Abstract
Progression of cancer from the earliest event of cell transformation through stages of tumor growth and metastasis at a distal site involves many complex biological processes. Underlying the numerous responses of cancer cells to the tumor microenvironment which support their survival, migration and metastasis are transcription factors that regulate the expression of genes reflecting properties of the tumor cell. A number of transcription factors have been identified that play key roles in promoting oncogenesis, tumor growth, metastasis and tissue destruction. Relevant to solid tumors and leukemias, tissue specific transcription factors that are deregulated resulting from mutations, being silenced or aberrantly expressed, have been well characterized. These are the master transcription factors of the Runx family of genes, the focus of this review, with emphasis placed on Runx2 that is abnormally expressed at very high levels in cancer cell lines that are metastatic to bone. Recent evidence has identified a correlation of Runx2 levels in advanced stages of prostate and breast cancer and demonstrated that effective depletion of Runx2 by RNA interference inhibits migration and invasive properties of the cells prevents metastatic bone disease. This striking effect is consistent with the broad spectrum of Runx2 properties in activating many genes in tumor cells that have already been established as indicators of bone metastasis in poor prognosis. Potential strategies to translate these findings for therapeutic applications are discussed.
Keywords: Runx/AML/Cbfa1 factors, Runx2, microRNA, breast and prostate tumors in bone, osteolytic disease, subnuclear targeting, Smads
Introduction
Initiation of the tumor cell phenotype, tumor growth and its metastasis to distal sites progresses through many stages involving responses of the tumor cell to systemic factors and the local tissue environment [1]. Each stage of tumor progression involves transcriptional regulation/deregulation with the activation of genes and secreted proteins that drive the proliferative and invasive properties of tumor cells. The earliest event is the epithelialmesenchymal transition mediated by well characterized transcription factors (e.g. HLH Factors, Twist, Snail) and signaling pathways (Notch, Wnt, TGF/BMP, Src) that result in a population of cells designated as tumor-initiating cells [2]. The developing tumor cells acquire properties that are increasingly mesenchymal-like and produce proteins that are components of the bone ECM. For more than a decade, the association of tumor cell expressed sibling proteins, integrins and matrix metalloproteinases have been correlated with bone metastasis. The discovery that the Runx2 transcription factor which controls bone development, is expressed in metastatic breast tumors, was a key finding in understanding the regulation of genes associated with metastatic bone disease. An overwhelming number of Runx2 gene targets are expressed at different stages along the osteoblast lineage; and together with the numerous Runx2 protein-protein interactions with chromatin remodeling factors, mediators of developmental signaling pathways (TGF/BMP, Hox, Wnt, Src, PTHrP) and tumor suppressor proteins, Runx2 has a broad spectrum of phenotype control of a cell. The functional characterization of abnormal and highly expressed levels of Runx2 in metastatic breast and prostate cancer cell lines emphasizes the significance of a master skeletal transcription factor in potentiating tumor cell progression and metastatic bone disease.
1. Runx Transcription Factors and Cancer
The Runx transcription factors (Runx1, Runx2, and Runx3) are essential for organogenesis and regulate phenotypic genes through successive cell divisions determining cell cycle progression or exit in progeny cells. Runx factors not only control lineage commitment and cell proliferation by regulating genes transcribed by RNA Pol II, but also acts as a repressor of RNA Pol I mediated ribosomal RNA (rRNA) synthesis by functional association with ribosomal genes that reside in large discrete foci at nucleolar organizing regions of metaphase chromosomes and in the nucleoli of interphase cells [3]. These Runx chromosomal foci are associated with open chromatin and undergo transition into nucleoli at sites of rRNA synthesis during interphase [3, 4]. Enlarged and increased numbers of nucleoli are hallmarks of the tumor cells and the abnormal expression levels of Runx factors in cancer cells and associated with multiple nucleoli implicates Runx factors in deregulated activities of the transformed phenotype [5].
Mutations in Runx genes have been linked to several types of cancer [6]. Runx1 is essential for definitive hematopoiesis; however, numerous translocations with other genes result in a spectrum of leukemias [7]. Runx3 contributes to gut and neural development and functional inactivation of RUNX3 through mutation, epigenetic silencing, or cytoplasmic mislocalization is highly correlated with gastric cancer(s) [8]. Runx2 is a key factor for bone formation [9-11] and is associated with osteosarcoma [12, 13]. The oncogenic potential of Runx2 was first indicated from T-cell lymphoma induced by retroviral insertion, synergism between Runx2 and c-Myc [6]. The finding of Runx2 expression in highly metastatic tumor cell lines and aggressive tumor growth in bone further implicated Runx2 activities in promoting tumor properties [14-16]. In normal bone development, Runx2 contributes to regulation of the balance between bone formation and bone resorption through repression and activation of genes that control osteoclast and osteoblast activity. A large number of Runx2 target genes essential for normal bone formation, are also documented to promote tumor growth and invasion [17, 18].
2. Runx2 function appears critical at early and late stages of tumor progression
The mechanism(s) by which each of the Runx factors switch their properties from a tumor suppressor role promoting differentiation to normal cell phenotypes to functioning as proteins with oncogenic properties in tumor cells are not clear [6, 19-21]. While Runx1 and Runx3 mutations are linked with leukemia and gastric cancer, respectively [6, 8] as established by at least a decade of research, Runx2 association with the cancer progression is relatively recent. We find in normal prostate and breast glandular tissue, Runx1 is expressed in the epithelial lining cells analogous to its expression in periosteum and perichondrium [22, 23], suggesting a critical function in supporting the phenotype of these cells. It is intriguing to postulate that EMT events may lead to deregulated expression of Runx1 and Runx2 in the resulting tumorigenic cell.
