Abstract
Background
This study examined effects of diabetes mellitus (DM) on cellular proliferation associated with vascular endothelial growth factor (VEGF)signaling in endothelial progenitor cells (EPC’s)and evaluated protein expression involved in cellular proliferation and pro-apoptotic signaling in chronically ischemic myocardium.
Methods
Insulin dependent DM was induced in yucatan miniswine with alloxan. Eight weeks after induction, chronic ischemia was induced by ameroid constrictor placement around the circumflex coronary artery. Seven weeks after ameroid constrictor, perfusion of ischemic territory was measured by isotope-labeled microspheres, and ischemic myocardium was harvested. Bone marrow (BM) samples were harvested from iliac bone and mononuclear cells (MNC’s) were cryopreserved. EPC’s were isolated from cryopreserved MNC’s in control (n=6) and DM swine (n=6). EPC proliferation was assessed.
Results
EPC proliferation was decreased in DM as compared to control (1.02±0.09, 0.40±0.04, p<0.01). VEGF induced EPC proliferation was impaired in DM as compared to control (p<0.01). Expression of ERK protein, an activator of VEGF induced cell proliferation, was decreased. AKT activation, an inhibitor of apoptosis, was decreased, while Bad, an activator of pro-apoptotic signaling, was elevated in the ischemic myocardium from DM. Collateral dependent perfusion was impaired in DM.
Conclusion
Impaired VEGF induced proliferation response in EPC as well as an increase in negative myocardial protein expression for cell proliferation and pro-apoptotic signaling via VEGF could be a therapeutic target to enhance the effects of pro-angiogenesis therapies in DM and other chronic illnesses.
Introduction
Cell transplantation is a therapeutic strategy for angiogenesis and myocyte functional improvement in patients with severe heart failure or inoperable coronary disease who have no options for intervention1, 2. However, we have recently shown that the effect of angiogenesis in the ischemic territory is markedly diminished in the setting of endothelial dysfunction associated with diabetes mellitus (DM) and hypercholestreromia3, 4. In particular, a decrease in endothelial microvascular reactivity in response to vascular endothelial growth factor (VEGF) as well as an increase in expression of anti-angiogenic proteins such as endostatin and angiostatin were found in diabetic pigs5. Unfortunately, patients who are candidates for pro-angiogenic therapies or other regenerative strategies nearly all have advanced atherosclerosis and endothelial dysfunction.
EPC’s enhance angiogenesis, promotes vascular repair and improve endothelial function 6 7. In this context, EPC’s are a promising candidate for angiogenic cell based therapies 8. However, the number of endothelial progenitor cells (EPC’s) in peripheral blood and their function are reduced in patients with DM. We have recently shown a method to obtain EPC from cryopreserved bone marrow derived mononuclear cells (MNCs) 9 Cryopreservation of MNC’s did not limit the potential of EPC’s to migrate or proliferate9. This strategy may allow the procurement of sufficient numbers of EPC’s via multiple harvests. However, the change in the number and function of bone marrow derived EPC’s from cryopreserved MNC’s is not known in DM.
VEGF signaling plays an important role in cell proliferation and pro-angiogenic signaling for angiogenesis10,11. VEGF activates cell proliferation via extracellular signal-regulated kinases (ERK). On the other hand, VEGF inhibits apoptosis via increase in AKT activation. In this study, we evaluated the feasibility of EPC collection from cryopreserved bone marrow derived MNC’s in a diabetic pig model. In addition, we examined the effects of DM on VEGF induced proliferation response in cryopreserved EPC’s, as well as protein expression involved in cell proliferation and pro-apoptotic signaling via VEGF in ischemic myocardium.
Materials and Method
Experimental model and sample collection
Yucatan miniswine (Sinclair Research Inc, Colombia, MO) were used for the study. To induce DM, animals at 8 months of age were given a single intravenous injection of alloxan, 150 mg/kg, and blood glucose levels were monitored daily thereafter (n= 10). Animals that achieved and maintained blood glucose levels greater than 200 mg/dL were included in the diabetes group. Animals with blood glucose levels exceeding 400mg/dL were treated with daily insulin given intramuscularly to maintain blood glucose levels between 250 and 400 mg/dL. Age matched swine receiving no alloxan served as controls (n= 6).
All animals underwent an identical experimental protocol involving 3 separate procedures on each animal. All animals received humane care in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee and National Research Council’s Guide for the Care and Use of Laboratory Animals, prepared by the Institutes of Health (NIH publication No.5377-3, 1996). Briefly, for all surgical procedures, anesthesia was induced with ketamine (10 mg/kg IM), thiopental (5–10 mg/kg IV), and thiopental 2.5% and maintained with a gas mixture of oxygen at 1.5–2 L/min and isoflurane at 0.75 to 3.0 %. The animals were intubated and mechanically ventilated at 12 – 20 breaths/min.
The first procedure, performed via small left anterolateral thoracotomy at 8 weeks post-diabetes induction, consisted of the placement of a 1.75-mm ameroid constrictor around the proximal circumflex artery and the injection of 1.5×107 gold-labeled microspheres into the left atrium during temporary circumflex coronary occlusion, to subsequently allow for identification, by shadow labeling, of the myocardial territory at risk.
At the second procedure, 3 weeks after ameroid placement, left coronary angiography was performed to document ameroid closure through an 8F sheath (Cordis Corporation, Miami, FL) surgically inserted in the femoral artery, using a catheter with the appropriate distal angulation and injection of radiographic contrast material (Mallinckrodt Inc., St. Louis, MO).
