Abstract
Sleep disruption is an important aspect of major depressive disorder but lacks an objective and inexpensive means of assessment. We evaluated the utility of electrocardiogram (ECG)-based cardiopulmonary coupling analysis to quantify physiologic sleep stability in patients with major depression. Relative to controls, unmedicated depressed patients had a reduction in high-frequency coupling, an index of stable sleep, an increase in low-frequency coupling, an index of unstable sleep, and an increase in very-low-frequency coupling, an index of wakefulness/REM sleep. The medicated depressed group showed a restoration of stable sleep to a level comparable with that of the control group. ECG-based cardiopulmonary coupling analysis may provide a simple, cost-efficient point-of-care method to quantify sleep quality/stability and to objectively evaluate the severity of insomnia in patients with major depression.
Descriptors: depression, insomnia, sleep stability, cyclic alternating pattern, cardiopulmonary coupling analysis, electrocardiogram-derived sleep spectrogram
Introduction
Insomnia, characterized by a perception of poor sleep quality, is a common symptom comorbid with major depression and other mental illness (Sateia & Nowell, 2004). However, unlike other sleep disorders, such as sleep apnea, in which the nature and severity of the illness are quantifiable, the assessment of insomnia is often subjective and is largely based on reports of perceived sleep quality. Currently, objective assessments of sleep physiology rely primarily on polysomnography (Buysse, ncoli-Israel, Edinger, Lichstein, & Morin, 2006). Sleep staging, a key component of polysomnography, is based on arbitrary criteria that relate to the amplitude and morphology of electroencephalographic (EEG) signals (Iber, 2007). The limitations of applying conventional EEG staging to assess insomnia include the findings that 1) EEG staging is poorly correlated with perceived sleep quality (Armitage, Trivedi, Hoffmann, & Rush, 1997; Saletu, 1975), 2) benzodiazepines may reduce “deep” sleep (i.e., decreased delta power in EEG signals) but still improve sleep continuity and subjective sleep quality (Achermann & Borbely, 1987), and 3) the fact that the majority of adult sleep is characterized by second-stage non-REM sleep.
A complementary approach used to quantify insomnia is the assessment of sleep “stability,” originally implemented with an EEG morphological marker that has been termed the cyclic alternating pattern (CAP) (Ferre, Guilleminault, & Lopes, 2006; Terzano, et al., 1985; Terzano & Parrino, 2000). CAP is a state of phasic EEG activity associated with microarousals during sleep and is, therefore, considered to be an index of sleep instability. Increased EEG-CAP during sleep has been found in patients with major depression and in those with primary insomnia. (Farina, et al., 2003; Lopes, Quera-Salva, & Guilleminault, 2007; Terzano, et al., 2003).
Recently, we demonstrated that the presence of EEG-CAP during sleep is associated with coupled modulations in respiratory and autonomic functions, thus raising the possibility of utilizing a continuous electrocardiographic (ECG) signal alone to quantify sleep stability (Thomas, Mietus, Peng, & Goldberger, 2005). This novel method, termed cardiopulmonary coupling (CPC) analysis, has been developed to measure sleep quality and to detect and phenotype sleep apnea based solely on the continuous ECG signal (Thomas, et al., 2007; Thomas, Mietus, Peng, & Goldberger, 2005). Improved sleep stability has also been quantified by CPC analysis in patients with heart failure undergoing a Tai Chi exercise program (Yeh, et al., 2008).
Since depression is associated with reduced sleep stability, as detected by the presence of increased EEG-CAP, and EEG-CAP is correlated with an alteration in autonomic function, an unstable, fragmented sleep pattern may be detectable by CPC analysis in depressed individuals. Consequently, in the present study, we tested the following two hypotheses: 1) stable sleep, as quantified by ECG-derived CPC analysis, is reduced in major depression, and 2) excessive physiologic sleep instability in depression is reversed by the use of hypnotics. We tested these hypotheses by employing the CPC method to investigate and quantify physiologic sleep stability in a group of depressed patients with comorbid insomnia who were either medication-free or were using hypnotics, and in a group of healthy controls.
