implants combined with collagen gels and, when present, were primarily associated with the periphery of the implant (Fig. 5 F). The microvasculature surrounding ePTFE implants with microvascular constructs were organized into a typical tree structure with conduit vessels that gradually branch into smaller vessels (Fig. 5 A – E). Furthermore, ink-filled vessels appeared to have penetrated into the material (Fig. 5 A – E). By starting at the surface of the polymer and focusing down stepwise, at a higher magnification, through the sample, it’s clear that perfusion-competent (i.e. ink-filled) vessels do extend into the ePTFE interstices (Fig. 6). Given that these vessels are filled with ink, they are likely not dead-ended. The occasional appearances of ink-filled loops that extend into the material space suggest that the interstitial vessels do not extend further, through the material but may loop down into the material and then back out to the surface through which they entered (Figs. 5 and 6).
Figure 5.
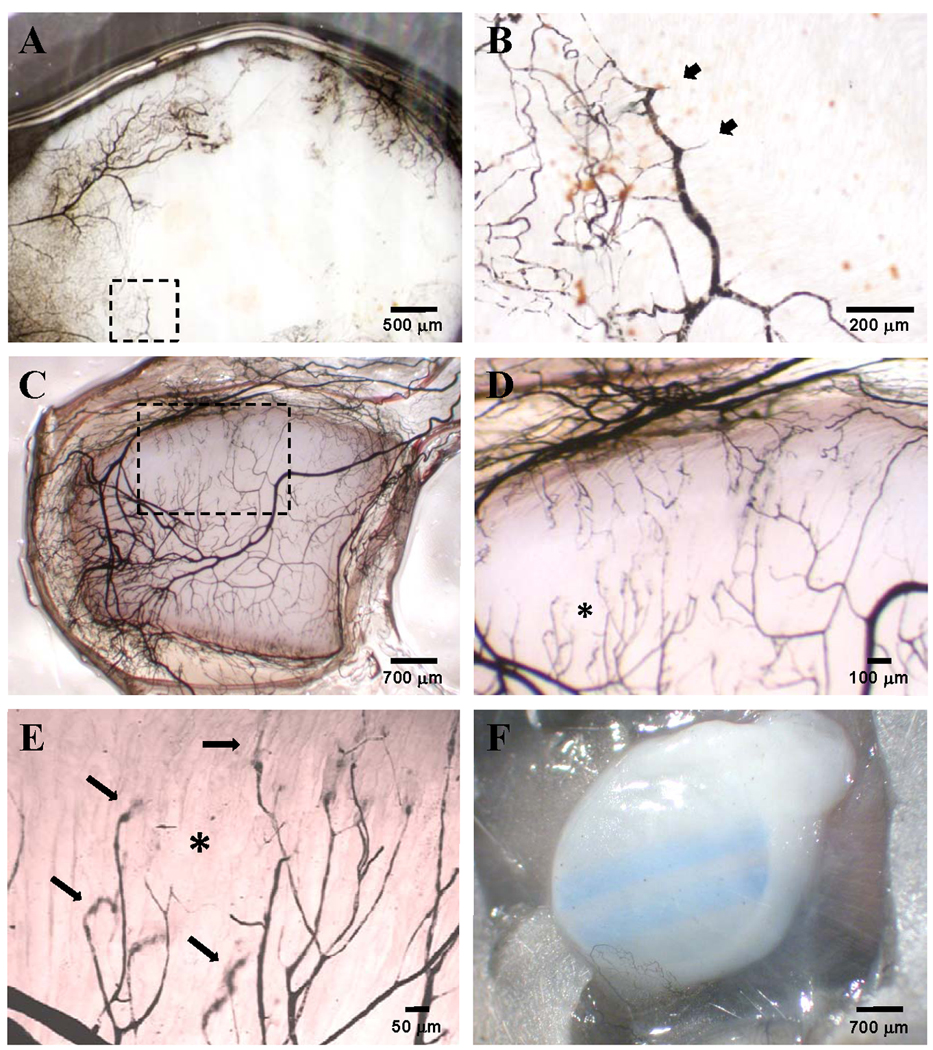
Vascular India ink casts of ePTFE discs implanted for 28 days used to visualize the perfusion-competent microvessel network in control bare ePTFE (A, B), control ePTFE embedded in a microvascular construct (C–E), or control ePTFE embedded in collagen alone (F). Panel B, a higher magnification of the area marked by the dotted box in panel A, shows short vessel branches that superficially enter the ePTFE interstices (arrow heads). The dotted box in panel C marks the area magnified and shown in panel D. The asterisk in panel D marks the area further magnified to better visualize perfused vessel ends plunging into the ePTFE interstices (arrows). The parallel blue stripes in panel F are markings printed onto the ePTFE by the manufacturer which are visible through the collagen gel.