Initial structural alterations characterizing breast cancer include loss of cell polarization and luminal filling of mammary glands [24, 25]. In normal mammary epithelial cells Runx2 is expressed at low levels and activates differentiation genes such as β-casein [26]. Ectopic Runx2 expression in normal mammary epithelial cells, induces several key cancer-related genes (Bcl-2, and IL8) and disorganizes acinar architecture to resemble a tumorigenic phenotype in the 3D culture model [16]. Depletion of endogenous Runx2 or expression of a dominant negative or point mutations that prevents fidelity of Runx2 intranuclear localization in highly aggressive metastatic breast cancer cells, reverts cancer cell aggregates into more normal acini-like structures in vitro. The significance of these in vitro studies was confirmed by limited tumor growth in the mammary fat when Runx2 was silenced in the MDA-MB-231 cells [16]. A recent study demonstrated a potential role for Runx2 in early phases of breast cancer by showing a positive association between nuclear Runx2 and estrogen-progesterone receptor gene expression in a small percentage of cells in human tissue samples and thus identifies a biological subtype of breast cancer [27]. These studies suggest that Runx2 not only promotes metastatic properties of cancer cells, but also can initiate tumorigenic properties in normal mammary epithelial cells.
Recent studies suggest possible mechanisms of Runx2 mediated early survival of tumor cells by directly activating survivin expression in prostate cancer cells [28]. Survivin is highly expressed in a range of human tumors and correlates with both accelerated relapse and chemotherapy resistance [29]. These findings indicate that Runx2 negatively affect the apoptotic pathway (by activating Bcl2 and survivin) leading to enhanced survival of tumor cells. Studies have also shown that Runx2 levels increased with breast and prostate tumor growth and are linked to protections of cancer cells against apoptosis by bone morphogenetic protein 7 (BMP7) [30-32]. For example, prostate tumors in the conditional Pten-knockout mouse exhibited higher Runx2 levels during prostate cancer progression, as did BMP7 [30]. The effects of BMP7 are complex being inhibitory to prostate cancer in the normal epithelial cell, but positively correlated with prostate metastasis and tumor growth in bone [30]. The prostate cell line C4-2B which produces osteoblastic lesions express both BMP7 and Runx2 at high levels [33]. This raises interesting possibilities of cross talk between BMP7-Smads and Runx2-Smad transcriptional pathways in prostate cancer progression, as Runx2 has a well established interaction with Smads [34]. Together, these observations support a novel role of Runx2 in regulating survivin expression in malignant epithelial cells and identifying Runx2 as a potential critical factor in BMP7 signaling that prevents prostate cancer cells from undergoing apoptosis.
Evidence for Runx2 involvement in the later metastatic process included the characterization of the bone-related matrix proteins (osteopontin, osteocalcin, bone sialoprotein and other sibling proteins) [35], vascular endothelial growth factor [36], TGFβ1R [37] and matrix metalloproteinases [35, 38, 39]. Each of these proteins is associated with stages of tumor progression and metastatic events [17]. Importantly, several Runx response elements are present in these genes identified in bone-seeking metastatic cancer cells. Runx2 is also responsive to integrin signaling [40] and the receptor integrins beta subunits are highly expressed in breast and prostate cancer cells [41, 42]. These secreted proteins regulate key cellular processes implicated in cancer progression by increasing the mobility and invasive properties of cancer cells.
A direct functional role for Runx2 in metastatic cell lines was provided by the intratibial model of bone metastasis. In these studies, the highly aggressive MDA-MB-231 cell line was modified with stably integrated mutant Runx2 proteins (dominant negative or a point mutation) or expression of shRNA-Runx2 [15, 43, 44]. Blocking Runx2 function either by expressing mutant Runx2 proteins or depletion of Runx2 decreases expression of metastatic and osteolytic genes, in vitro invasive properties of MDA-MB-231 breast and PC3 prostate cancer cells and inhibits bone osteolytic properties in vivo[43, 44]. These findings suggest that Runx2 function is obligatory for expression of target genes that mediate the osteolytic activity of metastatic breast cancer cells. These events are summarized in Figure 1. Tissue arrays that include biopsy samples of tumors at stages of progression of breast and prostate cancer have detected robust Runx 2 levels at more advanced stages by immunohistochemical studies [27, 44].
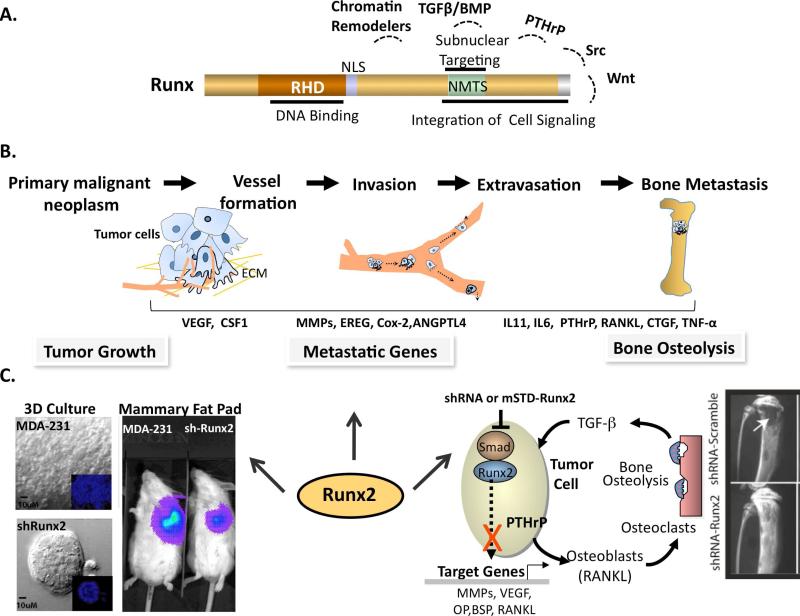
Figure 1. Central role of Runx2 in tumor growth and metastatic bone disease.
A: Domain organization of Runx proteins. DBD: DNA binding domain; NLS: nuclear localization signal; NMTS: nuclear matrix targeting signal is responsible for Runx protein sub-nuclear localization. The C-terminal interacts with chromatin remodelers including p300/CBP, HDACs and HATs and mediators of cell signaling pathways like TGFβ/BMP, Src, PTHrP, and Wnt and integrates extracellular responses for Runx function in various cell types.
B: Sequence of metastatic process where primary tumor attracts blood vessels, followed by migration and invasion of tumor cells through the lymphatic or hematogenous circulation and finally colonization of disseminated cells to distal organs such as bone. Among several genes shown to be activated during these events of metastatic process, are Runx target genes.