Methods
Participants and clinical assessment
One hundred Han-Chinese patients with major depressive disorder (63% female, aged 42.9 ± 10.7 years), as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, were recruited from the Taipei Veterans General Hospital, Taiwan. All subjects gave informed consent before commencement of the study. The protocol was approved by the institutional review board of the Taipei Veterans General Hospital (Taipei, Taiwan). Patient inclusion criteria were: 1) age of 20–65 years; 2) presence of a major depressive episode in either acute or early remission phase; 3) subjective complaints of difficulties initiating or maintaining sleep for a minimum of three nights per week associated with the current depressive episode. Patients were excluded if they met the following criteria: 1) remission of depression for over two months; 2) presence of major psychiatric disorders (except personality disorders) other than depression; 3) cardiac arrhythmia, including continuous atrial or ventricular bigeminy, atrial fibrillation or atrial flutter; 4) severe or acute medical illness within 3 months before the study; 5) other confounding medical conditions, including chronic pain, alcohol/substance abuse or dependence, pregnancy, sleep apnea, and systemic diseases (hypertension and diabetes mellitus), that are known to affect sleep or autonomic function. Moreover, to minimize the confounding effects of medication, patients were also excluded if they if they used medications which have known effects on autonomic function: anti-cholinergic medication, tricyclic antidepressants with anti-cholinergic effects, and beta-blockers.
Psychiatric diagnoses based on DSM-IV criteria were determined by a consensus of at least two psychiatrists. Depression severity was evaluated by the self-reported Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and the psychiatrist-rated Hamilton Depression Rating Scale (HAM-D, 17 items) (Hamilton, 1960). Subjective sleep quality was assessed by the Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) and subjective sleepiness by the Epworth Sleepiness Scale (ESS) (Johns, 1991).
Ninety-one age- and sex-matched healthy control subjects (63% female, aged 42.1 ± 12.6 years) were recruited during the same period using identical assessment procedures. Neither the control subjects nor their first-degree relatives had a history of mental illness. The control subjects reported no use of hypnotics in the past month and had no clinically significant insomnia as evaluated by a psychiatrist.
Continuous ECG monitoring
Holter recordings (MyECG E3-80 Portable Recorder, Microstar Inc., Taipei, Taiwan) were used to obtain continuous 24 hour ECG data. All healthy control subjects and 77% (n=77) of the depressed patients received ECG monitoring at home; the other depressed individuals (n=23; 23%) received ECG monitoring at an acute psychiatric ward. Participants were asked to maintain their usual daily activities and to avoid smoking and drinking alcoholic beverages when undergoing testing.
Half of the depressed subjects (n=50, 54% female, aged 41.7 ± 11.3 years) agreed to undergo ECG monitoring before initiating medication treatment and, therefore, were medication-free on the night of the ECG evaluation. The remainder of the depressed subjects (n=50, 72% female, aged 44.2 ± 10.0 years) had received short- (n=32), intermediate- (n=25) or long-acting benzodiazepines (n=24) before entering the study, and continued medication treatment during the night of the ECG monitoring. Most of these patients received a combination of short/intermediate- or short/long-acting hypnotics to treat insomnia. Forty-four of the 50 medicated patients who received hypnotics were also treated with antidepressants.
Cardiopulmonary coupling analysis
The autonomic nervous system has predictable characteristics that vary according to sleep depth and type (Dumont, et al., 2004; Kuo, Shaw, Lai, & Yang, 2008). CPC analysis is derived from an estimation of the coupling between the autonomic and respiratory drives, using heart rate and respiratory modulation of QRS amplitude, respectively. This dual information can be extracted from a single channel of ECG (Thomas, Mietus, Peng, & Goldberger, 2005). The ECG-derived respiration signal has been described in detail (Moody, Mark, Bump, & et al., 1986), and is highly correlated with the actual respiration waveforms (Yeragani, Appaya, Seema, Kumar, & Tancer, 2005). The algorithm of CPC analysis involves the following steps: the extraction of heart rate and respiration waveforms from the ECG signal, and a subsequent estimation of the cross-spectral power and coherence between the ECG-derived respiration and the heart rate signals to determine sleep state. The analysis window width is 512 seconds, moving forward in 128-second increments until the entire time series is analyzed.
Three physiological sleep states are derived from CPC analysis; namely, stable, unstable, and REM/wakeful states (Thomas, Mietus, Peng, & Goldberger, 2005). Specifically, stable sleep is associated with high-frequency coupling between the heart rate and the respiration at frequencies of 0.1 to 0.4 Hz, and it is correlated with an EEG non-CAP sleep state. In contrast, unstable sleep is associated with low-frequency coupling between the heart rate and the respiration over a range of 0.01 to 0.1 Hz, and it is correlated with an EEG CAP sleep state. The wakeful state and REM sleep are associated with the presence of very-low-frequency coupling between the heart rate and the respiration below 0.01 Hz correlates. Without the recording of muscle tone, REM sleep is not distinguishable from the wakeful state, and the detection of very-low-frequency coupling may reflect contributions from both states.
