Abstract
The vasculature of solid tumors is fundamentally different from that of normal vasculature and offers a unique target for anti-cancer therapy. Direct vascular-targeting with Tumor-Vascular Disrupting Agents (Tumor-VDAs) is distinctly different from anti-angiogenic strategies, and offers a complementary approach to standard therapies. Tumor-VDAs therefore have significant potential when combined with chemotherapy, radiotherapy, and angiogenesis-inhibiting agents. Preclinical studies with the different Tumor-VDA classes have demonstrated key tumor-selective anti-vascular and anti-tumor effects.
Keywords: Tumor-vascular disrupting agents, Tumor-VDAs, ASA404, Tubulin-binding agents, CA4P, Tumor vasculature, Anti-vascular therapy
Tumor Vasculature
The blood supply to the normal tissues of the body is maintained by an orderly and efficient vascular network. Blood vessels are regulated by the metabolic demand-driven balance of pro-angiogenic and anti-angiogenic molecular factors and a systematic network of lymphatic vessels which drain fluid and waste metabolic products from the interstitium. The resulting microarchitecture of normal vascular networks is hierarchically organized, with mature vessels that are evenly distributed to allow adequate perfusion of oxygen and other nutrients to all cells (Figure 1A).
Figure 1.
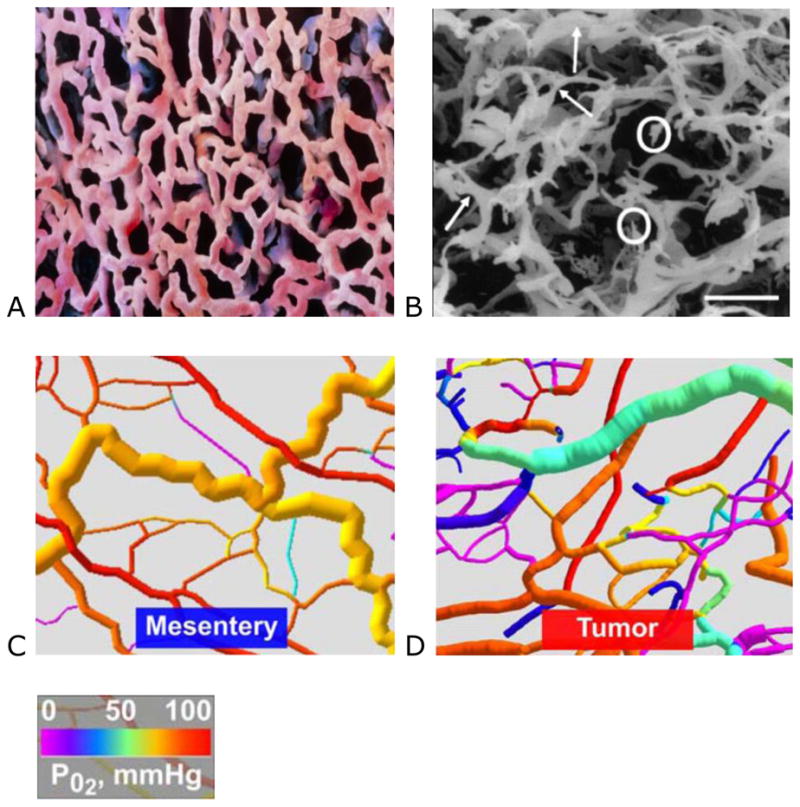
Panel A: scanning electron microscopy (SEM) image of a microvascular cast from normal lung tissue (Prof. P. Motta, G. Macchiarelli University, La Sapienza, Rome. Science Photo Library) and Panel B: An SEM of a human sigmoidal adenocarcinoma (bar = 100 μm), showing blind ends (circled) and abnormal bulges (arrowed) (used with permission from Macmillan Publishers Ltd: Br J Cancer 2001; 84:1354–1362. Copyright 2001).1 Panels C and D: Computer visualization of mesenteric and tumor vascular networks, color coded for pO2 showing poorer oxygenation in areas of restricted flow and blind endings (used with permission).12
In tumors, the aggressive growth of the neoplastic cell population and associated overexpression of pro-angiogenic factors leads to the development of disorganized blood vessel networks that are fundamentally different from normal vasculature. Tumor vasculature is typified by aberrant structural dynamics and vessels that are immature, tortuous, and hyperpermeable. The complex tumor vasculature is typically a disorganized labyrinth of vessels with a lack of conventional blood vessel hierarchy in which arterioles, capillaries, and venules are not clearly identifiable (Figure 1B).1 Blood vessels are of inconsistent diameter and uneven shape (arrowed) with abnormal bulges and blind ends (circled), arteriolar–venous shunts, and plasma channels lacking red blood cells.1–3 Similarly, the accompanying lymphatic vessels are dilated, leaky and discontinuous leading to dilated fluid-engorged vessels.4,5
Functionally, the ability of the tumor vasculature to deliver nutrients via blood vessels and remove waste products via the lymphatic system is drastically diminished. Tumor vessels are more permeable than normal vessels; their immature nature means they are poorly invested with smooth muscle cells and may have a discontinuous endothelial cell lining with an abnormal basement membrane.6,7 Increased vessel permeability results in aberrant osmotic forces, leading to accumulation of vascular contents and elevated interstitial fluid pressure.8,9 Geometric resistance caused by irregular vessel shape and diameter leads to impaired blood flow, consequently there is often an inadequate oxygen supply to tumor cells with micro-regional hypoxia.8–11 These consequences of high structural heterogeneity and uneven flow can readily be demonstrated by computer visualizations of normal and tumor vascular networks. Reductions in calculated oxygen tension (pO2) in areas of geometric resistance to blood flow and vessel bind ends are clearly identified (Figure 1C–D).12
The abnormal characteristics of tumor vasculature lead to aberrant micro-environmental conditions that obstruct traditional therapeutic anti-cancer strategies.9 Microregional hypoxia can result in resistance to both radiotherapy 13 and chemotherapy.14 Nonetheless, the unique features of tumor vasculature compared with that of normal tissues also present an opportunity for selective therapeutic intervention.
Selective Targeting of the Tumor Vasculature
Targeting the angiogenesis-driven sprouting of new vessels, 6,15 has seen a revolution in anti-cancer drug development in the past decade. The observation that tumors cannot grow beyond a size of approximately 2 mm3 without the support of neovascularization 16 has led to the clinical development of a plethora of angiogenesis-inhibiting agents (AIAs) that target vascular endothelial growth factor (VEGF) and its receptor (VEGFR).17–19 Ongoing anti-angiogenic drug development is also evaluating the potential benefits of targeting a number of other pro-angiogenic pathways, including those involving basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), placental growth factor (PlGF), insulin-like growth factor (IGF), mammalian target of rapamycin (mTOR), and histone deacetylases.20–25
A number of other approaches have sought to target tumor endothelial cells. These include the use of peptides, as well as antibodies directed toward tumor endothelial cell-specific antigens, to deliver bound endothelial cell-damaging agents.26–28 Gene therapy with endothelial cell-specific promoters has also been evaluated.29 A number of endothelial cell-specific vectors based on gene promoters are now known but clinical progress has not been documented.28,30–32
An alternative therapeutic approach that directly targets already established tumor vasculature has resulted in the evolution of a novel class of agents known as Tumor-Vascular Disrupting Agents (Tumor-VDAs). Tumor-VDAs selectively disrupt the immature and rapidly (>20 times faster than normal)33,34 proliferating endothelial cells of established tumor vasculature either by direct apoptotic effects or by effects related to endothelial cell reliance on a tubulin cytoskeleton to maintain cell shape. These agents aim to arrest the blood flow in tumors, with the resulting ischemia leading to a cascade of secondary tumor cell death in the central part of tumors.26,35,36 A clear division between Tumor-VDAs and anti-angiogenic therapies has now been established (Table 1).
Table 1.
Key preclinical differences between the Tumor-Vascular Disrupting Agents (Tumor-VDAs) and anti-angiogenic drug classes.
| Tumor-VDAs | Anti-angiogenic agents |
|---|---|
| Administered acutely | Administered chronically |
| Disrupt the established tumor vasculature | Inhibit neovascularization |
| Cause vessel occlusion and inhibition of blood flow | Induce vascular normalization with initial improved tumor blood flow |
| Cause extensive tumor necrosis | Prevent or limit tumor growth |
| Particularly active against large tumor masses, causing extensive central necrosis | Particularly active in peripheral tumor locations where nascent vessels are more predominant |
Tumor-VDAs: Comparison with AIAs
AIAs and Tumor-VDAs differ in three key respects: their physiologic target, the type or extent of disease that is likely to be susceptible, and the treatment scheduling.37 Since AIAs are cytostatic in nature, and designed to inhibit the progressive development of tumor neovasculature, they are likely to be inherently tailored toward the targeting of early-stage disease or newly developing metastases.37 The usual course of administration of AIAs is thus one of chronic exposure, where protracted administration or exposure restrains revascularization following initial inhibition, and results in disease stabilization rather than tumor shrinkage.20,38–40 In contrast, Tumor-VDAs exert a more immediate damaging effect on existing tumor vasculature, and are therefore suited to acute administration, requiring a shorter period of drug exposure. Tumor-VDAs lead to the collapse of existing tumor vasculature and secondary tumor cell death, with evidence for a superior effect on bulky disease.41,42
Preclinical studies have not established tumor necrosis as a predominant effect with AIAs, although there is clinical magnetic resonance imaging (MRI) and pathological evidence with some agents.43,44 Tumor-VDAs, on the other hand, are distinctive in their propensity for causing extensive centrally situated tumor necrosis.36,45–53 These key differences are conceptually illustrated in Figure 2.54,55
Figure 2.