C: In vivo evidence for Runx2 mediating tumor progression. Left panels: Runx2 knockdown (shRunx2) in bone metastatic MDA-MB-231 breast cancer cells show acini like structure after Runx2 depletion compared to parental cells control which form disorganized structure in 3D culture model. Runx2 knockdown (shRunx2) reduces tumor growth under mammary fat pads as shown by in vivo bioimaging of firefly luciferase labeled MDA-MB-231 cells. Right panel highlights the role of Runx2 in bone osteolytic disease associated with breast cancer metastasis. Breast cancer cells secrete PTHrP in response to TGFβ in the bone microenvironment, which further promotes activation of osteoclasts and causes osteolysis. Expression of shRunx2 or subnuclear targeting deficient mutants (mSTD-Runx2) in metastatic breast cancer cells inhibits osteolysis as shown in intratibial model of tumor growth by abrogating its interaction with co-regulatory proteins.
3. Runx2 Activities Promoting Bone Resorption at the Metastatic Site
Runx2 promotes breast and prostate tumor growth and associated osteolytic lesions in the bone microenvironment, in part through direct transcriptional activation of genes that promote bone degradation, MMP9, MMP13 and other MMPs [39, 44]. Understanding of the mechanism by which Runx2 participates in metastatic bone disease is indicated by the studies where Runx2 regulation of key components of the “vicious cycle” of tumor growth and bone resorption was investigated [43]. This cycle involves overproduction of PTHrP by breast cancercells that has a profound effect on tumor cell activities and survival and, when present in the bone microenvironment, results in osteoclastic bone resorption [1, 45]. The resorbed bone releases TGFβ-stimulating tumor cell proliferation and consequently increased PTHrP secretion, thus continuing the vicious cycle. Furthermore, PTHrP is regulated by Gli, a Hedgehog signaling factor, and this pathway leads to pathologic consequences in a variety of human tumors [46]. It was recently established thatRunx2 regulates TGFβ-mediated activation of PTHrP through interaction with Hedgehog signaling molecule Gli2 [43]. Runx2 binds to the Indian Hedgehog (IHH) promoter and activates its expression in cancer cells. This regulation further increases PTHrP levels, resulting in operation of the vicious cycle in cancer cells. Runx2 directly contributes to the osteolytic process by regulating the IHH-PTHrP pathway in breast cancer cells that leads to osteoclastogenesis in vivo. Importantly, Runx2 was shown to be required for TGFβ-mediated activation of cyclin D1 in breast cancer cells [43]. Thus Runx2 further impacts on the osteolytic vicious cycle as illustrated in Figure 1. Taken together, these studies suggest that metastatic cancer cells, by having higher levels of Runx2, are able to activate components of the vicious cycle and target genes that increase bone loss and promote tumor progression in bone, resulting in the metastatic bone disease.
Prostate cancer cells often metastasize to bone where osteolytic, osteoblastic or mixed lesions are formed [47]. The presence of Runx2 in prostate cancer tissues and bone metastatic cell lines is positively correlated to advanced stages of prostate cancer, in adenocarcinomas and metastatic tumors, as shown by human tissue microarray studies of prostate tumors at stages of cancer progression [44, 48]. Negligible Runx2 is found in normal prostate epithelial and nonmetastatic LNCaP prostate cancer cells. Our recent studies in three sublines of prostate cancer cells indicate that Runx2 is highly expressed in subclones of osteolytic PC3 bone metastatic cells. In osteolytic PC3 prostate cancer cells, endogenous elevation of Runx2 levels and diminished p57 protein levels are associated with faster proliferation in vitro and development of larger tumors in bone [49]. In the intra-tibial metastasis model, high Runx2 levels in PC3 cells are associated with development of large tumors, increased expression of metastasis-related genes (MMP9, MMP13, VEGF, Osteopontin) and secreted bone-resorbing factors (RANKL, PTHrP, IL8) promoting osteolytic disease [44]. These studies further identified the mechanisms of Runx2 function and show that PC3 cells promote osteoclastogenesis and inhibit osteoblast activity.
Of further significance, Runx2 siRNA treatment in PC3 prostate cells or MDA-MB-231 breast cancer decreases cell migration and invasion through Matrigel in vitro, and in vivo shRunx2 stable expression cells blocked the ability of these tumor cell lines to survive in the bone microenvironment ([43, 44] and see Fig. 1). The specific mechanisms for this striking effect of Runx2 depletion needs further elaboration, but the observation supports the concept that an osteoblast master transcription factor, having many biological activities, contributes to tumor cell properties in the bone microenvironment.
4. Runx2 Related Molecular Mechanisms in Tumor Cells
Runx family members form co-regulatory complexes with co-activator and co-repressor proteins that include chromatin remodeling factors, pathways and nuclear hormone receptors in both normal and tumor cells. These complexes are organized in unique subnuclear domains to regulate gene transcription. Runx is targeted to the nuclear scaffold and recruits co-regulators to these sites as a mechanism to provide cell and gene type specificity in response to signaling cascades and other regulators of tissue specifications [20]. The known pathways for which there is direct evidence of Runx involvement and which are relevant to the progression of breast and prostate tumor responses and associated metastatic bone disease include TGFβ/BMP [45], Wnt [50-52], PTHrP [45] and Src pathways [53]. Src signaling, which involves non-receptor tyrosine kinases and WW domain proteins, is a key component of osteoclastic resorption and is a strong stimulus of tumor growth. YAP is an intracellular mediator of Src signaling that shuttles to the nucleus, forms a transcriptional complex with Runx2 [54]. Inhibition of Src signaling is highly effective against bone metastatic tumor growth [55-57].
The protein-protein interactions of Runx with BMP and TGFβ/Smads are well documented in regulating normal cell differentiation (Runx1, hematopoietic cells; Runx2, osteoblasts) [58, 59]. Loss of the Runx1-Smad interaction in Runx1 translocations in leukemia is a significant contributing factor to loss of the normal differentiation pathway of the hematopoietic cells dependent on TGFβ [60]. Runx3, which has potent antiproliferative and proapoptotic effects through mediating TGFβ/Smad activities in gut epithelial cells, is deregulated in gastric cancer due to silencing of Runx3 or other loss-of-function of Runx3 [8, 61]. Runx2-Smad interactions have a critical role in bone formation mediating BMP and TGFβ effects [34, 62]. In breast and prostate tumor cells Runx2-Smad complexes form activating target genes that promote tumor growth and osteolytic disease.