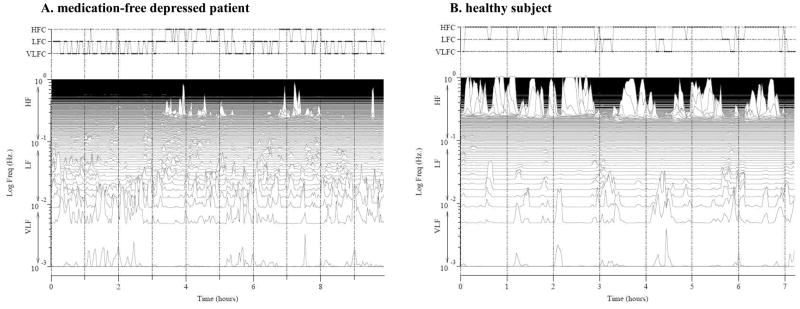
Additional information about sleep and wakefulness times reported by the subject was used to constrain the analysis to the approximate sleep period. The ECG signals were automatically processed and analyzed to generate a sleep spectrogram. These CPC-derived sleep states were then used to assess sleep structure. Figure 1 illustrates the comparison of sleep spectrograms between a healthy control subject and a depressed patient.
Figure 1.
A comparison of ECG-derived sleep spectrograms from a healthy control subject and a patient with depression. Left panel: A 34-year-old depressed female; high-frequency coupling (stable sleep): 21%; low-frequency coupling (unstable sleep): 46%; and very-low-frequency coupling (REM/wakefulness): 28%. Right panel: A 30-year-old healthy female; high-frequency coupling: 76%; low-frequency coupling: 13%; and very-low-frequency coupling: 12%. The spectrographic profiles of the two subjects are visually distinguishable: the depressed individual has fragmented sleep states whereas the healthy subject has more continuous high-frequency coupling sleep.
Statistical analysis
The STATISTICA program (Version 8.0; StatSoft, Inc.) was used for all statistical analyses. A p value of less than .05 (two-tailed) was required for statistical significance. Fisher’s exact test was used to compare categorical variables. Analysis was performed to compare the entire depression group with the healthy subjects and to compare the two depression groups (medicated and unmedicated) with healthy subjects. Between-group comparisons were performed with a one-way analysis of variance (ANOVA). Tukey’s honest significant difference post hoc test was used to test the significance among groups. Pearson’s correlation was applied to determine the associations between CPC sleep indices and scores from sleep/depression questionnaires.
Results
Subjects
Demographic and clinical assessment data for patient and control groups are presented in Table 1. There was no significant difference between the depression and control groups with regard to age and gender. Compared with controls, depressed patients had higher PSQI scores (p<0.001), higher insomnia scores derived from the BDI (p<0.001), and higher insomnia scores derived from the HAM-D (p<0.001). Daytime somnolence measured by the ESS score did not differ between the patient and control groups. Of note, 37% of the control subjects were also classified as having poor sleep (PSQI>5), compared with 93% of the depressed patients.
Table 1.
Demographic and Clinical Characteristics.
| Variable | Patients with Major Depressive Disorder |
Healthy Subjects (N=91) | ||
|---|---|---|---|---|
| Medication-free (N=50) | Hypnotics (N=50) | Total (N=100) | ||
| Age, years | 41.5 ± 12.9 | 44.2 ± 10.0 | 42.9 ± 10.7 | 42.1 ± 12.6 |
| Gender, Male/Female | 23/37 | 14/36 | 37/63 | 34/57 |
| Type of benzodiazepine used | ||||
| Short-acting, N (%) | 32 (64) | 32 (32) | ||
| Intermediate, N (%) | N/A | 25 (50) | 25 (25) | N/A |
| Long-acting, N (%) | 24 (48) | 24 (24) | ||
| Beck Depression Inventory | 20.7 ± 14.9* | 33.3 ± 14.1* | 27.2 ± 15.7* | 6.1 ± 6.6 |
| Hamilton Depression Rating Scale, 17 items | 14.3 ± 8.1* | 16.8 ± 7.9* | 15.6 ± 8.1* | 1.7 ± 2.6 |
| Pittsburgh Sleep Quality Index | 11.8 ± 4.0* | 14.0 ± 3.6* | 13.0 ± 4.0* | 4.7 ± 2.9 |
| Epworth Sleepiness Scale | 9.1 ± 5.9 | 7.2 ± 6.5 | 8.1 ± 6.3 | 9.1 ± 5.1 |
| Insomnia score derived from Beck Depression Inventory (item #16) | 1.5 ± 1.1* | 2.0 ± 0.9* | 1.8 ± 1.1* | 0.4 ± 0.7 |
| Insomnia score derived from Hamilton Depression Rating Scale (item #4–6) | 3.0 ± 1.8* | 3.3 ± 2.0* | 3.1 ± 1.9* | 0.4 ± 0.8 |
N/A: not applicable.