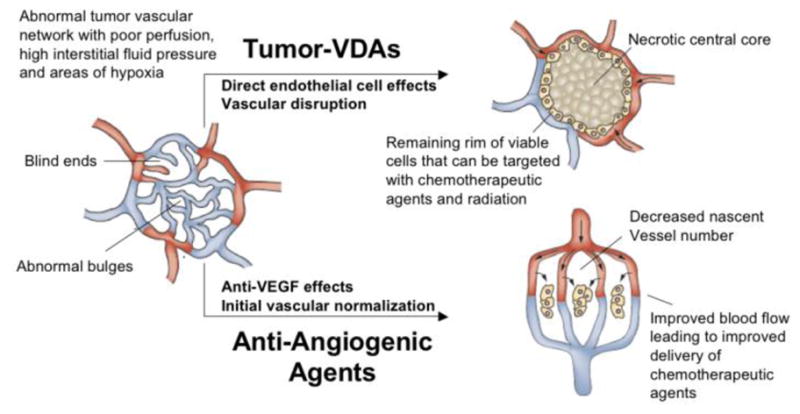
Diagram illustrating the different preclinical effects of angiogenesis-inhibiting agents (AIAs) and Tumor-Vascular Disrupting Agents (Tumor-VDAs) on abnormal tumor blood vessels. Treatment with AIAs leads to vessel normalization, allowing efficient delivery of chemotherapeutic agents and increased oxygenation to aid radiotherapy. In contrast, Tumor-VDA treatment leads to vascular disruption and extensive central necrosis, leaving a thin rim of surviving viable cells that can be targeted with standard therapies (adapted and redrawn with permission from Macmillan Publishers Ltd: Nat Rev Clin Oncol 2009; 6:395–404. Copyright 2009).54,55
Both classes of agents have found utility in combination with standard therapies, but for different reasons. Tumor-VDAs may be complimentary to radiotherapy and chemotherapy because they predominantly target the tumor core, a region of the tumor typically resistant to conventional anti-cancer therapies. AIAs on the other hand, selectively reduce immature vessel numbers, which may lead to normalization of the peripheral tumor vasculature and thus improved delivery of systemically administered chemotherapy.56
A prime target for AIAs is VEGF, and although VEGF is over-expressed by most solid tumors, it is also essential for the development of normal blood vessels. The wide expression of VEGF and its receptors in normal tissues therefore means that normal vascular networks may be affected. The degree of this inhibition is dependent upon the specificity of the inhibitor type. Preclinical studies in mice have shown that VEGF inhibitors may cause both the apoptosis of endothelial cells and regression of normal capillaries in various organs.57,58 Vascular effects that occur as a result of systemic VEGF inhibition include hypertension, proteinuria59,60 and impaired wound healing.61 A more selective targeting of fundamental structural differences between normal and tumor vasculature would potentially be of significant clinical therapeutic benefit. Tumor-VDAs seek to exploit these differences while minimizing concurrent effects on normal vasculature.
Classes of Tumor-VDAs and their Mechanisms of Action
There are currently two classes of Tumor-VDAs (Table 2). The tubulin-depolymerizing Tumor-VDAs comprise a large and diverse group of compounds that bind to the colchicine binding site of tubulin.62–64 These small-molecules are usually either stilbenes of the combretastatin family or heterocyclic compounds. Lead agents of this class include combretastatin A-4 phosphate (CA4P, fosbretabulin), 45,65,66 a serine-linked amino-derivative – AVE8062,48 and the combretastatin A-1 derivative OXi4503.67 Other Tumor-VDAs that also bind at the colchicine site include the N-acetyl colchinol ZD6126, the dolastatin-10 analogue TZT-1027 and other heterocyclic compounds such as MPC-6827, MN-029, NPI-2358 and ABT-751.50,68–70 In all cases, binding of these agents to tubulin causes microtubule depolymerization, cytoskeletal rearrangements and activation of actin stress fibers in endothelial cells, leading to changes in cell morphology (Figure 3).47,51,53,66,71–73
Table 2.
Current clinical status of Tumor-Vascular Disrupting Agents (Tumor-VDAs) in development (ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; HRMPC, hormone refractory metastatic prostate cancer; MBC, metastatic breast cancer; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma).
| Tumor-VDA | Company | Stage of clinical development |
|---|---|---|
| Flavonoid | ||
| ASA404 (vadimezan) | Novartis | Phase III (NSCLC), Phase II (HER2 –ve MBC; planned) |
| Tubulin binding | ||
| CA4P (fosbretabulin) | OXiGENE | Phase II/III (anaplastic thyroid cancer), Phase II (NSCLC) |
| AVE8062 (ombrabulin) | Sanofi-Aventis | Phase III (sarcoma), Phase I (NSCLC) |
| NPI-2358 (plinabulin) | Nereus | Phase II (NSCLC) |
| ABT-751 (E7010) | Abbott | Phase II (pediatric ALL, NSCLC, breast, colorectal, HRMPC, neuroblastoma, RCC) |
| TZT-1027 (soblidotin) | Daiichi | Phase II (sarcoma) |
| CYT997 | Cytopia | Phase II (multiple myeloma) |
| Dolastatin 10 | – | Phase II (RCC, sarcoma, pancreatic, HRMPC, liver/bile duct/gall bladder, lymphoma, CLL) |
| ZD6126 | Angiogene | Phase II |
| MPC-6827 | Myriad | Phase II (melanoma, glioblastoma) |
| OXi4503 (CA41P) | OXiGENE | Phase I |
| EPC2407 (crinobulin) | EpiCept | Phase I |
| MN-029 | Medicinova | Phase I |
| BNC105 | Bionomics | Phase I |
Figure 3.
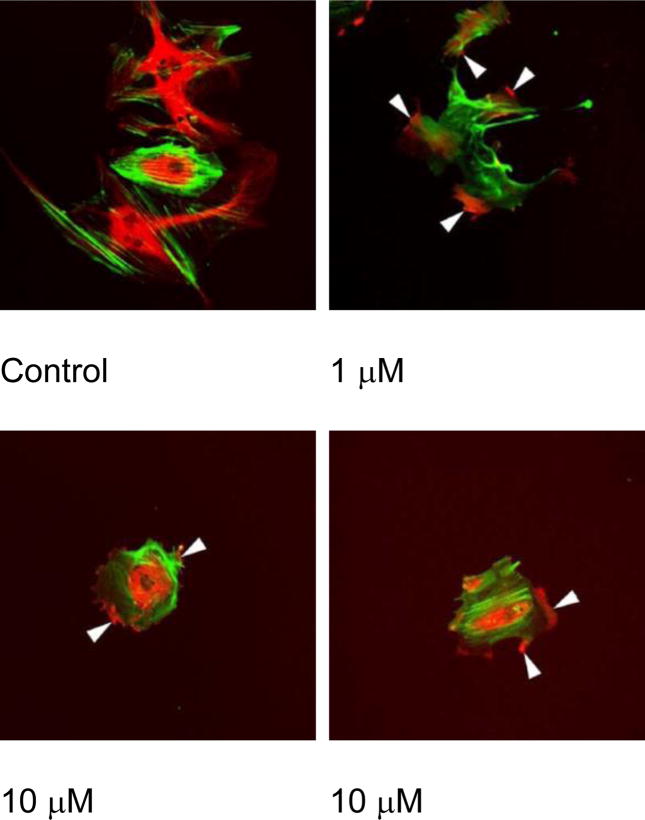
Effect of CA4P treatment on endothelial cell shape.73 Proliferating endothelial cells were untreated or treated with either 1 or 10 μM CA4P for a period of 2 h prior to staining microtubules (red, rotamine) and actin (green, fluorescein phalloidin). Arrows indicate the condensation of microtubules and rounding of endothelial cells (used with permission).73
Importantly, these agents selectively disrupt the cytoskeleton of proliferating endothelial cells.71 Both in vitro and in vivo studies in mice with the archetypal tubulin-binding Tumor-VDA, CA4P have demonstrated that the drug selectively induces regression of unstable tumor neovessels,74–76 in part by disruption of the signaling pathway of the endothelial cell-specific junctional protein, VE-cadherin.66 Activation of Rho signaling has been implicated in microtubule disruption and vessel collapse using selective inhibitors of Rho kinase to attenuate tubulin-dependent Tumor-VDA activity.77 The net result of these effects is a rounding up and surface blebbing of endothelial cells, together with increased vessel permeability and inhibition of blood flow.71,72,76,77 Rho-mediated active vasoconstriction and red cell stacking leads to further flow stagnation and vessel blockage.71,72,76 Normal vasculature (including smooth muscle cells) with a lower endothelial proliferation index and greater maturity, remains unaffected by tubulin-binding Tumor-VDAs (Figure 4).78,79
Figure 4.