The third procedure was carried out 4 weeks after the second procedure and 7 weeks after ameroid placement. BM samples were obtained from the iliac bone. After collection of BM samples, sternotomy was performed, and 1.5×107 Samarium microspheres were injected into the left atrium during rest conditions, and 1.5×107 Lanthanum microspheres were injected during pacing (150 bpm). Euthanasia was then performed with 10 mL/kg of a saturated KCl solution administered intravenously. Ischemic myocardium samples were harvested and snap frozen for molecular studies. Ameroid constrictors were resected along with a segment of circumflex artery and examined under low power magnification to confirm occlusion. This model is standard in our lab and that of others and is useful in studying chronic myocardial ischemia 12 13 14 15.
Ex-vivo expansion of EPCs
Bone marrow mononuclear cells (MNC) were isolated from BM samples by Histopaque 1077 (Sigma-Aldrich,St.Louis, MO) density-gradient centrifugation of buffy coats. After isolation of MNCs, they were hand counted by 0.2% trypan blue staining, and, 10 × 107 of MNCs were preserved in cryopreservation tubes at −80°C in a regular deep freezer at a concentration of 1.0 × 107 /ml by use of Bambanker (Lyphotec Inc, Tokyo, Japan). After 2–3 months cryopreserved MNCs were thawed in a 37°C water bath just to the point of achieving a liquid state (0–10°C), washed of cryopreservative solution, and then suspended into cell culture media. The number of MNCs after thawing were hand counted by a blinded investigator with 0.2% trypan blue. MNCs (10 × 107) were plated on fibronectin-coated 10 ml culture dishes and maintained in endothelial cell basal medium-2 (EBM-2, Clonetics, San Diego, CA) supplemented with EGM-2 microvascular single aliquots of:2.0 ml of recombinant human (rh) fibroblast growth factor-B, 0.5 ml of rh VEGF, 0.5 ml of R3-IGF-1, 0.5 ml of Ascorbic acid, 0.5 ml of rh epidermal growth factor, 0.5 ml of heparin, 0.5 ml of Gentamicin sulfate and Amphotericin-B, and 5% (10ml) fetal bovine serum (FBS). The recovery rate of MNCs after cryopreservation was calculated by the number of MNCs after cryopreservation divided by the number before cryopreservation. Four days after culture adherent cells were counted by 0.2% trypan blue staining and used for proliferation assay.
Fluorescent staining of EPC
To confirm isolated cells were EPC, direct fluorescent staining was performed using fluorescein giffonia simplicifolia lectine I, isolectin B4 (Vector Laboratories, Burlingame, CA), acylated low density lipoprotein labeled with 1,1’-dioctadecyl-3,3,3’-3’- tetramethylindo-carbocyanine perchlorate (DiI-acLDL) (Biomedical Technologies, Stoughton, Mass) and 4’,6-Diamidino-2-phenylindole dihydrochloride (SIGMA, St. Louis, Mis) with a fluorescent microscope, and adherent cells that stained positive by both isolectin B4, DiI-acLDL and DAPI were considered EPCs. The percentage of EPC was calculated by isolectin B4, DiI-acLDL and DAPI positive cells divided by DAPI positive cells.
EPC Proliferation
The proliferative activity of BM derived EPC’s taken from control and DM pigs with cryopreservation was examined with the use of CellTiter 96AQueous non-radioactive cell proliferation assay (Promega, Madison, WI) according to the manufacturer’s instructions. Briefly, 4 days after cell culture, 10,000 cells per well were reseeded on 96-well flat-bottomed plates with 100 µL of the cell culture media including EGM-2 microvascular single aliquots. In addition, in order to evaluate the response to VEGF, 10,000 EPC’s were also placed on plates in cell culture media including 0.2% of FBS, 0.2% of FBS plus 10 ng/ml VEGF (R&D Systems, Minneapolis, MN), and 0.2% of FBS plus 100 ng/ml VEGF, respectively. After 72 hours in culture at 37°C, tetrazolium compound and phenazine methosulfate solutions were added and the absorbance at 490-nm wavelength was recorded with the use of a 96-well ELISA plate reader (Bionetics Laboratory, Kensington, Md).
Western Blotting
Myocardial samples were homogenized in RIPA buffer (Boston Bioproducts, Worcester, MA) and protein concentration was determined (Pierce, Rockford, IL). Equal amounts of protein (40 µg) were subjected to SDS-PAGE and immunoblotting. Primary antibodies were used according to the manufacturer’s recommendation. Expression levels of Akt, phospho-Akt (Ser473), Erk 1/2 (p44/42 MAPK), and Bad were assessed. Myocardial samples from the ischemic territories were assessed separately. Gels were loaded with four samples from each group and second gel had an overlapping sample to normalize bands intensity between gels for analysis. Vinculin was used to confirm equal protein loading. Additionally, band intensities were normalized to Ponceau. Data are presented as mean ± SEM in arbitrary density units (AU).
Assessment of Myocardial Perfusion
Myocardial perfusion was assessed with isotope-labeled microspheres (ILM) (BioPAL, Worcester, MA) using methods previously reported 12, 14 13. Isotope-labeled microspheres, 15 µm in diameter, of different isotopic mass were used. Gold-labeled microspheres were injected during temporary circumflex occlusion at the time of ameroid placement to identify myocardial samples that originated from the circumflex coronary distribution (those with the lowest count of gold-labeled microspheres). Samarium and Lanthanum-labeled ILM were injected at rest and during pacing during the third procedure. Reference blood samples were obtained from the femoral artery during the third procedure. Following euthanasia, ten circumferential, transmural left ventricular sections were collected for ILM assays in each animal, weighed, and dried at 60°C for 24 hours. Each sample was exposed to neutron beams and microsphere densities were measured in a gamma counter.