Data represent mean ± 1 standard deviation unless otherwise noted.
Asterisk indicates statistical significance (p<0.05) compared with healthy controls.
Cardiopulmonary coupling analyses
Objective sleep indices derived from the CPC analysis are presented in Table 2. Medication-free depressed patients had a significantly lower percentage of stable sleep compared with healthy subjects (p<0.001), a higher percentage of unstable sleep (p=0.007), and a higher percentage of REM/wakeful state (p<0.001). Furthermore, compared with controls, medication-free depressed patients had a longer latency to the first epoch of stable sleep (p<0.001) and a shorter unstable sleep latency (p=0.02). Both medicated and unmedicated depressed individuals reported significantly more time in bed compared with healthy subjects (both p<0.001). Depressed and control groups did not differ on the REM/wakeful state latency (p=0.91).
Table 2.
Indices Derived From Cardiopulmonary Coupling Analysis
| Variable | Patients with Major Depressive Disorder |
Healthy Subjects (N=91) | ||
|---|---|---|---|---|
| Medication-free (N=50) | Hypnotics (N=50) | Total (N=100) | ||
| Stable sleep (high-frequency coupling), % a | 32.5 ± 12.0* | 48.1 ± 20.0 | 40.3 ± 18.2* | 49.9 ± 18.0 |
| Unstable sleep (low-frequency coupling), % a | 37.5 ± 10.9* | 29.3 ± 18.3 | 33.4 ± 15.6 | 29.5 ± 14.9 |
| REM/wakefulness (very-low-frequency coupling), % a | 28.0 ± 7.1* | 21.2 ± 7.1 | 24.6 ± 7.8* | 19.5 ± 7.4 |
| Stable sleep onset latency, minutes | 31.8 ± 32.3* | 24.0 ± 25.0* | 27.9 ± 29.0* | 14.4 ± 21.6 |
| Unstable sleep onset latency, minutes | 8.8 ± 13.8* | 19.2 ± 26.8 | 14.0 ± 21.8 | 18.9 ± 27.9 |
| REM/wakefulness onset latency, minutes | 19.9 ± 26.5* | 23.8 ± 31.4 | 21.8 ± 28.9 | 21.9 ± 26.9 |
| Time in bed, hours | 7.9 ± 2.1* | 8.2 ± 1.9* | 8.0 ± 2.0* | 6.7 ± 1.3 |
Data represent mean ± 1 standard deviation unless otherwise noted.
Asterisk indicates statistical significance (p<0.05) compared with healthy controls.
Represented as a % of sampling windows during the sleep log estimated sleep period
Correlations between CPC indices and age/questionnaires
When considering all study samples (medicated, unmedicated, and healthy controls), there was no significant correlation between CPC sleep indices and questionnaire scores. To reduce the confounding effect of medication on perceived sleep quality, we analyzed only the correlations between CPC sleep indices and questionnaire scores among the 50 medication-free depressed patients and the 91 healthy control subjects. Stable sleep was significantly correlated with the HAM-D (r=−0.35, p=0.002) and the BDI insomnia score (r=−0.33, p=0.001). REM/wakeful state was significantly correlated with the HAM-D score (r=0.42, p<0.001), the PSQI (r=0.31, p=0.001), the BDI (r=0.31, p=0.001), and the BDI insomnia score (r=0.41, p<0.001). Daytime somnolence, as measured by the ESS, did not correlate with any of CPC sleep indices. There was no significant correlation between age and CPC sleep states.