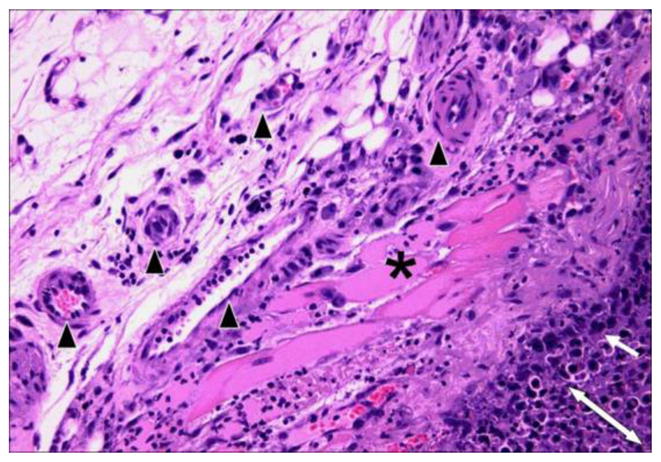
Hematoxylin and eosin stained section (at x 200 magnification) of KHT sarcoma treated with OXi4503 (25 mg/kg). The post-treatment necrotic tumor is defined by the double-headed arrow while the two- or three-cell layer of the viable tumor rim is shown with a single-headed arrow. Preserved muscle (*) and blood vessels (black arrowheads) are clearly evident in the surrounding, unaffected tissue (Siemann DW, unpublished results).
Flavonoid Tumor-VDAs have a tubulin-independent mechanism of action that results in both direct and indirect antivascular activity. This class is led by ASA404 (vadimezan, 5,6-dimethylxanthenone-4-acetic acid/DMXAA), an analog of flavone acetic acid. Direct disruption of the tumor vasculature by flavonoid Tumor-VDAs may be due to induction of apoptosis in tumor blood vessel endothelial cells. This effect has been detected within 30 minutes of administration in animal models (Figure 5).80,81 A large and early influx of neutrophils into subcutaneous Colon 38 tumors occurs following ASA404 treatment, and neutrophils have therefore been suggested as mediators of the drug’s rapid anti-vascular effects.82 Activated neutrophils are strongly implicated in endothelial cell damage and killing during inflammation.83 Increased myeloperoxidase activity, which is indicative of neutrophil activity, has also been reported following treatment with the tubulin-binding Tumor VDA CA4P in murine sarcomas.84
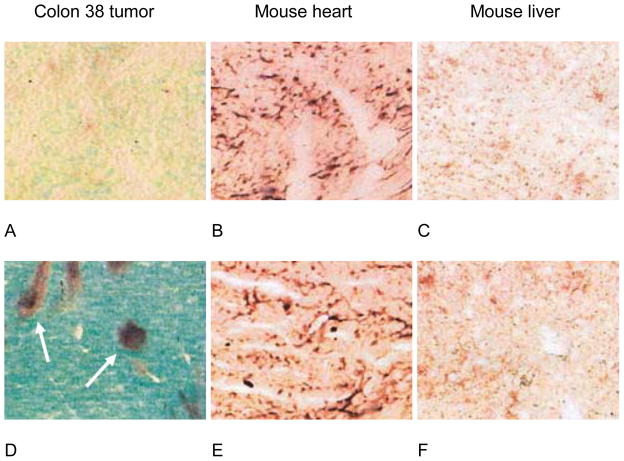
Figure 5.
Selective induction of tumor vascular endothelial cell apoptosis (arrows) in the Colon 38 tumor (A,D) compared with mouse heart (B,E) and mouse liver (C,F). Untreated (upper panels) and ASA404-treated (25 mg/kg, 3h) (lower panels). C57B1/6 mice were stained for TdT-mediated dUTP nick-end labeling (TUNEL) with alkaline phosphatase (used with permission from Macmillan Publishers Ltd: Br J Cancer 2004; 90:906–910. Copyright 2004).80
Endothelial cell death leads to exposure of the basement membrane, rupture of tumor blood vessels, and extravasation of erythrocytes into the surrounding tissues.80,81,85 Flavonoid Tumor-VDA-induced vascular damage leads to platelet accumulation within the damaged vessels, triggering the release of the vasoconstrictor 5-hydroxytryptamine (5-HT, serotonin), detected as its liver metabolite 5-hydroxyindole-3-acetic acid (5-HIAA).86–88 This direct disruption of the tumor vasculature leads to a rapid inhibition of tumor blood flow.80,81,85,87 Preclinical studies have revealed that flavonoid Tumor-VDAs can also indirectly affect the tumor vasculature by stimulating the production of cytokines such as tumor necrosis factor-α (TNF- α), interleukin 6 (IL-6), macrophage inflammatory 1α (MIP 1- α), interferon-γ, and chemokines such as interferon-inducible protein-10.81,82,87,89–93 Induction of these cytokines may also amplify the initial influx of neutrophils, providing sustained anti-vascular action.82 Evidence supporting the role of TNF-α in inducing vascular collapse is provided by the significant reductions in antivascular activity in TNF−/−or TNF receptor−/−(TNFR−/−) knockout mice.80,90
In Situ Effects of Tumor-VDA Therapy
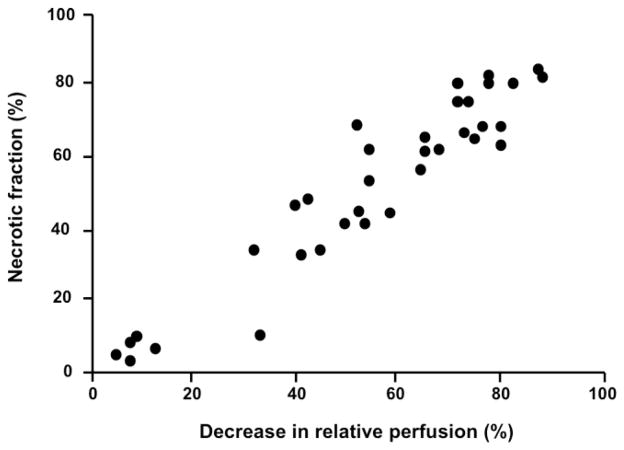
Tumor-VDAs have now been studied in a wide variety of preclinical tumor models, including transplanted and spontaneous rodent tumors, orthotopically transplanted tumors, and human tumor xenografts.28,72,75,94 Profound disruption of the tumor blood vessel network has been noted–effects include vascular shutdown, reductions in tumor blood flow, vessel permeability changes, and loss of patent blood vessels. Within minutes of Tumor-VDA treatment, tumor perfusion begins to be compromised. The suppression of tumor blood flow by both flavonoid and tubulin-binding Tumor-VDAs is rapid, dose dependent, and typically sustained for 24–48 hours, with maximal vessel shutdown and permeability changes occurring within 1–6 hours.36,47,50,74,80,81,91,95–103 In contrast, such extensive blood flow effects have not been seen in normal tissues.29,35 However, since these assessment endpoints are not practical in the clinic, efforts to monitor the effects of Tumor-VDA treatments utilizing non-invasive methods that could be applied in such a setting have begun. MRI studies in an orthotopic model of human head and neck cancer treated with the flavonoid Tumor-VDA ASA404 showed a marked decrease in enhancement within the tumor after contrast imaging, indicative of treatment-induced reduction in vascular perfusion 24 hours after infusion, together with hypo-intense regions within the tumor, indicating tumor hemorrhage, and no observable effects on surrounding tissues (Figure 6).104 In a study of a tubulin-binding Tumor-VDA, changes in tumor perfusion and tumor necrotic fraction after CA4P treatment were compared in the same individual animals.105 The results demonstrated that tumor perfusion as observed by MRI strongly correlated with tumor necrosis (Figure 7). Dynamic contrast-enhanced (DCE)-MRI measurements in patients also demonstrated specific changes in tumor perfusion after Tumor-VDA treatment,106–108 but these have as yet not been linked to a defined treatment outcome.
Figure 6.