Figure 6.
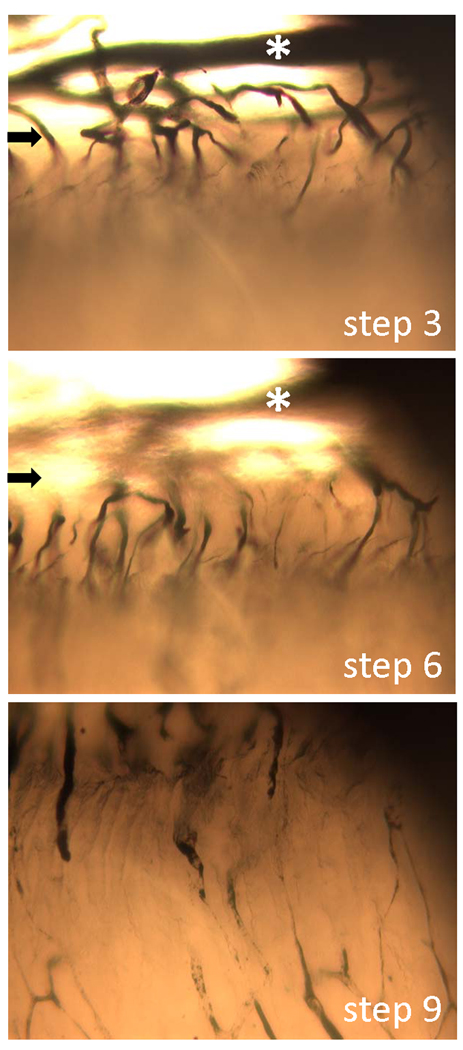
Three select micrographs from a Z-step series of an explanted, ink-casted ePTFE-microvascular construct implant (day 28) showing the penetration of perfusion-competent microvessels into the polymer. Micrographs were taken at 10 different focal planes from a slightly tilted, en face perspective. Focal planes (steps) 3, 6 and 9 are shown. The arrows mark the polymer edge while the white asterisk denotes a fiduciary vessel in the peri-implant tissue.
DISCUSSION
The long-term function of many implanted polymeric devices is compromised by the progressive development of avascular, fibrotic tissue around the implant. Maintaining a functional vasculature around the implant is thought to be a way of reducing fibrosis. Subcutaneous implantation of a microvascular construct (MVC) results in the formation of a new, perfusion-competent microcirculation 19. Furthermore, when placed on compromised tissue, a microvascular construct can serve as a means to bypass ischemic zones and preserve tissue function 20. Similarly, we have used a microvascular construct to establish a microcirculation immediately adjacent to an implanted material. The microcirculation that forms is perfusion competent, exhibits a good hierarchy of microvessel elements (i.e. large vessels progressing to small vessel progressing to large vessels), and is in close association with the material. Indeed, there were instances where perfused vessels of the surrounding microvascular construct penetrated the spaces of the material. In this regard, the use of a MVC in polymer implantation may prove effective in prolonging the function of implanted devices. Therefore, incorporation of a prevascularized collagen construct appears to maintain vascularity in the peri-implant tissue without a large cellular infiltrate, which often precedes fibrosis. In this regard, the use of a MVC in polymer implantation may prove effective in prolonging the function of implanted devices.
The density of vessels within the MVC-dependent peri-implant tissue is comparable to that observed in the pre-fibrotic, granulation tissue adjacent to a bare implant (i.e. implant without a MVC). We did not specifically determine whether or not the microvessels we incorporated into the construct persisted in the MVC-dependent peri-implant tissue. Previous work has shown that greater than 90% of the vessels present within an implanted microvascular construct after 4 weeks were derived from the vessels used to build the MVC 19. Clearly, host vessels do not populate implanted plain collagen gels (i.e. gels lacking incorporated microvessels at the time of implantation) indicating that the collagen matrix of the MVC is not a sufficient stimulus for host vessel in-growth. Therefore, it’s likely that a significant majority of the microvessels present around the polymer implant placed within the MVC are MVC-derived.