Effect of hypnotics on CPC sleep indices
Finally, post hoc analyses were conducted to test the differences in CPC sleep indices between the groups of healthy controls and depressed patients (medicated or drug-free). In medicated depressed patients, statistically significant improvements of CPC sleep indices were observed, with increases in stable sleep (p<0.001) and reductions in both unstable sleep (p<0.02) and REM/wakeful states (p<0.001), compared with medication-free patients. Moreover, this restoration was similar to controls in the percentages of stable sleep (p=0.84), unstable sleep (p=0.99), and REM/wakeful states (p=0.38). However, stable sleep latency was not fully restored in medicated depressed patients after receiving hypnotics, compared with controls (p=0.03).
Discussion
The key findings of this study, based on the CPC analysis, include the following: 1) reduced stable sleep and increased unstable sleep and wakeful/REM states were found in depressed patients compared with healthy controls; 2) medicated patients demonstrated partial restoration of stable sleep latency through the use of hypnotics, and 3) certain CPC indices correlated with subjective sleep quality and the severity of depression/insomnia.
The CPC analysis described here complements traditional approaches used to assess sleep stability/quality because it objectively incorporates features of physiological dynamics not accounted for by EEG-based techniques (Armitage, Trivedi, Hoffmann, & Rush, 1997; Tworoger, Davis, Vitiello, Lentz, & McTiernan, 2005). Our findings of decreased stable sleep and increased unstable sleep and REM/wakeful states in depressed individuals are consistent with well-known features of altered EEG sleep structures in major depression, namely an increase in sleep state fragmentation, a reduction in slow wave sleep, and an increase in REM pressure (Armitage, 1995; Germain, Nofzinger, Kupfer, & Buysse, 2004; Jindal, et al., 2002; Thase, Fasiczka, Berman, Simons, & Reynolds, 1998). Taken together, the results indicate that insomnia in depression may be not only a “brain” symptom but also a systemic phenomenon that represents inter-linked physiological processes, including autonomic, respiratory and electrocortical functions (Thomas, 2007). Moreover, the CPC indices were associated with subjective sleep quality and the severity of depression, particularly the stable sleep and REM/wakeful components. These findings may enhance the utility of this ECG-based method for evaluating insomnia in depressed patients.
Benzodiazepine hypnotics can reduce EEG-CAP states (Ozone, et al., 2008; Parrino, Boselli, Spaggiari, Smerieri, & Terzano, 1997; Terzano, et al., 1995) and, thus, presumably reduce the physiological unstable sleep state. The use of hypnotics may, therefore, produce “masking” effects that could make depressed patients appear to have more stable sleep than they might have without medication. In the present study, despite the restoration of the amount of stable sleep, medicated patients still showed a longer latency to stable sleep than healthy control subjects. However, due to ethical considerations regarding drug regimen modification solely for research purposes, the type and dosage of medication was not rigorously controlled in the present study. Thus, the results of the medicated group may need to be confirmed by future prospective, randomized trials.
Reduced high-frequency cardiopulmonary coupling and depression
The mechanisms of stable sleep reduction, as measured by high-frequency coupling in depressed patients, are unclear; however, they may open a new window into the pathophysiology of insomnia in depression. Our prior results suggest that stable sleep is associated with healthy conditions (Thomas, Mietus, Peng, & Goldberger, 2005). The findings of reduced stable sleep and increased unstable and REM/wakeful state in depression are in line with excessive wakefulness-promoting influences found in depressed patients. These changes could reflect hyperarousal from increased activity in the hypothalamo-pituitary-adrenal axis, enhanced central negative affective processing, or perhaps a personality trait that increases the vulnerability to depression (Adrien, 2002). Higher order autonomic control is mediated by the anterior cingulate, the ventromedial prefrontal cortex, the amygdala and the insular cortex. Altered activity within this network is well known in depression (Bae, et al., 2006; Drevets, Price, & Furey, 2008; van Eijndhoven, et al., 2008). These brain areas are also involved in the regulation of respiratory rate and heart rhythm and may be well positioned to impact the CPC analysis, which reflects the sleep-modulated interaction between heart rate and respiratory dynamics (Liotti, et al., 2001; von Leupoldt, et al., 2008).
Relationship of CPC-derived sleep indices and conventional spectral heart rate variability
Although the CPC analysis involves the measure of autonomic and respiratory drives, the spectrographic coupling metrics are distinct from conventional heart rate variability (HRV) spectral analysis in that CPC analysis incorporates both respiration and heart rate signals and measures the extent of coupling between them. A previous report has suggested that the CPC analysis can detect improved sleep stability in patients with heart failure undergoing Tai-Chi exercise, whereas this improvement was undetectable by conventional HRV techniques (Yeh, et al., 2008). In the present analysis, we also found generally low correlations (r-square < 0.15) between CPC-derived sleep indices and spectral HRV measures (see Appendix Table S1), suggesting an independent role for CPC analysis in the quantification of sleep physiology.