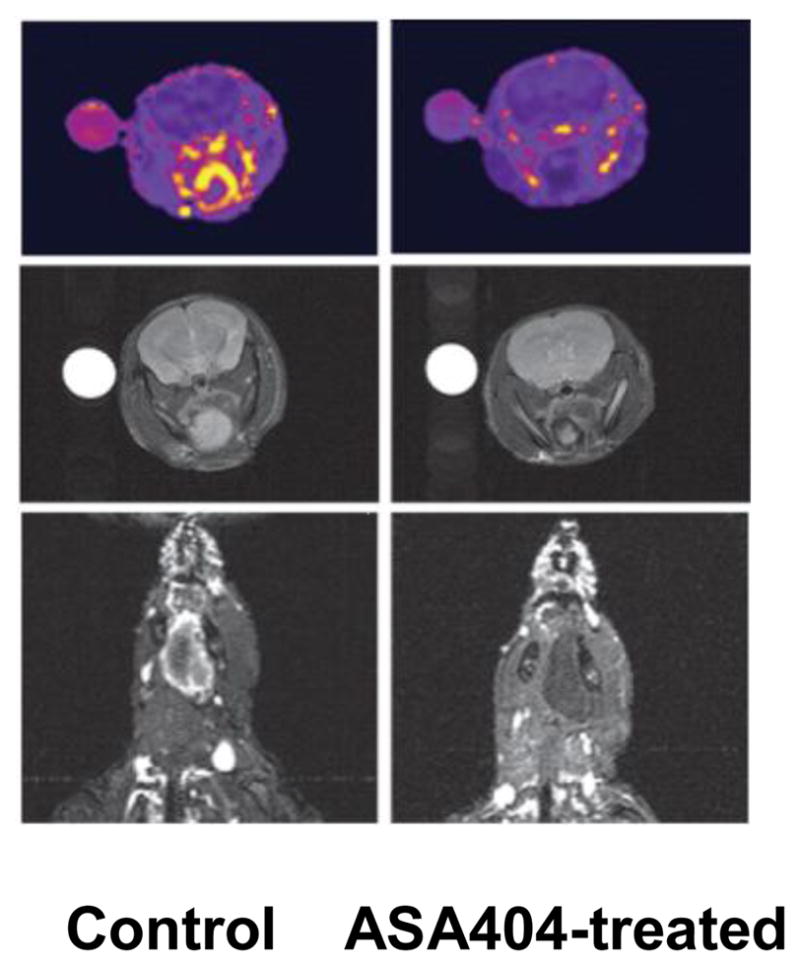
Contrast enhanced magnetic resonance imaging (MRI) images in an orthotopic head and neck cancer mouse model showing lack of enhancement in the ASA404-treated tumor indicating reduced vascular perfusion (top and bottom panels), and dark, hypo-intense regions in the tumor but not in the surrounding area (middle panels), suggesting selective tumor vascular hemorrhage (used with permission).104
Figure 7.
Comparison of the decrease in relative perfusion and necrotic fraction of KHT tumors in individual mice treated with CA4P.105 Dynamic contrast-enhanced magnetic resonance imaging measurements and histological analysis of tumor necrotic fraction were made on the same animal (data points represent individual animals); r=0.89, p<0.00001 (used with permission).105
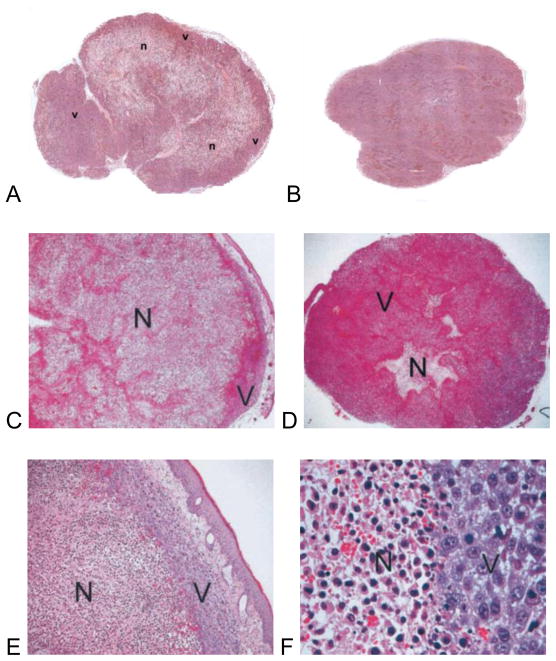
The impact of vascular disruption by Tumor-VDA treatments on tumor tissue has been readily demonstrated both by histologic assessments and measures of secondary cell death due to ischemia; two factors that are closely correlated.42,52,99,109 Typically, these show extensive, dose-dependent necrosis that can extend to within a few cell layers from the margin of the tumors (Figures 4 and 8).28,75,76,94 Histologic evidence for tumor necrosis induced by both flavonoid Tumor-VDAs (ASA40489,92) and tubulin-binding Tumor-VDAs (ZD6126,52,53,109 CA4P,36,45,46 AVE8062,47,48 OXi4503,49,67 MN-02950 and TZT-102751) has been reported in numerous preclinical tumor models. Importantly, vascular damage resulting from tubulin-binding Tumor-VDAs has been confined to tumor blood vessel networks (Figure 4). Similarly, immunostaining and histologic analyses have highlighted the selective nature of ASA404-induced vascular damage and necrosis in these preclinical studies, showing no toxicity in normal salivary gland, heart, liver and skeletal muscle tissues.104
Figure 8.
Comparison of the necrotic effects of a flavonoid Tumor-VDA (ASA404) and a tubulin-binding Tumor-VDA (ZD6126). Panel A: hematoxylin and eosin stained section of a G3H prolactinoma 24 hours post-treatment with ASA404 (350 mg/kg) Grade 4, extensive necrosis (n=necrotic tissue, v=viable tissue) and Panel B: control – Grade 1, no necrosis.92 Panel C: stained section of a Calu-6 lung cancer xenograft (x 16 magnification) post-treatment with ZD6126 (200 mg/kg) and Panel D: corresponding vehicle-treated control (N=necrotic tissue, V=viable tissue). Panels E (x 100) and F (x 400): magnifications showing a thin viable rim of cells remaining, and contrasting the necrotic and viable cells (used with permission).52
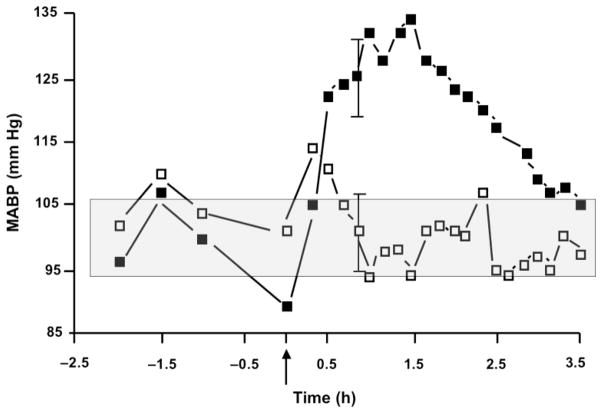
Blood pressure may be elevated by tumor blood vessel-directed anti-cancer treatments such as anti-angiogenic therapies108,110–112 and Tumor-VDAs.108,113 In mice and rats, tubulin-binding Tumor-VDAs can induce hypertension114,115 similar to that seen in humans.108,113 For example, treating tumor-bearing mice with a 100 mg/kg dose of CA4P raises mean arterial pressure to approximately 130 mm Hg within 1 hour of treatment before returning to near normal 3–4 hours later (Figure 9). Several strategies to counteract tubulin-binding Tumor-VDA-associated hypertension have been investigated preclinically. In mice, administering the vasodilator hydralazine (0.2 mg/kg) just prior to CA4P treatment inhibited the rise in blood pressure seen after CA4P exposure (127 ± 6 mm Hg) to pretreatment values (103 ± 6 mm Hg) (Siemann et al., unpublished results). In rats, infusions of the calcium channel blocker diltiazem and of the vasodilator nitroglycerin resulted in near complete blockage of CA4P-induced hypertension115 and co-administration of the channel blocker atenolol and the beta blocker nifedipine effectively inhibited transient hypertension induced by the tubulin-binding Tumor-VDA ZD6126.114 Gould et al. further noted that in susceptible strains of rats tubulin-binding Tumor-VDA-induced blood pressure elevation could lead to detectable cardiac damage; a result that could be prevented by inhibiting the hypertensive response.114 Taken together, these preclinical investigations suggest that treatment with anti-hypertensive agents may prove clinically valuable to prevent potential cardiovascular side effects of Tumor-VDAs. Perhaps most importantly, the anti-tumor efficacy of the tubulin-binding Tumor-VDAs was still maintained in the presence of anti-hypertensive medications.114 Non-dose-limiting hypertension in patients given the flavonoid Tumor-VDA ASA404 has only been seen at doses approaching the maximum tolerated dose in Phase I clinical trials,116,117 and was not observed in Phase II trials.118,119 Nonetheless, monitoring and controlling hypertension as well as excluding patients with a history of cardiovascular disease will be a key element in the Phase II/III protocols with both flavonoid and tubulin-binding Tumor-VDAs as it has been with the anti-angiogenic therapeutics bevacizumab and sorafenib.111
Figure 9.
Blood pressure measurements in mice made using thoracic aortic implantation of pressure-sensing catheters combined with the subcutaneous placement of transmitter bodies and monitoring by placing their cages on top of the telemetry receiver pads for data collection. Data are mean (± SD) arterial blood pressure (MABP) measurements in groups of 8 control (□) or CA4P-treated (100 mg/kg) (■) C3H mice; shaded area indicates normal range of MABP (Siemann DW, unpublished results).
The propensity of both classes of Tumor-VDAs to induce necrosis in the poorly perfused core regions of tumors leaving a thin layer of viable cells at the periphery is well documented (Figures 4 and 8).98,120–122 This residual rim of viable neoplastic cells is generally believed to survive because these cells derive their nutritional support from vasculature in the adjacent normal tissue which is unaffected by Tumor-VDA treatment.75 Recent studies have used spectral imaging of tumor microvessel hemoglobin saturation with mouse window chamber tumors to measure the real-time response of tumors to Tumor VDA treatments. These studies have revealed not only transient vessel collapse with time-dependent oxygenation changes followed by recovery but also extensive vascular remodeling and neovascularization of the tumor rim (Figure 10).123 Thus despite the extensive blood flow shutdown and central tumor necrosis observed with Tumor-VDAs, the surviving ‘viable rim’ can act as a source of tumor regrowth. Consequently, only repeated multiple-dose treatments with such agents impact tumor growth significantly52,75,94,124 and Tumor-VDA treatments alone are unlikely to eradicate the tumor mass. Nonetheless, the destruction of large tumor areas, particularly in the central regions and areas typically most resistant to radiation and chemotherapy, is clearly highly beneficial and desirable. Tumor-VDAs are therefore likely to be of greatest utility when applied in a combined modality setting with conventional anti-cancer therapies.