A notable difference between the vascular-rich, granulation tissue associated with the bare ePTFE implant and the vascular-rich, peri-implant tissue resulting from the microvascular construct is the relatively high density of macrophages within the polymer spaces in the implants without MVCs or collagen gels. Interestingly, the tissue around the implants that had high macrophage counts within the polymer space also had a high cellular content. Macrophages play a key role in the development of granulation tissue as well as the subsequent progression to an avascular scar by influencing fibroblast activity 7. Whether or not those macrophages specifically associated with the polymer, as observed in the bare implants, are involved in controlling peri-implant cellularity and eventual fibrosis, as these findings suggest, remains to be determined. Regardless, it appears that the presence of the collagen gel layer (with or without prevascularization) around the polymer appears to reduce the number of polymer-associated macrophages and cells within the surrounding tissue. Furthermore, even though the MVC is collagen-rich, it remains a loosely organized, non-compacted tissue with a relatively low density of cells. From this, the presence of the MVC appears to produce a vascularized, non-fibrotic tissue around the implant. Further studies are need to determine the specific roles of the collagen, the incorporated microvessels and the polymer-associated macrophages in MVC-mediate, polymer-associated healing.
Previously, we used a laminin-5-rich extracellular matrix derived from HaCaT cells to modify the surfaces of ePTFE to promote neovascularization and improve implant-associated healing 13. The HaCaT-derived matrix produced a tissue with an elevated vessel density and reduced fibrosity around the implant 13. With the exception of a small increase in macrophages within the polymer space, it appears that the presence of HaCaT-derived extracellular matrix molecules had little effect on the peri-implant tissue formed from the microvascular construct; vessel density and overall tissue organization were not different in MVC-associated implants when the polymer was modified with HaCaT-derived matrix. The absence of any obvious effect on the tissue suggests that the influence of HaCaT-derived matrix in the previous studies likely involved direct cell contact with the matrix-modified surface, something which appears to occur infrequently when a microvascular construct is present.
The general strategy involving the MVC was to create a tissue interface between the host tissue and the polymer. Previous work has shown that the MVC, comprised of isolated, intact microvessel elements assembled into a 3-D collagen gel, integrates with host tissue very effectively 19, 20. We anticipated that this relatively “seamless” integration may facilitate polymer-associated healing by surrounding the polymer with a more compatible tissue interface prior to implantation. Indeed, it appears that the MVC did integrate well with the host tissue/vasculature and the polymer. Unexpectedly, given the relative absence of a cellular infiltrate and polymer-associated macrophage associated with the MVC, it also appears that the MVC masked the implanted polymer from the host tissue. This masking effect may prove to be an added benefit in maintaining polymeric device implantation. Additionally, the MVC used here is a simple tissue construct comprised of microvessel elements and collagen gels. However, the construct itself is amenable to further manipulation and tissue engineering. The vast variety of techniques now available to build and modify tissue and tissue-like environments, many of which are performed in vitro, can be readily applied to the MVC to add complexity and flexibility. In this regard, control of the peri-implant space would not require manipulation of aspects of the material, which might compromise material properties or characteristics. With the MVC as a foundation, it should be possible to engineer a peri-implant environment customized to a device to improve functional capacity or even add new functionalities to the implant system.
ACKNOWLEDGEMENTS
This work was supported by NIH grants #EB007556 (JBH) and #DK078175 (SKW).
Footnotes
No benefit of any kind will be received either directly or indirectly by the author(s).
Reference List
- 1.Anderson JM. Inflammatory Response to Implants. ASAIO Transactions. 1988;34:101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Brauker J, Martinson LA, Hill RS, Young SK, Carr-Brendel VE, Johnson RC. Neovascularization of immunoisolation membranes: the effect of membrane architecture and encapsulated tissue. Transplant Proc. 1992;24:2924. [PubMed] [Google Scholar]
- 3.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29:1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 4.Emmez H, Kardes O, Dogulu F, Kurt G, Memis L, Baykaner MK. Role of antifibrotic cytokine interferon-gamma in the prevention of postlaminectomy peridural fibrosis in rats. Neurosurgery. 2008;62:1351–1357. doi: 10.1227/01.neu.0000333307.02802.04. [DOI] [PubMed] [Google Scholar]