Depression and the risk of cardiovascular disease
An emerging body of research suggests that nocturnal autonomic function, measured by heart rate variability, is altered in mood/anxiety disorders and is associated with poor sleep quality (Brosschot, Van Dijk, & Thayer, 2007; Irwin, Valladares, Motivala, Thayer, & Ehlers, 2006; Takahara, et al., 2008). The results of this study are in line with prior reports of increased sympathetic and reduced vagal tone in depression (Nahshoni, et al., 2004; Udupa, et al., 2007; Yeragani, et al., 2002), as the parasympathetic modulation is associated with physiological stable sleep state measured by high-frequency coupling between heart rate and respiration (Thomas, Mietus, Peng, & Goldberger, 2005). Reduced vagal and heightened sympathetic activity are known to be associated with an increased risk of cardiovascular diseases and cardiovascular mortality (Carney, et al., 2001; Carney & Freedland, 2003; Stein, et al., 2000). Our finding of reduced high-frequency coupling sleep in depressed patients may indicate a long-term risk factor for adverse cardiovascular events.
Limitations
The present study has a number of limitations. First, polysomnography was not performed; thus, the exact correlations with conventional sleep indices cannot be made in this population. Second, the detection of ECG-based stable and unstable sleep states reflects only an approximation of EEG non-CAP and CAP states, respectively (Thomas, Mietus, Peng, & Goldberger, 2005). Third, the role of REM sleep is not assessed in this study, and REM sleep may be attenuated in medicated depressed patients. The difficulty with differentiation of REM sleep from the wakeful state could possibly be addressed if muscle tone recordings are integrated into the algorithm. The addition of actigraphy, which is a simple and widely accepted tool used to assess sleep/wakeful states, may complement the CPC method and strengthen its value by better defining the sleep period. While actigraphy has better ability to estimate total sleep time and circadian sleep distribution, the CPC analysis is better at characterizing the quality of sleep.
Conclusions
In conclusion, the present study suggests that depressed individuals have disrupted sleep stability and continuity, as quantified by ECG-based CPC analysis. Despite the lack of comparative data from standard polysomnography, our study nevertheless provides a unique viewpoint on sleep stability in the context of cardiovascular physiology. This readily repeatable ECG-based method could provide a simple and objective way to evaluate insomnia in depression, and possibly track treatment effects.
Supplementary Material
Acknowledgments
The authors wish to thank Shan-Ing Chen and Chen-Ru Wang for their excellent technical assistance. This work was supported by the National Science Council of Taiwan (NSC 95-2314-B-075-111), Taipei Veterans General Hospital (V96C1-083, V97C1-132, V97F-005), the G. Harold and Leila Y. Mathers Foundation, the James S. McDonnell Foundation, the NIH-sponsored Research Resource for Complex Physiologic Signals (UO1EB008577), and from DynaDx Corporation, Mountain View, CA, USA.