Figure 10.
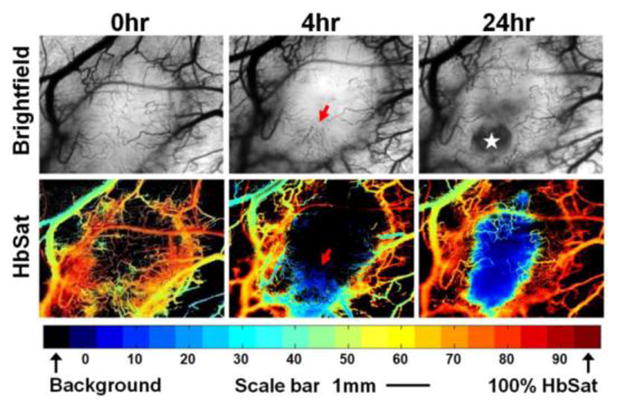
Typical OXi4503 treatment progression in a 4T1 tumor. The brightfield images (upper panels) and HbSat maps (lower panels) show structural alterations in the vasculature through the course of a single treatment. The oxygenation levels in the HbSat maps are color coded as indicated by the colorbar. Arrows highlight the disintegrating vasculature, while the star indicates the avascular regions created by the OXi4503 treatment (redrawn with kind permission from Springer Science+Business Media: Oncol Rep 2010; 23:685–692. Wankhede M, et al. Figure 1. Copyright 2010).123
Combination of Tumor-VDAs with Other Therapies
1. Radiotherapy
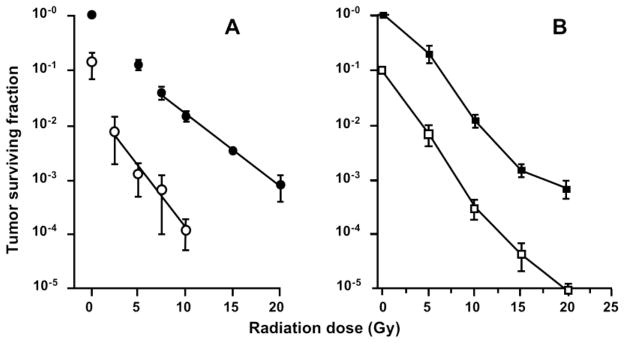
The cellular response to radiation has long been known to be strongly dependent upon oxygen concentration.125 Since Tumor-VDAs eliminate large portions of oxygen-deficient hypoxic cells from solid tumors, the combination of such agents with radiotherapy is logical. Indeed, it has now been well established that combining localized radiotherapy with various Tumor-VDAs results in significantly enhanced tumor cell killing and tumor growth inhibition compared with radiotherapy alone.42,74,94,120,126–128 Figure 11 illustrates the reduction in clonogenic cell survival in murine KHT sarcomas treated with increasing single doses of radiation administered in combination with ASA404 (A)126 or OXi4503 (B).74,79,94 Enhancement of radiation damage has also been reported for other tubulin-binding Tumor-VDAs including ABT-751, CA4P, MN-029 and TZT-1027. 42,74,94,127,128 In these studies the Tumor-VDA is typically administered 1–3 hours post radiation treatment – thus avoiding any possible negative effects on radiation efficacy that would arise if the Tumor-VDA treatment rendered some tumor cells hypoxic at the time of irradiation by inducing transient reductions in tumor blood flow.74,94 In the case of ASA404, the addition of hypoxia-selective bioreductive drugs such as tirapazamine and CI-1010 further enhanced the tumor response to ASA404 plus radiation, suggesting ASA404 treatment did not entirely eliminate the population of hypoxic cells affecting radiation response.98
Figure 11.
Enhancement of radiation damage by ASA404 (A) and OXi4503 (B) in the murine KHT sarcoma model, assessed by clonogenic cell survival assay: radiation alone (closed symbols) and radiation plus ASA404 or OXi4503 (open symbols) (Panel A redrawn with kind permission from Springer Science+Business Media: Radiat Res 2001; 156:503–509. Murata R, et al. Figure 3. Copyright 2001).79,126
Clinically most radiotherapy is delivered using daily fractionated dose treatments, therefore the incorporation of Tumor-VDA exposures into such a setting has also been evaluated. In the case of the tubulin-binding Tumor-VDAs CA4P and ZD6126, the drug was administered after the last radiation fraction at the end of each week of treatment. This resulted in a significantly enhanced tumor response to fractionated radiotherapy.35,42 Studies combining the flavonoid Tumor-VDA ASA404 with fractionated radiotherapy also reported improved treatment outcomes.120 Interestingly, when ASA404 was utilized it was administered successfully during the course of fractionated radiation.120 Importantly, Tumor-VDAs have shown neither significant effects on the radiation response of early-responding normal tissue such as skin,120,126,129 nor any effects on late-responding normal tissues such as bladder and lung.130 Taken together, these findings support the notion that combining Tumor-VDAs with radiotherapy may yield a therapeutic benefit.
2. Chemotherapy
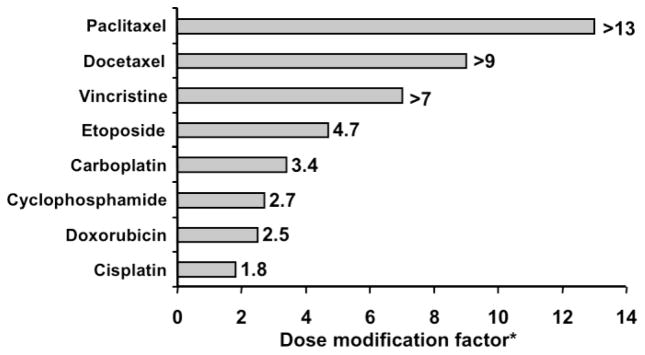
Preclinical studies on Tumor-VDAs combined with various chemotherapeutic agents have demonstrated improved anti-tumor activity compared with chemotherapy alone. Enhanced therapeutic interactions with the flavonoid Tumor-VDA ASA404 in combination with a number of different cytotoxic agents have been reported in the MDAH-MCa-4 mouse mammary tumor; most notably taxanes (Figure 12).102,131,132 Studies with paclitaxel in human non-small cell lung cancer (NSCLC) xenografts have also shown synergistic activity, as well as tumor cures.131,133 In contrast, no tumor cures were observed when either agent was used alone.133 Marked potentiation of docetaxel by ASA404 has also been observed in preclinical studies in human prostate cancer xenografts, resulting in a 43% cure rate with no significant increase in host toxicity.134 An additive or synergistic effect and thinning of the viable rim has been demonstrated with tubulin-binding Tumor-VDAs such as ZD612652 and CA4P29,102,135 when combined with various chemotherapeutic agents. Particular efficacy was noted for CA4P in combination with paclitaxel and manumycin A or carboplatin in anaplastic thyroid mouse xenografts.136 The related drug AVE8062 in combination with docetaxel significantly prolonged survival in HeyA8-injected mice.48
Figure 12.