Crude Blood flow (tissue sample) = Withdrawal rate (ml / min) / weight (tissue sample) × Isotope counts (tissue sample) / Isotope counts (reference blood sample)
Statistical analysis
Results are reported as mean ± SEM. Comparisons of 2 groups in each experiment were analyzed by Mann-Whiteney test. Comparisons of EPC proliferation in response to VEGF were analyzed by repeated measurement 2 way ANOVA. Probability values of <0.05 were considered statistically significant. Statistical analyses were conducted using JMP 5.0 (SAS institute Inc., Cary, NC) and Graph Pad Prism 4 (Graph Pad Software Inc., San Diego, CA).
Results
Experimental model
Diabetes was successfully induced in 8 of 10 miniswine (80%). Four animals required small doses of intramuscular insulin (10 to 20 u/d) to keep blood glucose levels below 400 mg/dl at certain points during the experimental period. Blood glucose levels at 8 weeks after alloxan injection were 319±31 mg/dl in diabetic animals compared with 69±2 mg/dl in control animals (p<0.001). In diabetic swine, EPC expansion was successfully performed in 6 of 8 samples. EPC expansion was not successful in 2 diabetic swine due to infection of cell culture medium during the culture of MNC and difficulty in collection of BM sample, respectively. Each 6 miniswine of control and DM were enrolled in the following experiment.
Ex-vivo expansion of EPC
There was a no difference in the numbers of MNC taken from DM swine as compared to control (Control=103.7±16.8 ×105/ml, DM= 142.7±19.6, p= 0.16). Recovery rate of MNC without cryopreservation was considered as 100%. No significant difference was found in the recovery rate of MNC’s after cryporeservation between Control and DM groups (Control 54.6±5.0 %, DM 40.0±6.3, p= 0.10). The percentage of EPC recovery after ex vivo expansion from MNC’s with cryopreservation was 0.82 ± 0.22 in control, and 1.30 ± 0.45 in DM. There was no significant difference between the 2 groups (p= 0.69).
EPC Fluorescent staining
To confirm the attached cells were characteristic of EPC, we stained the attached cells 4 days after cell culture by Isolectine B4, DiI-acLDL and DAPI. Attached cells were stained by Isolectine B4 (Fig 1-A, E), DiI-acLDL(Fig 1-B, F) and DAPI (Fig 1-C,G). Merge pictures are shown in Fig 1-D. As DAPI is a marker for cell nuclei, we calculated the EPC displaying positive staining for Isolectine B4, DiI-acLDL, and DAPI as a percentage of total DAPI staining cells. The percentage of EPC in Control was 95.3±1.2% (Fig 1-D). The percentage of EPC in DM was 93.4±0.8% (Fig 1-H). There was no significant difference in the percentage of EPC between Control and DM (p=0.20).
Figure 1. Direct fluorescent staining of EPC from cryopreserved MNC in Control and DM swine.
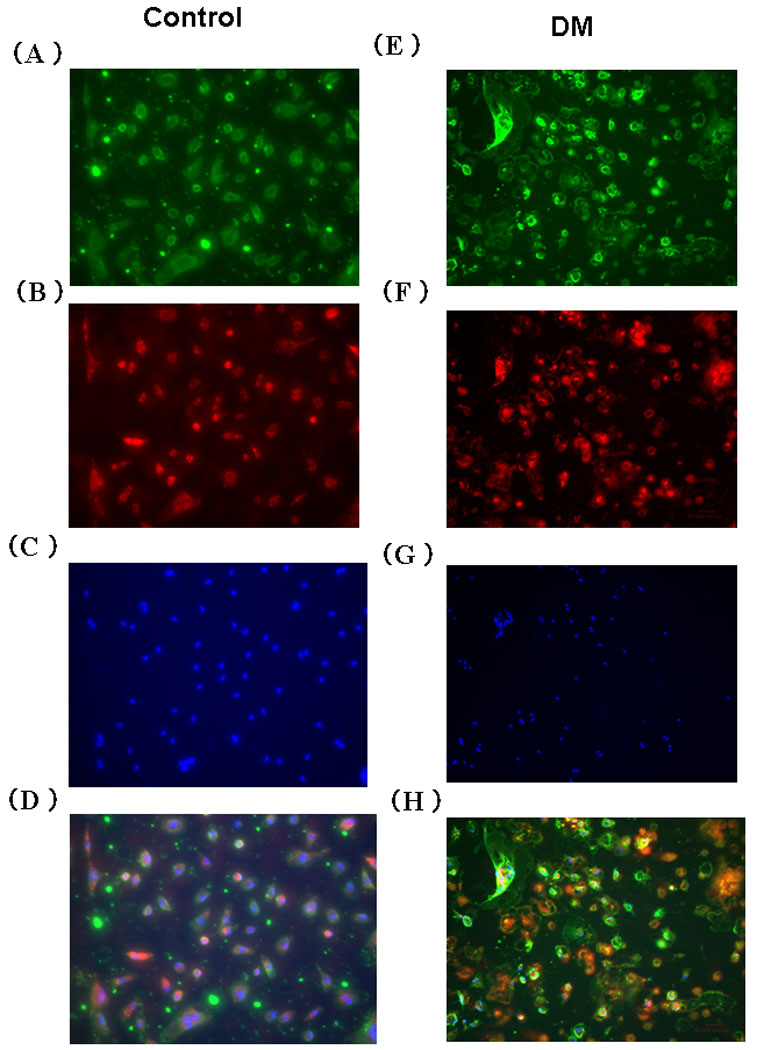
(A, E) Isolectin B4 (B, F) DiI-acLDL (C, G) DAPI (D, H) Merge EPC; endothelial progenitor cell, MNC; mononuclear cell, DiI-acLDL; acethlated low density lipoprotein labeled with 1,1’-dioctadecyl-3,3,3’-3’- tetramethylindo-carbocyanine perchlorate, DAPI; 4’,6-Diamidino-2-phenylindole dihydrochloride
Impaired proliferation response to VEGF in DM
EPC proliferation of cells harvested from pigs with and without DM was evaluated. Proliferation of EPC’s from DM swine using cell culture media was decreased as compared to that from control swine (Control 1.02 ± 0.09, DM 0.40 ± 0.04, p<0.01) (Figure 2).