References
- Achermann P, Borbely AA. Dynamics of EEG slow wave activity during physiological sleep and after administration of benzodiazepine hypnotics. Human Neurobiology. 1987;6:203–210. [PubMed] [Google Scholar]
- Adrien J. Neurobiological bases for the relation between sleep and depression. Sleep Medicine Review. 2002;6:341–351. [PubMed] [Google Scholar]
- Armitage R. Microarchitectural findings in sleep EEG in depression: diagnostic implications. Biological Psychiatry. 1995;37:72–84. doi: 10.1016/0006-3223(94)00082-E. [DOI] [PubMed] [Google Scholar]
- Armitage R, Trivedi M, Hoffmann R, Rush AJ. Relationship between objective and subjective sleep measures in depressed patients and healthy controls. Depression and Anxiety. 1997;5:97–102. doi: 10.1002/(sici)1520-6394(1997)5:2<97::aid-da6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biological Psychiatry. 2006;60:1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Van Dijk E, Thayer JF. Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. International Journal of Psychophysiology. 2007;63:39–47. doi: 10.1016/j.ijpsycho.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, ncoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biological Psychiatry. 2003;54:241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure & Function. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Jurysta F, Lanquart JP, Migeotte PF, van de Borne P, Linkowski P. Interdependency between heart rate variability and sleep EEG: linear/non-linear? Clinical Neurophysiology. 2004;115:2031–2040. doi: 10.1016/j.clinph.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Farina B, Della Marca G, Grochocinski VJ, Mazza M, Buysse DJ, Di Giannantonio M, Francesco Mennuni G, De Risio S, Kupfer DJ, Frank E. Microstructure of sleep in depressed patients according to the cyclic alternating pattern. Journal of Affective Disorders. 2003;77:227–235. doi: 10.1016/s0165-0327(02)00147-7. [DOI] [PubMed] [Google Scholar]
- Ferre A, Guilleminault C, Lopes M. Cyclic alternating pattern as a sign of brain instability during sleep. Neurologia. 2006;21:304–311. [PubMed] [Google Scholar]
- Germain A, Nofzinger EA, Kupfer DJ, Buysse DJ. Neurobiology of non-REM sleep in depression: further evidence for hypofrontality and thalamic dysregulation. The American Journal of Psychiatry. 2004;161:1856–1863. doi: 10.1176/ajp.161.10.1856. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology Neurosurgery Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C. AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Irwin MR, Valladares EM, Motivala S, Thayer JF, Ehlers CL. Association between nocturnal vagal tone and sleep depth, sleep quality, and fatigue in alcohol dependence. Psychosomatic Medicine. 2006;68:159–166. doi: 10.1097/01.psy.0000195743.60952.00. [DOI] [PubMed] [Google Scholar]
- Jindal RD, Thase ME, Fasiczka AL, Friedman ES, Buysse DJ, Frank E, Kupfer DJ. Electroencephalographic sleep profiles in single-episode and recurrent unipolar forms of major depression: II. Comparison during remission. Biological Psychiatry. 2002;51:230–236. doi: 10.1016/s0006-3223(01)01226-4. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Kuo TB, Shaw FZ, Lai CJ, Yang CC. Asymmetry in sympathetic and vagal activities during sleep-wake transitions. Sleep. 2008;31:311–320. doi: 10.1093/sleep/31.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa FE, Fox PT, Denton D. Brain responses associated with consciousness of breathlessness (air hunger) Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MC, Quera-Salva MA, Guilleminault C. Non-REM sleep instability in patients with major depressive disorder: subjective improvement and improvement of non-REM sleep instability with treatment (Agomelatine) Sleep Medicine. 2007;9:33–41. doi: 10.1016/j.sleep.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Moody GB, Mark RG, Bump MA, et al. Clinical validation of the ECG-derived respiration (EDR) technique. Computing in Cardiology. 1986;13:507–510. [Google Scholar]
- Nahshoni E, Aravot D, Aizenberg D, Sigler M, Zalsman G, Strasberg B, Imbar S, Adler E, Weizman A. Heart rate variability in patients with major depression. Psychosomatics. 2004;45:129–134. doi: 10.1176/appi.psy.45.2.129. [DOI] [PubMed] [Google Scholar]
- Ozone M, Yagi T, Itoh H, Tamura Y, Inoue Y, Uchimura N, Sasaki M, Shimizu T, Terzano MG, Parrino L. Effects of zolpidem on cyclic alternating pattern, an objective marker of sleep instability, in Japanese patients with psychophysiological insomnia: a randomized crossover comparative study with placebo. Pharmacopsychiatry. 2008;41:106–114. doi: 10.1055/s-2008-1058104. [DOI] [PubMed] [Google Scholar]