Interaction of ASA404 with chemotherapy. The dose modification factor was defined as the ratio of the effect with ASA404 plus cytotoxic agent versus cytotoxic agent alone (adapted with kind permission from Springer Science+Business Media: Cancer Chemother Pharmacol 2003; 51:43–52. Siim BG, et al. Table 1. Copyright 2003).132
The improved treatment response to chemotherapy upon addition of Tumor-VDAs has been attributed to the elimination of those poorly perfused regions of the tumor that are either inaccessible for effective drug delivery or resistant to chemotherapeutic agents due to their proliferation status.29,52,74,102,132,135,137 Blood flow reductions caused by vascular disruption may also lead to drug entrapment and an improved response through increased tumor exposure to the drug.102,136–138 As with radiotherapy, the schedule of administration of chemotherapeutic agents and Tumor-VDAs is critical since rapid vascular disruption may render tumor cells inaccessible to chemotherapy.102,139 Preclinical studies with the flavonoid Tumor-VDA ASA404 suggest that a chemotherapeutic agent should be given either before or shortly after Tumor-VDA administration to avoid compromised delivery.132 Scheduling studies with tubulin-binding Tumor-VDAs indicate that administering the chemotherapy a few hours before may be optimal.102,109 When the tubulin-binding Tumor-VDA ZD6126 was combined with a microtubule stabilizing drug, maximum benefit was obtained when the Tumor-VDA was given 72 hours after taxane treatment.140 Importantly, the inclusion of the antivascular agents did not increase bone marrow stem cell toxicity associated with these anti-cancer drugs, thus giving rise to a therapeutic gain.102
Nitric oxide generation has been shown to protect tumor vasculature against Tumor-VDA-induced injury through anti-neutrophil action.84 Tumor-VDAs have therefore also been investigated in combination with nitric oxide synthase inhibitors. Repeated dosing of N-nitro-L-arginine with CA4P produced significantly enhanced growth delay in p22, CaNT and mouse mammary tumors. Nitric oxide synthase inhibitors may therefore have utility in combination with other Tumor-VDAs in development.141,142
Demonstrating improved tumor responses through the combination of Tumor-VDAs and chemotherapy will only be of benefit if such a combined modality treatment does not enhance the response of critical normal tissues. Results from preclinical investigations addressing this question indicate the enhancements in anti-tumor efficacy generally occur without any significant increase in host toxicity.29,52,98,102,132,138,139 Data on chemotherapeutic agent-specific side effects are more limited but the absence of enhanced bone marrow toxicity is encouraging.102
3. AIAs
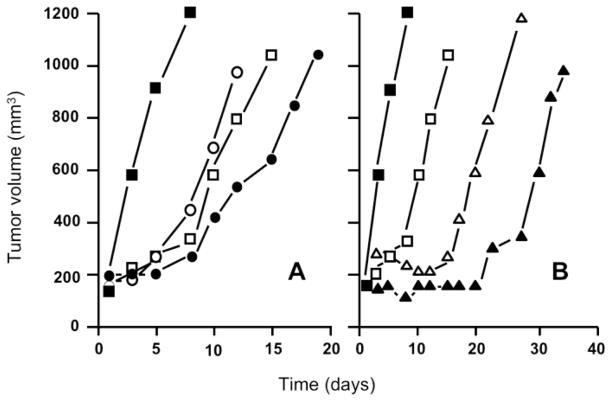
Vascular-targeted therapies have shown impressive anti-tumor effects in preclinical tumor models, and recent clinical observations are encouraging. Nevertheless, the complexity of pathways available for neovascularization implies that impairing only a single aspect of angiogenesis with AIAs will probably not suffice, while Tumor-VDAs will not be able to eliminate pockets of tumor cells with a nutritional supply derived from blood vessels in the surrounding normal tissues. A logical extension in vascular targeting is therefore the application of anti-angiogenic and vascular disrupting therapies in concert. Since both the initiation of new vessel formation and the integrity of the existing blood vessel network are critical to tumor growth and survival, such a double assault on the tumor vasculature should hold considerable promise. In view of their disparate modes of action, the combined application of AIAs and Tumor-VDAs is likely to lead to complimentary anti-tumor effects.37 This possibility has been supported by observations in preclinical tumor models. For example, the combination of VEGFR2-associated tyrosine kinase inhibition (ZD6474) and Tumor-VDA therapy (ZD6126) was found to lead to marked improvements in treatment outcomes even in tumors demonstrating only a modest response to single-agent therapy.143,144 Studies in which the anti-VEGF antibody bevacizumab was combined with the tubulin-binding Tumor-VDAs CA4P or OXi4503 to treat human clear cell renal carcinoma xenografts showed that when two vascular-targeted therapies were combined, a significantly greater tumor response could be attained compared with that achieved with single agent treatments (Figure 13).145 Enhanced anti-tumor activity has also been reported for the flavonoid Tumor-VDA ASA404 in combination with bevacizumab in lung and colon cancer xenografts.146,147
Figure 13.
Response of Caki-1 tumors to bevacizumab (2 mg/kg, twice a week for 2 weeks), CA4P (A) or OXi4503 (B) (100 mg/kg or 25 mg/kg respectively, three times a week for 2 weeks) or the combination of an angiogenesis-inhibiting agent and Tumor-Vascular Disrupting Agent (median tumor responses of groups of 8–10 mice). Controls (■), bevacizumab (□), CA4P (○), OXi4503 (△), bevacizumab + CA4P (●), bevacizumab + OXi4503 (▲) (redrawn with permission).145
Conclusions, Clinical Status, and Future Perspective
The direct vascular-targeted approach to anti-cancer drug development offers a complementary approach to both standard chemotherapy and other targeted therapies. A wealth of preclinical data has provided proof of concept for selective disruption of established tumor vasculature. Decreases in vascular perfusion and even tumor shrinkage have been observed by techniques such as DCE-MRI, together with immunostaining and histologic evidence for selective and extensive tumor necrosis. These studies have demonstrated the efficacy of Tumor-VDAs in various tumor types, however, because microvessels can acquire organ-specific specialization in response to local tissue-derived signals types,148 it is conceivable that there may be some differences in the response to such agents depending on the tumor site of origin. Importantly the preclinical investigations have concluded that Tumor-VDAs hold significant potential when combined with other therapies, most notably taxane chemotherapy, radiotherapy, and anti-angiogenic drugs. Selectivity in a clinical setting has been demonstrated by MRI techniques, and a number of Tumor-VDAs have now been evaluated in Phase I and II clinical trials (Table 2). In Phase II trials ASA404 resulted in an apparent 5 month survival advantage in NSCLC patients when administered in combination with cytotoxic drugs. 118,119 These observations led to two Phase III clinical trials investigating ASA404 in combination with taxane-based chemotherapy for first-line (ATTRACT-1) or second-line (ATTRACT-2) treatment of NSCLC. 149 The former, which combined paclitaxel (200 mg/kg), carboplatin (AUC 6) and ASA404 (1800 mg/m2) was halted when the planned interim analysis showed little prospect of demonstrating a survival benefit with ASA404 in this setting. The ATTRACT-2 trial for the second-line treatment of patients with non-small cell lung cancer (doxetaxel, 75 mg/m2 and ASA404, 1800 mg/m2) is ongoing. Following Phase II clinical trial evidence of potential clinical benefit150 the tubulin-binding Tumor-VDA, CA4P (fosbretabulin, 60 mg/m2) is currently being studied in a Phase II trial (FALCON) in combination with bevacizumab (15 mg/kg), carboplatin (AUC 6) and paclitaxel (200 mg/m2) as first-line treatment of advanced NSCLC. A Phase III trial (FACT) in anaplastic thyroid cancer is comparing the effects of carboplatin (AUC 6) and paclitaxel (200 mg/m2) with carboplatin (AUC 6) and paclitaxel (200 mg/m2) plus CA4P (60 mg/m2).151 These pivotal trials will determine the future potential of Tumor-VDAs in cancer treatment.
Acknowledgments
The author’s work is supported by the National Cancer Institute (Public Health Service grant CA084408); the funding sponsor had no involvement in the preparation of this manuscript. Writing assistance was provided by Articulate Science, London, UK (funded by Novartis Pharmaceuticals Corporation).