Runx2 also responds to canonical Wnt signaling, a pathway particularly enhanced in prostate tumors [50-52]. In normal mesenchymal stem cells and osteoprogenitors, Runx2 expression is activated by canonical Wnt /β-Catenin signaling, while in mature osteoblasts the TCF/LEF transcriptional target of Wnts forms a co-regulatory complex with Runx2 to attenuate genes, such as osteocalcin [63, 64]. Several studies show stimulated canonical Wnt signaling mediates osteoblastic lesions produced by PCa 2b and C4-2B cell lines, both of which are associated with Runx2 /Cbfa1. Interestingly, the osteolytic cell line PC3 is characterized by high level of the Wnt inhibitor DDK1, proposed to be the indictor of osteoblastic versus osteolytic lesions induced by prostate cancer cells [65, 66], as well as associated with breast cancer cell mediated the osteolytic lesions [67, 68]. How Runx2-interfaces with Wnt signaling in breast and prostate tumor cells to mediate the metastatic bone disease, is not clear. It is likely that both dependent and independent pathways are operative.
Runx2 coregulatory protein interactions include nuclear hormone receptors as another mechanism for regulating tumor cell activities. The androgen receptor binds to Runx2 and abrogates its binding to DNA [69, 70]. In the PCa LNCaP cells, Runx2 stimulates androgen receptor (AR) responsive expression of the prostate-specific marker PSA [49]. Runx factors cooperate with an Ets transcription factor to regulate PSA gene expression through 4 RUNX sites in the PSA gene regulatory region [71]. Studies also show that Runx2 is mechanistically linked to TGFβ and androgen responsive pathways that support prostate cancer cell growth [49]. These distinct observations indicate that Runx2 and AR are integrated at several levels to regulate gene expression by DNA binding activity, protein-protein interactions, and through TGFβ signaling. In breast cancer cells, in the presence of estradiol, estrogen receptor alpha interacts with Runx2 and suppresses its transcriptional activity [72]. What is not yet understood is how repression of Runx2 by AR and ER affects tumor cell activities in response to the bone microenvironment.
Mechanisms underlying the upstream global changes in chromatin structure and related gene expression during metastasis progression are poorly understood. Studies in normal osteoblasts suggest that Runx2 regulates differentiation through chromatin remodeling by interacting with homeobox and Hox proteins [73-76] and similar control might exist in cancer cells as these transcription factors are also implicated in cancer progression [77-80]. Alsoexpressed in aggressive metastatic breast cancer cells and tumors is SATB1, a genome organizer that anchors multiple genomic loci and recruits chromatin-remodeling enzymes to regulate chromatin structure and downstream gene expression. The knockdown of SATB1 in MDA-MB-231breast cancer cells altered the expression of >1,000 genes and blocks tumorigenesis by restoring glandular structure-like acinar polarity and inhibits tumor growth and metastasis in vivo [81]. Interestingly, Runx2 is one of the important SATB1 regulated genomic loci shown in breast cancer cells in this study.
The interactions of Runx2 with histone acetyl transferases, as p300 and histone deacetylases (HDACs) is an important component of Runx2 regulation of gene activation and repression of specific target genes at different stages of skeletal development [82]. It is likely that in cancer cells Runx2-HDAC interactions, as well as Runx2 interactions that promote acetylation of histone to increase target gene transcription, alters the landscape of expressed genes in a tumor cells compared to a normal cell. Genetic deletions of different HDACs in the skeleton of mice have shown either anabolic or catabolic effects on bone. HDAC inhibitors (as trichostatin A) promote osteoblast differentiation in vitro [83], reduce osteoclastogenesis in vitro [84] and have beneficial effects in vivo by inhibiting inflammatory arthritis [85]. Studies using HDAC inhibitors clinically (vorinostat) are very effective in reducing tumor volume [86], but their effects on the skeleton are not fully realized.
5. Translational Approaches Based on Unique Properties of Runx2
A unique feature of Runx proteins is a common protein module in the C-terminus, designated the nuclear matrix targeting signal (NMTS). Runx proteins are targeted and reside in subnuclear foci within the nucleus where they recruit co-regulatory proteins and form complexes with co-activator and co-repressor proteins, as well as with nuclear hormone receptors in breast and prostate tumor cells on gene promoters. This is an elegant mechanism for adapting tissue specificity of gene regulation [20]. Each Runx family member is dependent on this module for normal functions. Striking evidence include the loss of this C-terminal module in Runx1 due to translocations with other genes causes leukemias; and deletion of the Runx2 C-terminus prevents bone formation and results in embryonic lethality equivalent to a null phenotype in mice [11]. Disruption of Runx2 subnuclear targeting with expression of point mutants of nuclear matrix targeting signal (NMTS) or deletion of C-terminal containing NMTS in bone metastatic breast cancer cells showed a significant loss of its transcriptional activity, loss of invasive potential and inhibition in associated bone osteolytic disease in the intratibial mouse model of tumor growth [14, 15]. Understanding of subnuclear association of Runx2 in metastatic cancer cells will not only identify novel mechanisms of bone metastatic disease but will also provide opportunity for developing targeted therapies as small molecule inhibition of the subnuclear targeting domain responsible for transcriptional activity.
The inhibition of osteolytic bone disease by inactivation of Runx2 using RNA interference approaches in bone metastatic and prostate breast cancer cells again provides a strong basis for developing Runx2 as a therapeutic target to inhibit tumor metastasis [16, 43, 44]. Tumor growth was reduced by MDA-MB-231 with shRNARunx2 in the fat pad and in breast and prostate bone tumors with inhibition of osteolytic disease and loss of activation of metastatic genes. These in vivo studies in which Runx2 was completely depleted by an shRNA approach, clearly demonstrate the feasibility of RNA interference for developing specific therapies for bone metastasis.