- 5.Gerritsen M. Problems associated with subcutaneously implanted glucose sensors [editorial; comment]. [Review] [26 refs] Diabetes Care. 2000;23:143–145. doi: 10.2337/diacare.23.2.143. [DOI] [PubMed] [Google Scholar]
- 6.Gruionu G, Hoying JB, Pries AR, Secomb TW. Structural remodeling of mouse gracilis artery after chronic alteration in blood supply. Am J Physiol Heart Circ Physiol. 2005;288:H2047–H2054. doi: 10.1152/ajpheart.00496.2004. [DOI] [PubMed] [Google Scholar]
- 7.Hering TM, Suzuki Y, Anderson JM. Collagen type distribution in healing of synthetic arterial prostheses. Connect Tissue Res. 1986;15:141–154. doi: 10.3109/03008208609167139. [DOI] [PubMed] [Google Scholar]
- 8.Hetrick EM, Prichard HL, Klitzman B, Schoenfisch MH. Reduced foreign body response at nitric oxide-releasing subcutaneous implants. Biomaterials. 2007;28:4571–4580. doi: 10.1016/j.biomaterials.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jay SM, Saltzman WM. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J Control Release. 2009;134:26–34. doi: 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidd KR, Dal Ponte DB, Kellar RS, Williams SK. A comparative evaluation of the tissue responses associated with polymeric implants in the rat and mouse. J Biomed Mater Res. 2002;59:682–689. doi: 10.1002/jbm.10032. [DOI] [PubMed] [Google Scholar]
- 11.Kidd KR, Dal PD, Stone AL, Hoying JB, Nagle RB, Williams SK. Stimulated endothelial cell adhesion and angiogenesis with laminin-5 modification of expanded polytetrafluoroethylene. Tissue Eng. 2005;11:1379–1391. doi: 10.1089/ten.2005.11.1379. [DOI] [PubMed] [Google Scholar]
- 12.Kidd KR, Nagle RB, Williams SK. Angiogenesis and neovascularization associated with extracellular matrix-modified porous implants. J Biomed Mater Res. 2002;59:366–377. doi: 10.1002/jbm.1253. [DOI] [PubMed] [Google Scholar]
- 13.Kidd KR, Williams SK. Laminin-5-enriched extracellular matrix accelerates angiogenesis and neovascularization in association with ePTFE. J Biomed Mater Res A. 2004;69:294–304. doi: 10.1002/jbm.a.20133. [DOI] [PubMed] [Google Scholar]
- 14.Ono M, Torisu H, Fukushi J, Nishie A, Kuwano M. Biological implications of macrophage infiltration in human tumor angiogenesis. Cancer Chemother Pharmacol. 1999;43 Suppl:S69–S71. doi: 10.1007/s002800051101. S69–S71. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-de-Erenchun R, Dotor de las HJ, Hontanilla B. Use of the transforming growth factor-beta1 inhibitor peptide in periprosthetic capsular fibrosis: experimental model with tetraglycerol dipalmitate. Plast Reconstr Surg. 2005;116:1370–1378. doi: 10.1097/01.prs.0000181694.07661.0d. [DOI] [PubMed] [Google Scholar]
- 16.Salzmann DL, Kleinert LB, Berman SS, Williams SK. Inflammation and Neovascularization Associated with Clinically Used Vascular Prosthetic Materials. Cardiovascular Pathology. 1999;7:63–71. doi: 10.1016/s1054-8807(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 17.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. II. Plasma-tissue exchange properties. Journal of Biomedical Materials Research. 1998;40:586–597. doi: 10.1002/(sici)1097-4636(19980615)40:4<586::aid-jbm10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd BR, Chen HY, Smith CM, Gruionu G, Williams SK, Hoying JB. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler Thromb Vasc Biol. 2004;24:898–904. doi: 10.1161/01.ATV.0000124103.86943.1e. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd BR, Chen HY, Smith CM, Gruionu G, Williams SK, Hoying JB. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler Thromb Vasc Biol. 2004;24:898–904. doi: 10.1161/01.ATV.0000124103.86943.1e. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd BR, Hoying JB, Williams SK. Microvascular transplantation after acute myocardial infarction. Tissue Eng. 2007;13:2871–2879. doi: 10.1089/ten.2007.0025. [DOI] [PubMed] [Google Scholar]
- 21.Updike SJ, Shults MC, Gilligan BJ, Rhodes RK. A subcutaneous glucose sensor with improved longevity, dynamic range, and stability of calibration [see comments] Diabetes Care. 2000;23:208–214. doi: 10.2337/diacare.23.2.208. [DOI] [PubMed] [Google Scholar]
- 22.Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, DiPietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008;88:579–590. doi: 10.1038/labinvest.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams SK, Berman SS, Kleinert LB. Differential healing and neovascularization of ePTFE implants in subcutaneous versus adipose tissue. J Biomed Mater Res. 1997;35:473–481. doi: 10.1002/(sici)1097-4636(19970615)35:4<473::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Zhou M, Liu Z, Wei Z, Liu C, Qiao T, Ran F, Bai Y, Jiang X, Ding Y. Development and validation of small-diameter vascular tissue from a decellularized scaffold coated with heparin and vascular endothelial growth factor. Artif Organs. 2009;33:230–239. doi: 10.1111/j.1525-1594.2009.00713.x. [DOI] [PubMed] [Google Scholar]