Footnotes
Conflict of Interest Statement
The author is a member of the Scientific Advisory Board of OXiGENE, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer. 2001;84:1354–1362. doi: 10.1054/bjoc.2001.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dewhirst MW, Kimura H, Rehmus S, et al. Microvascular studies on the origins of perfusion-limited hypoxia. Br J Cancer. 1996;27:S247–S251. [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald D, Choyke P. Imaging of angiogenesis: from microscope to clinic. Nature Med. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 4.Leu A, Berk D, Lymboussaki A, et al. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60:4324–4327. [PubMed] [Google Scholar]
- 5.Padera T, Kadambi A, di Tomaso E, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 7.Gee MS, Procopio WN, Makonnen S, et al. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol. 2003;162:183–193. doi: 10.1016/S0002-9440(10)63809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong RT, Boucher Y, Kozin SV, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 9.Vaupel P, Fortmeyer HP, Runkel S, et al. Blood flow, oxygen consumption, and tissue oxygenation of human breast cancer xenografts in nude rats. Cancer Res. 1987;47:3496–3503. [PubMed] [Google Scholar]
- 10.Vaupel P, Schlenger K, Knoop C, et al. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- 11.Vaupel P, Hockel M. Blood supply, oxygenation status and metabolic micromilieu of breast cancers: characterization and therapeutic relevance. Int J Oncol. 2000;17:869–879. doi: 10.3892/ijo.17.5.869. [DOI] [PubMed] [Google Scholar]
- 12.Pries A, Cornelissen A, Sloot A, et al. Structural adaptation and heterogeneity of normal and tumor microvascular networks. PLoS Computational Biology. 2009;5:1–11. doi: 10.1371/journal.pcbi.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohindra J, Rauth A. Increased cell killing by metronidazole and nitrofurazone of hypoxic compared to aerobic mammalian cells. Cancer Res. 1976;36:930–936. [PubMed] [Google Scholar]
- 14.Koch S, Meyer F, Honecker F, et al. Efficacy of cytotoxic agents used in the treatment of testicular germ cell tumors under normoxic and hypoxic conditions in vitro. Br J Cancer. 2003;89:2133–2139. doi: 10.1038/sj.bjc.6601375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randal J. Antiangiogenesis drugs target specific cancers, mechanisms. J Natl Cancer Inst. 2000;92:520–522. doi: 10.1093/jnci/92.7.520. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 18.Hicklin D, Ellis M. Role of the vascular endothelial growth factor pathway in tumour growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 19.Ma W, Hidalgo M. Exploiting novel molecular targets in gastrointestinal cancers. World J Gastroenterol. 2007;13:5845–5856. doi: 10.3748/wjg.v13.i44.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskens FA. Angiogenesis inhibitors in clinical development; where are we now and where are we going? Br J Cancer. 2004;90:1–7. doi: 10.1038/sj.bjc.6601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapoor A, Figlin R. Targeted inhibition of mammalian target of rapamycin for the treatment of advanced renal cell carcinoma. Cancer. 2009;115:3618–3630. doi: 10.1002/cncr.24409. [DOI] [PubMed] [Google Scholar]
- 22.Melillo D. Inhibiting hypoxia-inducible factor 1 for cancer therapy. Mol Cancer Res. 2006;4:601–605. doi: 10.1158/1541-7786.MCR-06-0235. [DOI] [PubMed] [Google Scholar]
- 23.Michaelis M, Michaelis U, Fleming I, et al. Valproic acid inhibits angiogenesis in vitro and in vivo. Mol Pharmacol. 2004;65:520–527. doi: 10.1124/mol.65.3.520. [DOI] [PubMed] [Google Scholar]
- 24.Qian D, Wang X, Kachhap S, et al. The histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2004;64:6626–6634. doi: 10.1158/0008-5472.CAN-04-0540. [DOI] [PubMed] [Google Scholar]
- 25.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Molema G, King S, et al. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science. 1997;275:547–550. doi: 10.1126/science.275.5299.547. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson F, Kosmehl H, Zardi L, et al. Targeted delivery of tissue factor to the ED-B domain of fibronectin, a marker of angiogenesis, mediates the infarction of solid tumors in mice. Cancer Res. 2001;61:711–716. [PubMed] [Google Scholar]
- 28.Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res. 2004;10:415–427. doi: 10.1158/1078-0432.ccr-0642-03. [DOI] [PubMed] [Google Scholar]
- 29.Chaplin DJ, Dougherty GJ. Tumour vasculature as a target for cancer therapy. Br J Cancer. 1999;80 (Suppl 1):57–64. [PubMed] [Google Scholar]
- 30.Jin N, Chen W, Blazar BR, et al. Gene therapy of murine solid tumors with T cells transduced with a retroviral vascular endothelial growth factor - immunotoxin target gene. Hum Gene Ther. 2002;13:497–508. doi: 10.1089/10430340252809793. [DOI] [PubMed] [Google Scholar]
- 31.Masood R, Gordon EM, Whitley MD, et al. Retroviral vectors bearing IgG-binding motifs for antibody-mediated targeting of vascular endothelial growth factor receptors. Int J Mol Med. 2001;8:335–343. doi: 10.3892/ijmm.8.4.335. [DOI] [PubMed] [Google Scholar]
- 32.Savontaus MJ, Sauter BV, Huang TG, et al. Transcriptional targeting of conditionally replicating adenovirus to dividing endothelial cells. Gene Ther. 2002;9:972–979. doi: 10.1038/sj.gt.3301747. [DOI] [PubMed] [Google Scholar]
- 33.Denekamp J. Endothelial cell proliferation as a novel approach to targeting tumour therapy. Br J Cancer. 1982;45:136–139. doi: 10.1038/bjc.1982.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denekamp J. Vascular endothelium as the vulnerable element in tumours. Acta Radiol Oncol. 1984;23:217–225. doi: 10.3109/02841868409136015. [DOI] [PubMed] [Google Scholar]
- 35.Chaplin D, Pettit G, Hill S. Anti-vascular approaches to solid tumor therapy: evaluation of combretastatin A4 phosphate. Anticancer Res. 1999;19:189–195. [PubMed] [Google Scholar]
- 36.Dark GG, Hill SA, Prise VE, et al. Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res. 1997;57:1829–1834. [PubMed] [Google Scholar]
- 37.Siemann DW, Bibby MC, Dark GG, et al. Differentiation and definition of vascular-targeted therapies. Clin Cancer Res. 2005;11:416–420. [PubMed] [Google Scholar]
- 38.Cai W, Rao J, Gambhir S, et al. How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther. 2006;5:2624–2633. doi: 10.1158/1535-7163.MCT-06-0395. [DOI] [PubMed] [Google Scholar]
- 39.Korn E, Arbuck S, Pluda J, et al. Clinical trial designs for cytostatic agents: are new approaches needed? J Clin Oncol. 2001;19:265–272. doi: 10.1200/JCO.2001.19.1.265. [DOI] [PubMed] [Google Scholar]
- 40.Muruganandham M, Lupu M, Dyke J, et al. Preclinical evaluation of tumor microvascular response to a novel antiangiogenic/antitumor agent RO0281501 by dynamic contrast-enhanced MRI at 1.5 T. Mol Cancer Ther. 2006;5:1950–1957. doi: 10.1158/1535-7163.MCT-06-0010. [DOI] [PubMed] [Google Scholar]
- 41.Landuyt W, Verdoes O, Drijkoningen M, et al. Vascular targeting of solid tumours: a major ‘inverse’ volume-response relationship following combretastatin A-4 phosphate treatment of rat rhabdomyosarcomas. Eur J Cancer. 2000;36:1833–1843. doi: 10.1016/s0959-8049(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 42.Siemann DW, Rojiani AM. The vascular disrupting agent ZD6126 shows increased antitumor efficacy and enhanced radiation response in large, advanced tumors. Int J Radiat Oncol Biol Phys. 2005;62:846–853. doi: 10.1016/j.ijrobp.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 43.Baccala A, Jr, Hedgepeth R, Kaouk J, et al. Pathological evidence of necrosis in recurrent renal mass following treatment with sunitinib. Int J Urol. 2007;14:1095–1097. doi: 10.1111/j.1442-2042.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- 44.Horger M, Lauer UM, Schraml C, et al. Early MRI response monitoring of patients with advanced hepatocellular carcinoma under treatment with the multikinase inhibitor sorafenib. BMC Cancer. 2009;9 doi: 10.1186/1471-2407-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaplin DJ, Hill S. The development of combretastatin A4 phosphate as a vascular targeting agent. Int J Radiat Oncol Biol Phys. 2002;54:1491–1496. doi: 10.1016/s0360-3016(02)03924-x. [DOI] [PubMed] [Google Scholar]
- 46.Grosios K, Holwell SE, McGown AT, et al. In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate prodrug. Br J Cancer. 1999;81:1318–1327. doi: 10.1038/sj.bjc.6692174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hori K, Saito S. Microvascular mechanisms by which the combretastatin derivative AC7700 (AVE8062) induces tumor blood flow stasis. Br J Cancer. 2003;89:1334–1344. doi: 10.1038/sj.bjc.6601261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim TJ, Ravoori M, Landen CN, et al. Antitumor and antivascular effects of AVE8062 in ovarian carcinoma. Cancer Res. 2007;67:9337–9345. doi: 10.1158/0008-5472.CAN-06-4018. [DOI] [PubMed] [Google Scholar]
- 49.Sheng Y, Hua J, Pinney KG, et al. Combretastatin family member OXI2503 induces tumor vascular collapse through the induction of endothelial apoptosis. Int J Cancer. 2004;111:604–610. doi: 10.1002/ijc.20297. [DOI] [PubMed] [Google Scholar]
- 50.Shi W, Siemann D. Preclinical studies of the novel vascular disrupting agent MN-029. Anticancer Res. 2005;25:3899–3904. [PubMed] [Google Scholar]
- 51.Otani M, Natsume T, Watanabe J, et al. TZT-1027, an antimicrotubule agent, attacks tumor vasculature and induces tumor cell death. Jpn J Cancer Res. 2000;91:837–844. doi: 10.1111/j.1349-7006.2000.tb01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blakey D, Westwood F, Walker M, et al. Antitumor activity of the novel vascular targeting agent ZD6126 in a panel of tumor models. Clin Cancer Res. 2002;8:1974–1983. [PubMed] [Google Scholar]
- 53.Davis PD, Dougherty GJ, Blakey DC, et al. ZD6126: a novel vascular targeting agent that causes selective destruction of tumor vasculature. Cancer Res. 2002;62:7247–7253. [PubMed] [Google Scholar]