Another approach to preventing tumor growth by targeting multiple pathways at the same time is through microRNA control. Evidence is accumulating regarding a key role of microRNAs in cancer progression and bone metastasis [87]. Recent study shows that systemic treatment of tumor-bearing mice with miR-10b antagomirs specifically suppresses breast cancer metastasis to lungs but not the primary mammary tumor growth [88] and systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors in bone via downregulation of multiple cell-cycle genes [89]. miRNAs inhibiting breast cancer metastasis were also identified like miR-31, whose expression correlates inversely with metastasis in human breast cancer patients [90]. Recently, miR-200 family and miR-9 have been shown as a suppressor and promoter of metastasis cascade by influencing epithelial-mesenchymal transition respectively [91, 92].
We have performed miRNA profiling in metastatic breast cancer cell lines and leukemic cell lines where Runx levels were modulated and identified several potential Runx regulated miRNAs ([93] and our unpublished results). We find that both Runx1 and the t(8;21)-encoded AML1-ETO occupy the miR-24-23-27 locus with Runx1 decreasing and AML-ETO increasing transcription of miR-24. Expression of miR-24 stimulates myeloid cell growth, renders proliferation independent of interleukin-3, and blocks granulocytic differentiation [93]. Thus, a miR-24 inhibitor (antagomir) could have potential in reversing or reducing the AML-ETO phenotype. Several studies have validated miRs targeting Runx2 in bone cells [94, 95], but it is not known if these miRs are present in tumor cells. Nonetheless, as a future therapeutic approach for inhibiting bone metastasis, high protein levels of Runx2 in metastatic cancer cells could effectively be reduced by expression of miRs that directly bind to the Runx2 3’ UTR to inhibit translation.
Closing remarks
Treating cancer cells, tumor growth and metastasis is a complex process, and combinatorial approaches offer promise where conventional strategies have been ineffective. In summary, mounting evidence supports the role of Runx factors in contributing to oncogenesis by multiple mechanisms that include silencing, mutations or aberrant expression levels of Runx in tumor cells. A direct role of Runx2 in control of tumor metastasis to bone is consistent with a spectrum of genes in metastatic cancer cells that are linked to mobility, invasiveness and angiogenesis. Just as microRNAs (miRNAs) attenuate gene expression to regulate biological processes and involve the targeting of many genes that affect the responsiveness of tumor cells, Runx factor regulation converges on many target genes and integrates transcriptional control with signaling pathways that promote tumor growth and metastatic bone disease. The potential to capture this opportunity for targeting Runx2 as an approach to treat tumors can amplify 1) therapeutic effectiveness through repression of multiple gene targets, and 2) the different parameters of biological responses used by tumor cells for their survival, growth and metastasis.
Acknowledgements
This review was supported in part by NIH grants P01CA082834 (to G.S.S.), R37DE012528 (to J.B.L), and R03CA123599 (J.P.). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health. We thank the investigators carrying out the studies in our Cancer Biology program, John Wixted, Christopher Dowdy, Ricardo Medina, Sadiq Hussain, Jennifer Colby, S. Kaleem Zaidi, Jason Dobbs, Jacqueline Akech and our colleagues and collaborators for their valuable contributions and stimulating discussions: Drs. Janet Stein (UMass Med Sch), Andre van Wijnen (UMass Med Sch), Jennifer Westendorf (Mayo Clinic), Lucia Languino (UMass Med Sch), Dario Altieri (UMass Med Sch), Carlo Croce (Ohio State Univ), Rami Aqeilan (Ohio State Univ and The Hebrew University-Hadassah Med Sch) and Yoshiaki Ito (National Univ of Singapore).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 2.Creighton CJ, Chang JC, Rosen JM. Epithelial-Mesenchymal Transition (EMT) in Tumor-Initiating Cells and Its Clinical Implications in Breast Cancer. J Mammary Gland Biol Neoplasia. 2010 doi: 10.1007/s10911-010-9173-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Young DW, Hassan MQ, Pratap J, Galindo M, Zaidi SK, Lee SH, et al. Mitotic occupancy and lineage-specific transcriptional control of rRNA genes by Runx2. Nature. 2007;445:442–6. doi: 10.1038/nature05473. [DOI] [PubMed] [Google Scholar]
- 4.Ali SA, Zaidi SK, Dacwag CS, Salma N, Young DW, Shakoori AR, et al. Phenotypic transcription factors epigenetically mediate cell growth control. Proc Natl Acad Sci U S A. 2008;105:6632–7. doi: 10.1073/pnas.0800970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakshi R, Zaidi SK, Pande S, Hassan MQ, Young DW, Montecino M, et al. The leukemogenic t(8;21) fusion protein AML1-ETO controls ribosomal RNA genes and associates with nucleaolar organizing regions at mitotic chromosomes. J Cell Sci. 2008;21:3981–90. doi: 10.1242/jcs.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blyth K, Cameron ER, Neil JC. The runx genes: gain or loss of function in cancer. Nat Rev Cancer. 2005;5:376–87. doi: 10.1038/nrc1607. [DOI] [PubMed] [Google Scholar]
- 7.Osato M. Point mutations in the RUNX1/AML1 gene: another actor in RUNX leukemia. Oncogene. 2004;23:4284–96. doi: 10.1038/sj.onc.1207779. [DOI] [PubMed] [Google Scholar]
- 8.Chuang LS, Ito Y. RUNX3 is multifunctional in carcinogenesis of multiple solid tumors. Oncogene. 2010 doi: 10.1038/onc.2010.88. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 10.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–71. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 11.Choi J-Y, Pratap J, Javed A, Zaidi SK, Xing L, Balint E, et al. Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc Natl Acad Sci , USA. 2001;98:8650–5. doi: 10.1073/pnas.151236498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas DM, Johnson SA, Sims NA, Trivett MK, Slavin JL, Rubin BP, et al. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biol. 2004;167:925–34. doi: 10.1083/jcb.200409187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan SS, Pereira BP, Zhou YF, Gupta A, Dombrowski C, Soong R, et al. Elevated expression of Runx2 as a key parameter in the etiology of osteosarcoma. Mol Biol Rep. 2009;36:153–8. doi: 10.1007/s11033-008-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes GL, Hebert KE, Kamal M, Javed A, Einhorn TA, Lian JB, et al. Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases associated osteolytic disease. Cancer Res. 2004;64:4506–13. doi: 10.1158/0008-5472.CAN-03-3851. [DOI] [PubMed] [Google Scholar]