Figure 2. Effects of DM on EPC proliferation.
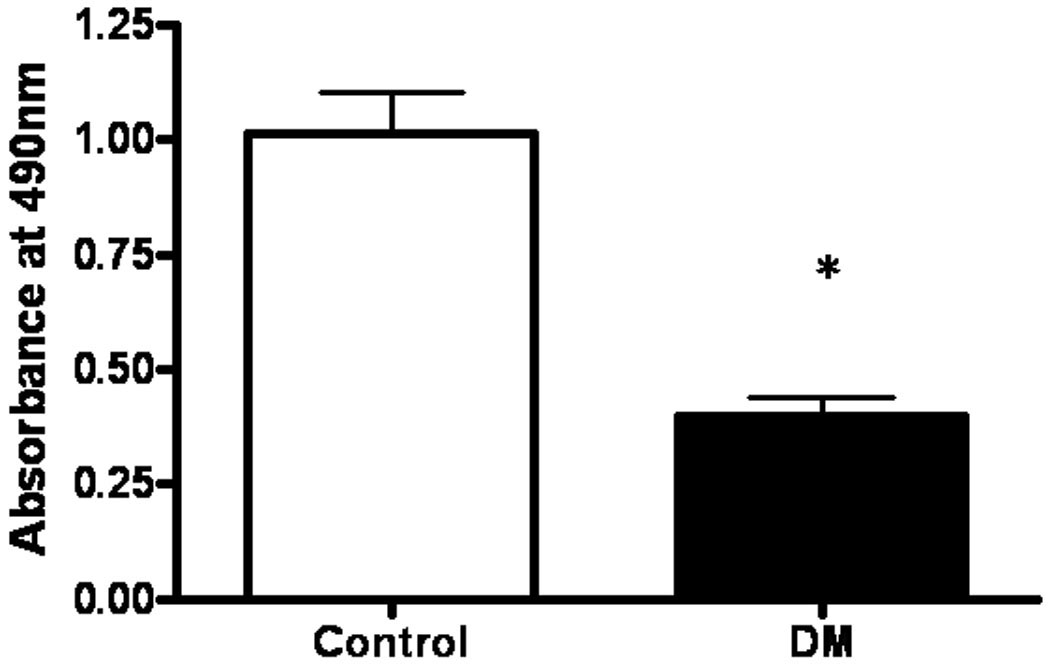
EPC proliferation was evaluated measuring absorbance at 490 nm using tetrazoum compound. Proliferation was impaired in EPC from DM as compared to Control. Results are expressed as mean ± SEM. *p < 0.01 versus Control.
In order to clarify the impaired response in DM, we evaluated EPC proliferation in response to VEGF. VEGF induced proliferation activity was impaired in DM as compared to Control (P<0.01, interaction 0.02) (Figure 3).
Figure 3. VEGF induced proliferation using EPC from cryopreserved MNCs.
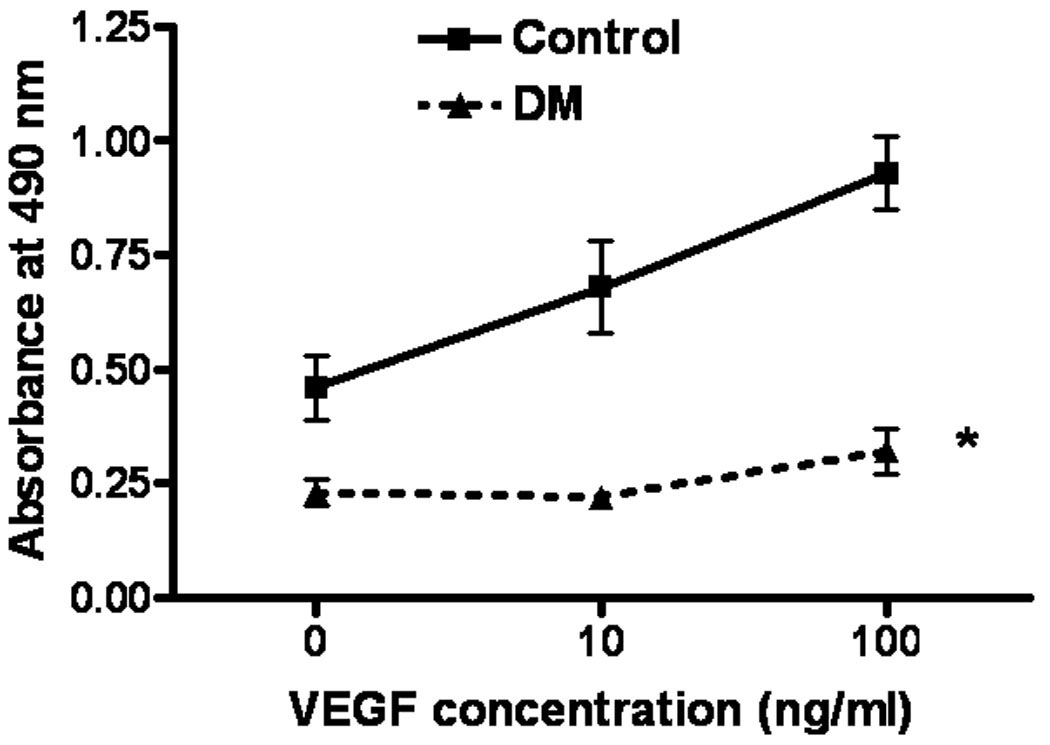
VEGF induced proliferation was impaired in EPC from DM as compared to Control (p<0.01).