- Parrino L, Boselli M, Spaggiari MC, Smerieri A, Terzano MG. Multidrug comparison (lorazepam, triazolam, zolpidem, and zopiclone) in situational insomnia: polysomnographic analysis by means of the cyclic alternating pattern. Clinical Neuropharmacology. 1997;20:253–263. doi: 10.1097/00002826-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Saletu B. Is the subjectively experienced quality of sleep related to objective sleep parameters? Behavioral Biology. 1975;13:433–444. doi: 10.1016/s0091-6773(75)91009-3. [DOI] [PubMed] [Google Scholar]
- Sateia MJ, Nowell PD. Insomnia. Lancet. 2004;364:1959–1973. doi: 10.1016/S0140-6736(04)17480-1. [DOI] [PubMed] [Google Scholar]
- Stein PK, Carney RM, Freedland KE, Skala JA, Jaffe AS, Kleiger RE, Rottman JN. Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. Journal of Psychosomatic Research. 2000;48:493–500. doi: 10.1016/s0022-3999(99)00085-9. [DOI] [PubMed] [Google Scholar]
- Takahara M, Mizuno K, Hirose K, Sakai K, Nishii K, Onozuka M, Sato S, Shirakawa S. Continuous recording of autonomic nervous activity at nighttime effectively explains subjective sleep reports in postmenopausal women. Sleep and Biological Rhythms. 2008;6:215–221. [Google Scholar]
- Terzano MG, Mancia D, Salati MR, Costani G, Decembrino A, Parrino L. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep. 1985;8:137–145. doi: 10.1093/sleep/8.2.137. [DOI] [PubMed] [Google Scholar]
- Terzano MG, Parrino L. Origin and Significance of the Cyclic Alternating Pattern (CAP) Sleep Medicine Review. 2000;4:101–123. doi: 10.1053/smrv.1999.0083. [DOI] [PubMed] [Google Scholar]
- Terzano MG, Parrino L, Boselli M, Dell’Orso S, Moroni M, Spaggiari MC. Changes of cyclic alternating pattern (CAP) parameters in situational insomnia under brotizolam and triazolam. Psychopharmacology (Berl) 1995;120:237–243. doi: 10.1007/BF02311169. [DOI] [PubMed] [Google Scholar]
- Terzano MG, Parrino L, Spaggiari MC, Palomba V, Rossi M, Smerieri A. CAP variables and arousals as sleep electroencephalogram markers for primary insomnia. Clinical Neurophysiology. 2003;114:1715–1723. doi: 10.1016/s1388-2457(03)00136-6. [DOI] [PubMed] [Google Scholar]
- Thase ME, Fasiczka AL, Berman SR, Simons AD, Reynolds CF., 3rd Electroencephalographic sleep profiles before and after cognitive behavior therapy of depression. Archives of General Psychiatry. 1998;55:138–144. doi: 10.1001/archpsyc.55.2.138. [DOI] [PubMed] [Google Scholar]
- Thomas RJ. Effective Sleep Homeostasis - oscillations during sleep, and the function of sleep in health and disease. Cellscience Review. 2007;3:49–62. [Google Scholar]
- Thomas RJ, Mietus JE, Peng CK, Gilmartin G, Daly RW, Goldberger AL, Gottlieb DJ. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep. 2007;30:1756–1769. doi: 10.1093/sleep/30.12.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28:1151–1161. doi: 10.1093/sleep/28.9.1151. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Davis S, Vitiello MV, Lentz MJ, McTiernan A. Factors associated with objective (actigraphic) and subjective sleep quality in young adult women. Journal of Psychosomatic Research. 2005;59:11–19. doi: 10.1016/j.jpsychores.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Udupa K, Sathyaprabha TN, Thirthalli J, Kishore KR, Lavekar GS, Raju TR, Gangadhar BN. Alteration of cardiac autonomic functions in patients with major depression: a study using heart rate variability measures. Journal of Affective Disorders. 2007;100:137–141. doi: 10.1016/j.jad.2006.10.007. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P, van Wingen G, van Oijen K, Rijpkema M, Goraj B, Jan Verkes R, Oude Voshaar R, Fernandez G, Buitelaar J, Tendolkar I. Amygdala Volume Marks the Acute State in the Early Course of Depression. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.10.027. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Buchel C. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. American Journal of Respiratory and Critical Care Medicine. 2008;177:1026–1032. doi: 10.1164/rccm.200712-1821OC. [DOI] [PubMed] [Google Scholar]
- Yeh GY, Mietus JE, Peng CK, Phillips RS, Davis RB, Wayne PM, Goldberger AL, Thomas RJ. Enhancement of sleep stability with Tai Chi exercise in chronic heart failure: preliminary findings using an ECG-based spectrogram method. Sleep Medicine. 2008;9:527–536. doi: 10.1016/j.sleep.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeragani V, Appaya S, Seema K, Kumar R, Tancer M. QRS Amplitude of ECG in Normal Humans: Effects of Orthostatic Challenge on Linear and Nonlinear Measures of Beat-to-Beat Variability. Cardiovascular Engineering. 2005;5:135–140. [Google Scholar]
- Yeragani VK, Rao KA, Smitha MR, Pohl RB, Balon R, Srinivasan K. Diminished chaos of heart rate time series in patients with major depression. Biological Psychiatry. 2002;51:733–744. doi: 10.1016/s0006-3223(01)01347-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.