- 15.Javed A, Barnes GL, Pratap J, Antkowiak T, Gerstenfeld LC, van Wijnen AJ, et al. Impaired intranuclear trafficking of Runx2 (AML3/CBFA1) transcription factors in breast cancer cells inhibits osteolysis in vivo. Proc Natl Acad Sci , USA. 2005;102:1454–9. doi: 10.1073/pnas.0409121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pratap J, Imbalzano K, Underwood J, Cohet N, Gokul KD, Akech J, et al. Ectopic Runx2 expression in mammary epithelial cells disrupts formation of normal acini structure: Implications for breast cancer progression. Cancer Res. 2009;69:6807–14. doi: 10.1158/0008-5472.CAN-09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratap J, Lian JB, Javed A, Barnes GL, van Wijnen AJ, Stein JL, et al. Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev. 2006;25:589–600. doi: 10.1007/s10555-006-9032-0. [DOI] [PubMed] [Google Scholar]
- 18.Casimiro S, Guise TA, Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Mol Cell Endocrinol. 2009;310:71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Zaidi SK, Pande S, Pratap J, Gaur T, Grigoriu S, Ali SA, et al. Runx2 deficiency and defective subnuclear targeting bypass senescence to promote immortalization and tumorigenic potential. Proc Natl Acad Sci U S A. 2007;104:19861–6. doi: 10.1073/pnas.0709650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidi SK, Young DW, Javed A, Pratap J, Montecino M, van WA, et al. Nuclear microenvironments in biological control and cancer. Nat Rev Cancer. 2007;7:454–63. doi: 10.1038/nrc2149. [DOI] [PubMed] [Google Scholar]
- 21.Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, et al. Cell growth regulatory role of Runx2 during proliferative expansion of pre-osteoblasts. Cancer Res. 2003;63:5357–62. [PubMed] [Google Scholar]
- 22.Lian JB, Balint E, Javed A, Drissi H, Vitti R, Quinlan EJ, et al. Runx1/AML1 hematopoietic transcription factor contributes to skeletal development in vivo. J Cell Physiol. 2003;196:301–11. doi: 10.1002/jcp.10316. [DOI] [PubMed] [Google Scholar]
- 23.Smith N, Dong Y, Lian JB, Pratap J, Kingsley PD, van Wijnen AJ, et al. Overlapping expression of Runx1(Cbfa2) and Runx2(Cbfa1) transcription factors supports cooperative induction of skeletal development. J Cell Pysiol. 2005;203:133–43. doi: 10.1002/jcp.20210. [DOI] [PubMed] [Google Scholar]
- 24.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–88. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 25.Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757–1763s. [PubMed] [Google Scholar]
- 26.Inman CK, Li N, Shore P. Oct-1 counteracts autoinhibition of Runx2 DNA binding to form a novel Runx2/Oct-1 complex on the promoter of the mammary gland-specific gene beta-casein. Mol Cell Biol. 2005;25:3182–93. doi: 10.1128/MCB.25.8.3182-3193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das K, Leong DT, Gupta A, Shen L, Putti T, Stein GS, et al. Positive association between nuclear Runx2 and oestrogen-progesterone receptor gene expression characterises a biological subtype of breast cancer. Eur J Cancer. 2009;45:2239–48. doi: 10.1016/j.ejca.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim M, Zhong C, Yang S, Bell AM, Cohen MB, Roy-Burman P. Runx2 regulates survivin expression in prostate cancer cells. Lab Invest. 2010;90:222–33. doi: 10.1038/labinvest.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 30.Morrissey C, Brown LG, Pitts TE, Vessella RL, Corey E. Bone morphogenetic protein 7 is expressed in prostate cancer metastases and its effects on prostate tumor cells depend on cell phenotype and the tumor microenvironment. Neoplasia. 2010;12:192–205. doi: 10.1593/neo.91836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S, Lim M, Pham LK, Kendall SE, Reddi AH, Altieri DC, et al. Bone morphogenetic protein 7 protects prostate cancer cells from stress-induced apoptosis via both Smad and c-Jun NH2-terminal kinase pathways. Cancer Res. 2006;66:4285–90. doi: 10.1158/0008-5472.CAN-05-4456. [DOI] [PubMed] [Google Scholar]
- 32.Alarmo EL, Parssinen J, Ketolainen JM, Savinainen K, Karhu R, Kallioniemi A. BMP7 influences proliferation, migration, and invasion of breast cancer cells. Cancer Lett. 2009;275:35–43. doi: 10.1016/j.canlet.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Lin DL, Tarnowski CP, Zhang J, Dai J, Rohn E, Patel AH, et al. Bone metastatic LNCaP-derivative C4-2B prostate cancer cell line mineralizes in vitro. Prostate. 2001;47:212–21. doi: 10.1002/pros.1065. [DOI] [PubMed] [Google Scholar]
- 34.Javed A, Bae JS, Afzal F, Gutierrez S, Pratap J, Zaidi SK, et al. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem. 2008;283:8412–22. doi: 10.1074/jbc.M705578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8:212–26. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zelzer E, Glotzer DJ, Hartmann C, Thomas D, Fukai N, Soker S, et al. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech Dev. 2001;106:97–106. doi: 10.1016/s0925-4773(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 37.Ji C, Casinghino S, Chang DJ, Chen Y, Javed A, Ito Y, et al. CBFa(AML/PEBP2)-related elements in the TGF-beta type I receptor promoter and expression with osteoblast differentiation. J Cell Biochem. 1998;69:353–63. [PubMed] [Google Scholar]