Expression of myocardial pro-angiogenic and cell survival proteins
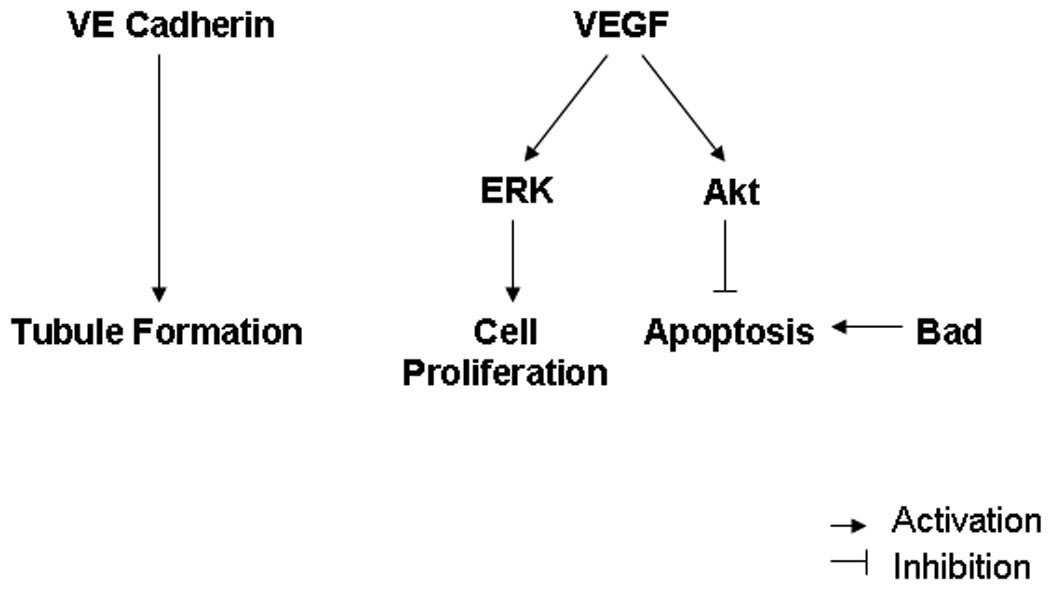
Protein expression of VE Cadherin, important in tubule formation of newly formed blood vessels, was significantly decreased in the DM swine (Control 1.2 fold higher than DM) (p=0.01). The expression of ERK was 2.2 fold greater in control than in DM swine(p<0.01). Protein levels of phospho-Akt (thr473) were 1.9 fold greater in control than in DM swine (p=0.03). The expression of Bad, a pro-apoptotic protein, was increased 1.8 fold in the DM swine as compared to controls (p=0.03) (Figure 4A-D).
Figure 4. Expression of pro-angiogenic and pro-apoptotic proteins in ischemic myocardium.
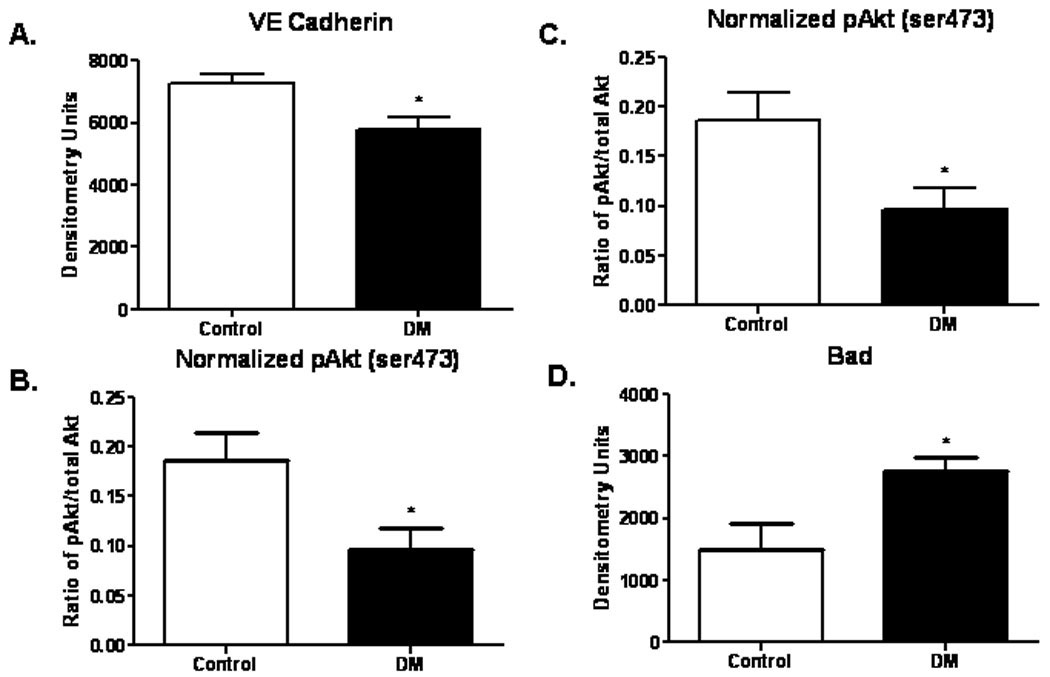
(A)Expression of VE Cadherin was significantly decreased in the DM group (p=0.01). (B) Levels of ERK were significantly decreased in the DM group (p= 0.004). (C) Protein expression of phospho-Akt (ser473) was also decreased in the DM group (p= 0.03). (D) Expression of the pro-apoptotic protein Bad was increased in the DM group (p= 0.03).