- 38.Nannuru KC, Futakuchi M, Varney ML, Vincent TM, Marcusson EG, Singh RK. Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-beta signaling at the tumor-bone interface. Cancer Res. 2010;70:3494–504. doi: 10.1158/0008-5472.CAN-09-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratap J, Javed A, Languino LR, van Wijnen AJ, Stein JL, Stein GS, et al. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol. 2005;25:8581–91. doi: 10.1128/MCB.25.19.8581-8591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franceschi RT, Xiao G, Jiang D, Gopalakrishnan R, Yang S, Reith E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect Tissue Res. 2003;44(Suppl 1):109–16. [PMC free article] [PubMed] [Google Scholar]
- 41.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clezardin P. Integrins in bone metastasis formation and potential therapeutic implications. Curr Cancer Drug Targets. 2009;9:801–6. doi: 10.2174/156800909789760348. [DOI] [PubMed] [Google Scholar]
- 43.Pratap J, Wixted JJ, Gaur T, Zaidi SK, Dobson J, Gokul KD, et al. Runx2 transcriptional activation of Indian hedgehog and a downstream bone metastatic pathway in breast cancer cells. Cancer Res. 2008;68:7795–802. doi: 10.1158/0008-5472.CAN-08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, et al. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. 2010;29:811–21. doi: 10.1038/onc.2009.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clines GA, Guise TA. Molecular mechanisms and treatment of bone metastasis. Expert Rev Mol Med. 2008;10:e7. doi: 10.1017/S1462399408000616. [DOI] [PubMed] [Google Scholar]
- 46.Sterling JA, Oyajobi BO, Grubbs B, Padalecki SS, Munoz SA, Gupta A, et al. The hedgehog signaling molecule Gli2 induces parathyroid hormone-related peptide expression and osteolysis in metastatic human breast cancer cells. Cancer Res. 2006;66:7548–53. doi: 10.1158/0008-5472.CAN-06-0452. [DOI] [PubMed] [Google Scholar]
- 47.Roudier MP, Morrissey C, True LD, Higano CS, Vessella RL, Ott SM. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol. 2008;180:1154–60. doi: 10.1016/j.juro.2008.04.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brubaker KD, Vessella RL, Brown LG, Corey E. Prostate cancer expression of runt-domain transcription factor Runx2, a key regulator of osteoblast differentiation and function. Prostate. 2003;56:13–22. doi: 10.1002/pros.10233. [DOI] [PubMed] [Google Scholar]
- 49.van der Deen M, Akech J, Wang T, FitzGerald TJ, Altieri DC, Languino LR, et al. The cancer-related Runx2 protein enhances cell growth and responses to andogen and TGFβ in prostate cancer cells. J Cell Biochem. 2010;109:828–37. doi: 10.1002/jcb.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson DR, Zylstra CR, Williams BO. Wnt signaling and prostate cancer. Curr Drug Targets. 2008;9:571–80. doi: 10.2174/138945008784911831. [DOI] [PubMed] [Google Scholar]
- 51.Emami KH, Corey E. When prostate cancer meets bone: control by wnts. Cancer Lett. 2007;253:170–9. doi: 10.1016/j.canlet.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 52.Hall CL, Keller ET. The role of Wnts in bone metastases. Cancer Metastasis Rev. 2006;25:551–8. doi: 10.1007/s10555-006-9022-2. [DOI] [PubMed] [Google Scholar]
- 53.Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223:14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 54.Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL, et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23:790–9. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabbani SA, Valentino ML, Arakelian A, Ali S, Boschelli F. SKI-606 (Bosutinib) Blocks Prostate Cancer Invasion, Growth, and Metastasis In vitro and In vivo through Regulation of Genes Involved in Cancer Growth and Skeletal Metastasis. Mol Cancer Ther. 2010 doi: 10.1158/1535-7163.MCT-09-0962. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Araujo J, Logothetis C. Dasatinib: A potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010 doi: 10.1016/j.ctrv.2010.02.015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saad F, Lipton A. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev. 2010;36:177–84. doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Pouliot F, Blais A, Labrie C. Overexpression of a dominant negative type II bone morphogenetic protein receptor inhibits the growth of human breast cancer cells. Cancer Res. 2003;63:277–81. [PubMed] [Google Scholar]
- 59.Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-beta superfamily and Runx proteins. Oncogene. 2004;23:4232–7. doi: 10.1038/sj.onc.1207131. [DOI] [PubMed] [Google Scholar]
- 60.Ford AM, Palmi C, Bueno C, Hong D, Cardus P, Knight D, et al. The TEL-AML1 leukemia fusion gene dysregulates the TGF-beta pathway in early B lineage progenitor cells. J Clin Invest. 2009;119:826–36. doi: 10.1172/JCI36428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ito Y, Miyazono K. RUNX transcription factors as key targets of TGF-beta superfamily signaling. Curr Opin Genet Dev. 2003;13:43–7. doi: 10.1016/s0959-437x(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 62.Lee KS, Hong SH, Bae SC. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene. 2002;21:7156–63. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- 63.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PVN, Komm BS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating RUNX2 gene expression. J Biol Chem. 2005;280:33132–40. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 64.Kahler RA, Galindo M, Lian J, Stein GS, van Wijnen AJ, Westendorf JJ. Lymphocyte enhancer-binding factor 1 (Lef1) inhibits terminal differentiation of osteoblasts. J Cell Biochem. 2006;97:969–83. doi: 10.1002/jcb.20702. [DOI] [PubMed] [Google Scholar]
- 65.Li ZG, Yang J, Vazquez ES, Rose D, Vakar-Lopez F, Mathew P, et al. Low-density lipoprotein receptor-related protein 5 (LRP5) mediates the prostate cancer-induced formation of new bone. Oncogene. 2008;27:596–603. doi: 10.1038/sj.onc.1210694. [DOI] [PubMed] [Google Scholar]
- 66.Yang J, Fizazi K, Peleg S, Sikes CR, Raymond AK, Jamal N, et al. Prostate cancer cells induce osteoblast differentiation through a Cbfa1-dependent pathway. Cancer Res. 2001;61:5652–9. [PubMed] [Google Scholar]
- 67.Dai J, Hall CL, Escara-Wilke J, Mizokami A, Keller JM, Keller ET. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008;68:5785–94. doi: 10.1158/0008-5472.CAN-07-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bu G, Lu W, Liu CC, Selander K, Yoneda T, Hall C, et al. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int J Cancer. 2008;123:1034–42. doi: 10.1002/ijc.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baniwal SK, Khalid O, Sir D, Buchanan G, Coetzee GA, Frenkel B. Repression of Runx2 by androgen receptor (AR) in osteoblasts and prostate cancer cells: AR binds Runx2 and abrogates its recruitment to DNA. Mol Endocrinol. 2009;23:1203–14. doi: 10.1210/me.2008-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu XH, Kirschenbaum A, Yao S, Liu G, Aaronson SA, Levine AC. Androgen-induced Wnt signaling in preosteoblasts promotes the growth of MDA-PCa-2b human prostate cancer cells. Cancer Res. 2007;67:5747–53. doi: 10.1158/0008-5472.CAN-07-0478. [DOI] [PubMed] [Google Scholar]
- 71.Fowler M, Borazanci E, McGhee L, Pylant SW, Williams BJ, Glass J, et al. RUNX1 (AML-1) and RUNX2 (AML-3) cooperate with prostate-derived Ets factor to activate transcription from the PSA upstream regulatory region. J Cell Biochem. 2006;97:1–17. doi: 10.1002/jcb.20664. [DOI] [PubMed] [Google Scholar]
- 72.Khalid O, Baniwal SK, Purcell DJ, Leclerc N, Gabet Y, Stallcup MR, et al. Modulation of Runx2 activity by estrogen receptor-alpha: implications for osteoporosis and breast cancer. Endocrinology. 2008;149:5984–95. doi: 10.1210/en.2008-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hassan MQ, Saini S, Gordon JA, van Wijnen AJ, Montecino M, Stein JL, et al. Molecular switches involving homeodomain proteins, HOXA10 and RUNX2 regulate osteoblastogenesis. Cells Tissues Organs. 2009;189:122–5. doi: 10.1159/000151453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hassan MQ, Tare RS, Lee S, Mandeville M, Morasso MI, Javed A, et al. BMP2 commitment to the osteogenic lineage involves activation of Runx2 by Dlx3 and a homeodomain transcriptional network. J Biol Chem. 2006;281:40515–26. doi: 10.1074/jbc.M604508200. [DOI] [PubMed] [Google Scholar]
- 75.Hassan MQ, Tare R, Lee SH, Mandeville M, Weiner B, Montecino M, et al. HOXA10 controls osteoblastogenesis by directly activating bone regulatory and phenotypic genes. Mol Cell Biol. 2007;27:3337–52. doi: 10.1128/MCB.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gordon JA, Hassan MQ, Saini S, Montecino M, van Wijnen AJ, Stein GS, et al. Pbx1 represses osteoblastogenesis by blocking Hoxa10-mediated recruitment of chromatin remodeling factors. Mol Cell Biol. 2010 doi: 10.1128/MCB.00889-09. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barnes GL, Javed A, Waller SM, Kamal MH, Hebert KE, Hassan MQ, et al. Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 2003;63:2631–7. [PubMed] [Google Scholar]
- 78.Ruhin-Poncet B, Ghoul-Mazgar S, Hotton D, Capron F, Jaafoura MH, Goubin G, et al. Msx and dlx homeogene expression in epithelial odontogenic tumors. J Histochem Cytochem. 2009;57:69–78. doi: 10.1369/jhc.2008.951707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H, Lindsey S, Konieczna I, Bei L, Horvath E, Huang W, et al. Constitutively active SHP2 cooperates with HoxA10 overexpression to induce acute myeloid leukemia. J Biol Chem. 2009;284:2549–67. doi: 10.1074/jbc.M804704200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ko SY, Lengyel E, Naora H. The Mullerian HOXA10 gene promotes growth of ovarian surface epithelial cells by stimulating epithelial-stromal interactions. Mol Cell Endocrinol. 2010;317:112–9. doi: 10.1016/j.mce.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han HJ, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452:187–93. doi: 10.1038/nature06781. [DOI] [PubMed] [Google Scholar]
- 82.Westendorf JJ. Histone deacetylases in control of skeletogenesis. J Cell Biochem. 2007;102:332–40. doi: 10.1002/jcb.21486. [DOI] [PubMed] [Google Scholar]
- 83.Schroeder TM, Westendorf JJ. Histone deacetylase inhibitors promote osteoblast maturation. J Bone Miner Res. 2005;20:2254–63. doi: 10.1359/JBMR.050813. [DOI] [PubMed] [Google Scholar]
- 84.Kim HN, Ha H, Lee JH, Jung K, Yang D, Woo KM, et al. Trichostatin A inhibits osteoclastogenesis and bone resorption by suppressing the induction of c-Fos by RANKL. Eur J Pharmacol. 2009;623:22–9. doi: 10.1016/j.ejphar.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 85.Grabiec AM, Krausz S, de JW, Burakowski T, Groot D, Sanders ME, et al. Histone deacetylase inhibitors suppress inflammatory activation of rheumatoid arthritis patient synovial macrophages and tissue. J Immunol. 2010;184:2718–28. doi: 10.4049/jimmunol.0901467. [DOI] [PubMed] [Google Scholar]
- 86.Ma X, Ezzeldin HH, Diasio RB. Histone deacetylase inhibitors: current status and overview of recent clinical trials. Drugs. 2009;69:1911–34. doi: 10.2165/11315680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 87.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–14. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341–7. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther. 2010;18:181–7. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC, et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–46. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Korpal M, Kang Y. The emerging role of miR-200 family of microRNAs in epithelialmesenchymal transition and cancer metastasis. RNA Biol. 2008;5:115–9. doi: 10.4161/rna.5.3.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khew-Goodall Y, Goodall GJ. Myc-modulated miR-9 makes more metastases. Nat Cell Biol. 2010;12:209–11. doi: 10.1038/ncb0310-209. [DOI] [PubMed] [Google Scholar]
- 93.Zaidi SK, Dowdy CR, van Wijnen AJ, Lian JB, Raza A, Stein JL, et al. Altered Runx1 subnuclear targeting enhances myeloid cell proliferation and blocks differentiation by activating a miR-24/MKP-7/MAPK network. Cancer Res. 2009;69:8249–55. doi: 10.1158/0008-5472.CAN-09-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, et al. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci USA. 2008;105:13906–11. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–64. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]