Myocardial Perfusion
At 7 weeks after placement of ameroid constrictor, collateral dependent circumflex territory myocardial blood flow was reduced in DM as compared to Control (0.69 ± 0.07 vs 0.15 ± 0.02, p<0.001). The reduction in collateral dependent myocardial blood flow in DM was also observed with pacing (0.55 ± 0.11 vs 0.16 ± 0.02, p<0.0003). (Figure 5)
Figure 5. Schematic of the roles pro-angiogenic and pro-apoptotic signaling proteins in ischemic myocardium.
Discussion
The main findings of this study were (1) no difference was found in the percentage of EPC’s collected by ex-vivo expansion between control and DM groups. The recovery rate of MNC after cryopreservation was 55% in control and 40% in DM swine. (2) EPC proliferation was impaired by DM. In particular, the VEGF induced proliferation was reduced in the DM groups. (3) In ischemic myocardium, ERK protein expression, which is involved in VEGF induced proliferation, was decreased. (4) AKT protein activation, which is involved in VEGF induced anti-apoptotic signaling, was decreased, while Bad, a pro-apoptotic marker, was increased in ischemic myocardium. These results suggest that ex-vivo expansion of EPC’s from cryopreserved MNC’s could be a useful method to prepare sufficient numbers of EPC’s by multiple harvesting of BM in advance. However, the impairment of VEGF induced EPC proliferation as well as negative myocardial environment in ischemic myocardium could be a therapeutic target to enhance effects of angiogenesis in DM. This study exemplifies that DM and likely other illnesses associated with coronary artery disease and heart failure diminishes the potential for angiogenesis and the utility of cell therapy in these patients. Unfortunately, these are the patients most in need of these therapies.
Cryopreservation is a common method to preserve isolated cells. Little is reported about cryopreservation of BM derived EPC’s. There are several publications reporting on the effects of cryopreservation of human cord blood samples 16, 17. Ketheesan et al. demonstrated that the cooling speed of human cord blood MNC at a rate of 1, 5, 10°C/min shows 44, 76, 93% recovery rates respectively. In this study the recovery rate of MNCs after cryopreservation was 55% in control and 40% in DM swine, and the results were not statistically significant between the two groups. In addition, the recovery of EPC’s after ex vivo expansion from MNC was similar between control and DM groups. These results suggest that DM did not affect the recovery of MNC’s or the ex vivo expansion of EPC’s following cryopreservation. Recently, we reported that cryopreservation did not affect the proliferation or migration potential of EPCs from healthy swine 9. However, further studies will be required to improve the recovery rate of MNC’s, and also to further evaluate the effects of DM and cryopreservation on cell viability and function.
In this study we evaluated the percentage of EPC’s after ex vivo expansion in BM, not peripheral blood. Most studies have evaluated the peripheral number of EPC’s. The percentage of progenitor cells in the MNC population is limited to less than 0.1% 18. In addition, the circulating quantity of EPC’s is decreased in patients with DM 19. We have previously reported that the percentage of EPC’s after expansion collected from BM sample was 0.83 %9. This percentage is more than 8 times higher than that observed in peripheral blood. Interestingly, we showed that the percentage of EPC’s in BM samples was unchanged between control and DM groups in this study. Recent reports have demonstrated that EPC’s are embedded in a microenviroment of BM stromal cells, and translocate to the circulation upon matrix metalloproteinase-9 (MMP-9) mediated release of soluble c-kit ligand 20. Increased reactive oxygen species and matrix metalloproteinase, extracellular signal-regulated kinases (ERK) 21 and VEGF/nitric oxide-mediated pathways 6 22 are involved in the process of the EPC mobilization 23. The reduced number of circulating EPC’s found in peripheral blood with DM may be associated with the alteration of mobilization process. In this study we demonstrated that DM did not affect the number of EPC and MNC in the BM sample, and that BM collection is suitable for obtaining sufficient numbers of EPC for cell therapy with or without DM.
VEGF response plays a crucial role in EPC proliferation. VEGF exerts its biological effects through binding to two high affinity tyrosine kinase receptors, Flt-1 (VEGFR1) and KDR/Flk-1 (VEGFR2) 24. KDR/Flk-1 is implicated in the recruitment, differentiation and proliferation of EPC’s 25 26. Imanishi et al. demonstrated that an increase in KDR/Flk-1 mRNA and membrane expression is associated with VEGF induced proliferation 27. In addition, recent siRNA techniques demonstrated that inhibition of hypoxia-inducible factor-1 α (HIF 1α) reduces KDR/Flk-1 protein expression leading to inhibition of EPC function 28. In our study, VEGF induced EPC proliferation was impaired in DM swine. These lines of evidence suggest that decreased KDR/Flk-1 expression may be associated with impaired EPC proliferation function.
A negative myocardial environment for cell proliferation as well as pro-apoptotic signaling via VEGF was observed in ischemic myocardium under diabetic conditions. We have previously shown that VEGF protein expression is decreased in this diabetic swine model 3. As shown in Fig 4 and 5, we observed a change in protein expression such that a relatively negative myocardial environment is evident for proliferation and anti-apoptotic signaling via VEGF in ischemic myocardium. Little has been reported regarding effects of DM on pro-angiogeneic and pro-apoptotic protein alterations in chronic ischemic myocardium. Our findings indicate that DM negatively alters VEGF related protein expression involved in proliferation associated with angiogenesis, and sustained VEGF related pro-apoptotic protein expression in ischemic myocardium at 7weeks after induction of myocardial ischemia. In another set of experiments, protein expression involved in VEGF proliferation such as phospho-ERK and VEGF receptor 2 was potentially decreased in non-ischamic territory with DM (data not shown). Under diabetic condition, collateral dependent myocardial blood flow was markedly reduced in this ischemic territory. This negative myocardial environment may limit the effects of pro-angiogenic therapies in DM.
The findings in the present study may give us new insights for cell based therapy using EPC in diabetic patients. Recent studies have demonstrated that EPCs promote angiogenesis in ischemic tissue, as well as promote vascular repair leading to improvement of endothelial function 1 6. In addition, we demonstrated that ex vivo expansion of EPC using cryopreserved MNC could be a useful method for autologous EPC transplantation. However, as is consistant with our study, EPC’s from DM patients have been reported to exhibit impaired pro-angiogenesis signaling 19. This suggests that EPC’s from DM patients may benefit from treatment to help restore the function related to angiogenesis. Recently, peroxisome proliferators-activated receptor (PPAR)-γ agonist (thiazolidinediones) have been shown to lower serum glucose levels and improve insulin sensitivity in DM patients 29. In addition, PPAR-γ agonists have been reported to increase ex-vivo EPC function as well as angiogenesis in-vivo30. Treatment with a PPAR-γ agonist may be an option for DM patients requiring EPC transplantation. However, this treatment and all such treatments will require clinical trials for verification.
Study limitations
The animal model we used in this study corresponds to insulin dependent type-1 DM patients. DM swine were exposed to hyperglycemia for 15 weeks. Diabetes-associated metabolic factors may differ between type 1 and type 2 DM. In addition, the difference in the periods and degree of DM should be evaluated in future studies. Furthermore, we performed only ex vivo experiments to evaluate the feasibility of EPC expansion from cryopreserved MNC’s. As a next step, we will perform autologous EPC transplantation after expansion using cryopreserved MNC’s by multiple collections, and attempt to clarify the optimum number and technique for EPC transplantation into chronically ischemic myocardium. Lastly, change of lineage markers are found in the process of proliferation and differentiation of EPC’s. However, we did not show results of lineage marker by flow cytometry, because it is difficult to get commercially available anti-swine antibodies such as CD34, VEGFR2, and AC133. Evaluation of the change of lineage markers of EPC may be helpful to decide the timing of cell transplantation. Finally, we only examined a small number of proteins involved in angiogenic signaling. However, since nearly all cell therapy trials have been negative, or nearly so, we feel that other signaling proteins will also contribute negatively to the engraftment and function of EPC and other cell therapies.
Conclusion
The results of the present study suggest that the ex vivo expansion of EPC’s from cryopreserved MNC’s could be a useful method to obtain sufficient numbers of EPC’s. However, the impairment of VEGF signaling in EPC proliferation as well as negative myocardial environment in the diabetic or ischemic myocardium could hinder the engraftment and function of EPC’s and other cell therapies. Countering these negative factors could be a therapeutic target to enhance effects of angiogenesis in patients with diabetes.
Figure 6. Effects of DM on collateral dependent myocardial blood flow.
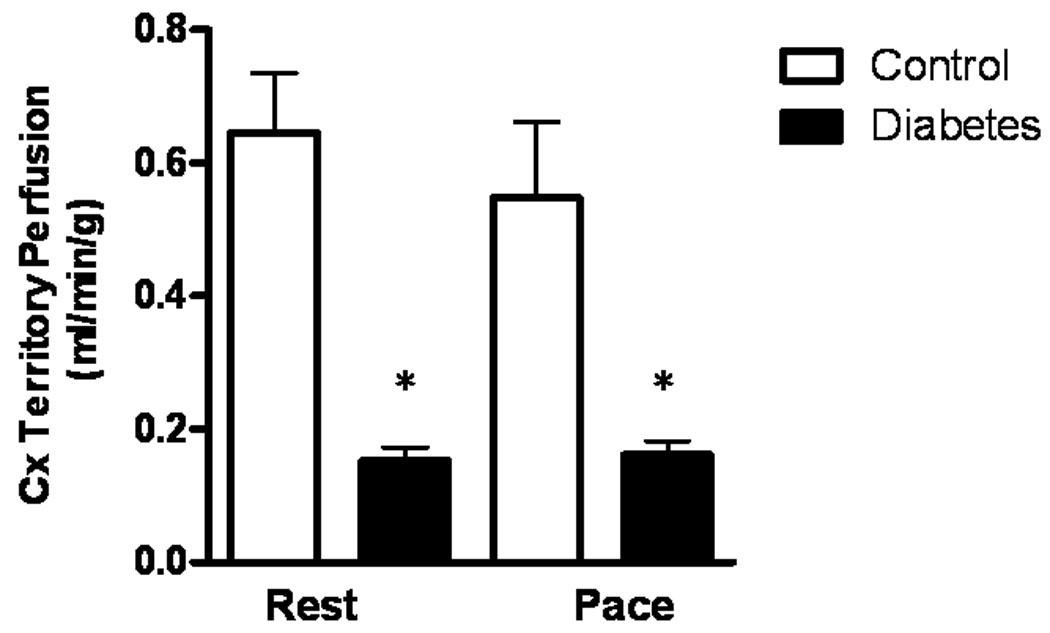
Collateral dependent myocardial blood flow is markedly impaired at rest as well as with pacing in DM. Results are expressed as mean ± SEM. *p < 0.01 versus Control.
Acknowledgement
This work was supported by National Institutes of Health grants R01 HL46716 (F.W.S.) and R01 HL69024 (F.W.S.) and R01HL085647 (F.W.S).
References
- 1.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 2.Hamano K, Nishida M, Hirata K, et al. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: clinical trial and preliminary results. Jpn Circ J. 2001;65:845–847. doi: 10.1253/jcj.65.845. [DOI] [PubMed] [Google Scholar]
- 3.Boodhwani M, Sodha NR, Mieno S, et al. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116(11Suppl):I31–I37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boodhwani M, Nakai Y, Mieno S, et al. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. The Annals of thoracic surgery. 2006;81:634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 5.Sodha NR, Clements RT, Boodhwani M, et al. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296:H428–H434. doi: 10.1152/ajpheart.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 7.Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 8.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mieno S, Clements RT, Boodhwani M, et al. Characteristics and function of cryopreserved bone marrow-derived endothelial progenitor cells. The Annals of thoracic surgery. 2008;85:1361–1366. doi: 10.1016/j.athoracsur.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Lin KT, Lien JC, Chung CH, Kuo SC, Huang TF. A novel compound, NP-184, inhibits the vascular endothelial growth factor induced angiogenesis. European journal of pharmacology. 630:53–60. doi: 10.1016/j.ejphar.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 11.Cai J, Ahmad S, Jiang WG, et al. Activation of vascular endothelial growth factor receptor-1 sustains angiogenesis and Bcl-2 expression via the phosphatidylinositol 3-kinase pathway in endothelial cells. Diabetes. 2003;52:2959–2968. doi: 10.2337/diabetes.52.12.2959. [DOI] [PubMed] [Google Scholar]
- 12.Ruel M, Wu GF, Khan TA, et al. Inhibition of the cardiac angiogenic response to surgical FGF-2 therapy in a Swine endothelial dysfunction model. Circulation. 2003;108 Suppl 1:II335–II340. doi: 10.1161/01.cir.0000087903.75204.ad. [DOI] [PubMed] [Google Scholar]
- 13.Nakai Y, Voisine P, Bianchi C, et al. Effects of L-arginine on the endogenous angiogenic response in a model of hypercholesterolemia. Surgery. 2005;138:291–298. doi: 10.1016/j.surg.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Voisine P, Li J, Bianchi C, et al. Effects of L-arginine on fibroblast growth factor 2-induced angiogenesis in a model of endothelial dysfunction. Circulation. 2005;112(9 Suppl):I202–I207. doi: 10.1161/CIRCULATIONAHA.104.526350. [DOI] [PubMed] [Google Scholar]
- 15.Voisine P, Bianchi C, Khan TA, et al. Normalization of coronary microvascular reactivity and improvement in myocardial perfusion by surgical vascular endothelial growth factor therapy combined with oral supplementation of l-arginine in a porcine model of endothelial dysfunction. The Journal of thoracic and cardiovascular surgery. 2005;129:1414–1420. doi: 10.1016/j.jtcvs.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 16.Hubel A, Carlquist D, Clay M, McCullough J. Cryopreservation of cord blood after liquid storage. Cytotherapy. 2003;5:370–376. doi: 10.1080/14653240310003035. [DOI] [PubMed] [Google Scholar]
- 17.Nicol A, Nieda M, Donaldson C, Denning-Kendall P, Bradley B, Hows J. Analysis of cord blood CD34+ cells purified after cryopreservation. Exp Hematol. 1995;23:1589–1594. [PubMed] [Google Scholar]
- 18.Powell TM, Paul JD, Hill JM, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 19.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 20.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung TW, Lee YC, Kim CH. Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential. Faseb J. 2004;18:1123–1125. doi: 10.1096/fj.03-1429fje. [DOI] [PubMed] [Google Scholar]
- 22.Landmesser U, Engberding N, Bahlmann FH, et al. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 23.Thum T, Fraccarollo D, Galuppo P, et al. Bone marrow molecular alterations after myocardial infarction: Impact on endothelial progenitor cells. Cardiovasc Res. 2006;70:50–60. doi: 10.1016/j.cardiores.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Millauer B, Wizigmann-Voos S, Schnurch H, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 25.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 26.Larrivee B, Lane DR, Pollet I, Olive PL, Humphries RK, Karsan A. Vascular endothelial growth factor receptor-2 induces survival of hematopoietic progenitor cells. J Biol Chem. 2003;278:22006–22013. doi: 10.1074/jbc.M212158200. [DOI] [PubMed] [Google Scholar]
- 27.Imanishi T, Hano T, Nishio I. Angiotensin II potentiates vascular endothelial growth factor-induced proliferation and network formation of endothelial progenitor cells. Hypertens Res. 2004;27:101–108. doi: 10.1291/hypres.27.101. [DOI] [PubMed] [Google Scholar]
- 28.Jiang M, Wang B, Wang C, et al. Inhibition of Hypoxia-Inducible Factor-1alpha and Endothelial Progenitor Cell Differentiation by Adenoviral Transfer of Small Interfering RNA in vitro. J Vasc Res. 2006;43:511–521. doi: 10.1159/000095964. [DOI] [PubMed] [Google Scholar]
- 29.Pistrosch F, Herbrig K, Oelschlaegel U, et al. PPARgamma-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 2005;183:163–167. doi: 10.1016/j.atherosclerosis.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 30.Grensch C, Clever YP, Werner C, Hanhoun M, Bohm M, Laufs U. The PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192:67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]