- 54.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 55.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6:395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 56.Dickson P, Hamner J, Sims T, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systematically administered chemotherapy. Clin Cancer Res. 2007;13:3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 57.Baffert F, Thurston G, Rochon-Duck M, et al. Age-related changes in vascular endothelial growth factor dependency and angiopoietin-1-induced plasticity of adult blood vessels. Circ Res. 2004;94:984–992. doi: 10.1161/01.RES.0000125295.43813.1F. [DOI] [PubMed] [Google Scholar]
- 58.Baffert F, Le T, Sennino B, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 59.Hurwitz H, Fehrenbacher L, Novotny WF, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 60.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 61.Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 62.Pettit G, Singh S, Hamel E, et al. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A4. Experimentia. 1989;45:205–211. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- 63.Lippert JW., III Vascular disrupting agents. Bioorg Med Chem. 2007;15:605–615. doi: 10.1016/j.bmc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 64.McGowan AT, Fox B. Structural and biochemical comparison of the anti-mitotic agents colchicine, combretastatin A4 and amphethinile. Anticancer Drug Des. 1989;3:249–254. [PubMed] [Google Scholar]
- 65.Gaya A, Daley F, Taylor N, et al. Relationship between human tumour angiogenic profile and combretastatin-induced vascular shutdown: an exploratory study. Br J Cancer. 2008;99:321–326. doi: 10.1038/sj.bjc.6604426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vincent L, Kermani P, Young LM, et al. Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J Clin Invest. 2005;115:2992–3006. doi: 10.1172/JCI24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salmon HW, Siemann DW. Effect of the second-generation vascular disrupting agent OXi4503 on tumor vascularity. Clin Cancer Res. 2006;12:4090–4094. doi: 10.1158/1078-0432.CCR-06-0163. [DOI] [PubMed] [Google Scholar]
- 68.Horti J, Juhasz E, Monostori Z, et al. Phase I study of TZT-1027, a novel synthetic dolastatin 10 derivative, for the treatment of patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2008;62:173–180. doi: 10.1007/s00280-007-0665-7. [DOI] [PubMed] [Google Scholar]
- 69.Kasibhatla S, Baichwal V, Cai S, et al. MPC-6827: a small-molecule inhibitor of microtubule formation that is not a substrate for multidrug resistance pumps. Cancer Res. 2007;67:5865–5871. doi: 10.1158/0008-5472.CAN-07-0127. [DOI] [PubMed] [Google Scholar]
- 70.Segreti J, Polakowski J, Koch K, et al. Tumor selective antivascular effects of the novel antimitotic compound ABT-751: an in vivo rat regional hemodynamic study. Cancer Chemother Pharmacol. 2004;54:273–281. doi: 10.1007/s00280-004-0807-0. [DOI] [PubMed] [Google Scholar]
- 71.Galbraith SM, Chaplin DJ, Lee F, et al. Effects of combretastatin A4 phosphate on endothelial cell morphology in vitro and relationship to tumour vascular targeting activity in vivo. Anticancer Res. 2001;21:93–102. [PubMed] [Google Scholar]
- 72.Kanthou C, Tozer GM. The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells. Blood. 2002;99:2060–2069. doi: 10.1182/blood.v99.6.2060. [DOI] [PubMed] [Google Scholar]
- 73.Siemann DW. Therapeutic strategies that selectively target and disrupt established tumor vasculature. Hematol Oncol Clin North Am. 2004;18:1023–1037. doi: 10.1016/j.hoc.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 74.Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006;66:11520–11539. doi: 10.1158/0008-5472.CAN-06-2848. [DOI] [PubMed] [Google Scholar]
- 75.Siemann DW, Chaplin DJ, Horsman MR. Vascular-targeting therapies for treatment of malignant disease. Cancer. 2004;100:2491–2499. doi: 10.1002/cncr.20299. [DOI] [PubMed] [Google Scholar]
- 76.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 77.Kanthou C, Tozer GM. Microtubule depolymerizing vascular disrupting agents: novel therapeutic agents for oncology and other pathologies. Int J Exp Pathol. 2009;90:284–294. doi: 10.1111/j.1365-2613.2009.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rojiani AM, Rojiani M. Morphologic manifestations of vascular-disrupting agents in preclinical models. In: Siemann DW, editor. Vascular-Targeted Therapies in Oncology. London: WileyBlackwell; 2006. pp. 81–94. [Google Scholar]
- 79.Siemann D, Horsman M. Small molecule vascular disrupting agents in cancer therapy. In: Ellis L, Teicher B, editors. Antiangiogenic Agents in Cancer Therapy. Totowa: Humana; 2008. pp. 297–310. [Google Scholar]
- 80.Ching LM, Zwain S, Baguley BC. Relationship between tumour endothelial cell apoptosis and tumour blood flow shutdown following treatment with the antivascular agent DMXAA in mice. Br J Cancer. 2004;90:906–910. doi: 10.1038/sj.bjc.6601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seshadri M, Spernyak JA, Maiery PG, et al. Visualizing the acute effects of vascular-targeted therapy in vivo using intravital microscopy and magnetic resonance imaging: correlation with endothelial apoptosis, cytokine induction, and treatment outcome. Neoplasia. 2007;9:128–135. doi: 10.1593/neo.06748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang L, Thomsen L, Sutherland R, et al. Neutrophil influx and chemokine production during the early phases of the antitumor response to the vascular disrupting agent DMXAA (ASA404) Neoplasia. 2009;11:793–803. doi: 10.1593/neo.09506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lentsch A, Ward P. Regulation of inflammatory vascular damage. J Pathol. 2000;190:343–348. doi: 10.1002/(SICI)1096-9896(200002)190:3<343::AID-PATH522>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 84.Parkins C, Holder A, Hill S, et al. Determinants of anti-vascular action by combretastatin A-4 phosphate: role of nitric oxide. Br J Cancer. 2000;83:811–816. doi: 10.1054/bjoc.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ching LM, Cao Z, Kieda C, et al. Induction of endothelial cell apoptosis by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid. Br J Cancer. 2002;86:1937–1942. doi: 10.1038/sj.bjc.6600368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baguley BC, Zhuang L, Kestell P. Increased plasma serotonin following treatment with flavone-8-acetic acid, 5,6-dimethylxanthenone-4-acetic acid, vinblastine, and colchicine: relation to vascular effects. Oncol Res. 1997;9:55–60. [PubMed] [Google Scholar]
- 87.Baguley B. Antivascular therapy of cancer: DMXAA. Lancet Oncol. 2003;4:141–148. doi: 10.1016/s1470-2045(03)01018-0. [DOI] [PubMed] [Google Scholar]
- 88.Kestell P, Zhao L, Jameson MB, et al. Measurement of plasma 5-hydroxyindole acetic acid as a possible clinical surrogate marker for the action of antivascular agents. Clin Chim Acta. 2001;314:159–166. doi: 10.1016/s0009-8981(01)00692-1. [DOI] [PubMed] [Google Scholar]
- 89.Ching LM, Goldsmith D, Joseph WR, et al. Induction of intratumoral tumor necrosis factor (TNF) synthesis and hemorrhagic necrosis by 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF knockout mice. Cancer Res. 1999;59:3304–3307. [PubMed] [Google Scholar]
- 90.Zhao L, Baguley BC, Kestell P. The antitumor activity of 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF receptor-1 knockout mice. Br J Cancer. 2002;87:465–470. doi: 10.1038/sj.bjc.6600479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao L, Ching LM, Kestell P, et al. Mechanisms of tumor vascular shutdown induced by 5,6-dimethylxanthenone-4-acetic acid (DMXAA): Increased tumor vascular permeability. Int J Cancer. 2005;116:322–326. doi: 10.1002/ijc.21005. [DOI] [PubMed] [Google Scholar]
- 92.McPhail LD, McIntyre DJ, Ludwig C, et al. Rat tumor response to the vascular-disrupting agent 5,6-dimethylxanthenone-4-acetic acid as measured by dynamic contrast-enhanced magnetic resonance imaging, plasma 5-hydroxyindoleacetic acid levels, and tumor necrosis. Neoplasia. 2006;8:199–206. doi: 10.1593/neo.05739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang LC, Reddy CB, Baguley BC, et al. Induction of tumour necrosis factor and interferon-gamma in cultured murine splenocytes by the antivascular agent DMXAA and its metabolites. Biochem Pharmacol. 2004;67:937–945. doi: 10.1016/j.bcp.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 94.Siemann D, Horsman M. Vascular targeted therapies in oncology. Cell Tissue Res. 2009;335:241–248. doi: 10.1007/s00441-008-0646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Breidahl T, Nielsen FU, Stodkilde-Jorgensen H, et al. The effects of the vascular disrupting agents combretastatin A-4 disodium phosphate, 5,6-dimethylxanthenone-4-acetic acid and ZD6126 in a murine tumour: a comparative assessment using MRI and MRS. Acta Oncol. 2006;45:306–316. doi: 10.1080/02841860600570465. [DOI] [PubMed] [Google Scholar]
- 96.Hill S, Tozer G, Pettit G, et al. Preclinical evaluation of the antitumour activity of the novel vascular targeting agent Oxi 4503. Anticancer Res. 2002;22:1453–1458. [PubMed] [Google Scholar]
- 97.Hua J, Sheng Y, Pinney K. Oxi4503, a novel vascular targeting agent: effects on blood flow and antitumor activity in comparison to combretastatin A-1 phosphate. Anticancer Res. 2003;23:1433–1440. [PubMed] [Google Scholar]
- 98.Lash CJ, Li AE, Rutland M, et al. Enhancement of the anti-tumour effects of the antivascular agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) by combination with 5-hydroxytryptamine and bioreductive drugs. Br J Cancer. 1998;78:439–445. doi: 10.1038/bjc.1998.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murata R, Overgaard J, Horsman M. Comparative effects of combretastatin A-4 disodium phosphate and 5,6-dimethylxanthenone-4 acetic acid on blood perfusion in a murine tumour and normal tissues. Int J Radiat Biol. 2001;77:195–204. doi: 10.1080/09553000010007695. [DOI] [PubMed] [Google Scholar]
- 100.Murata R, Overgaard J, Horsman MR. Potentiation of the anti-tumor effect of hyperthermia by combining with the vascular targeting agent 5,6-dimethyl-xanthenone-4-acetic acid. Anticancer Res. 2001;21:93–102. doi: 10.1080/02656730110087040. [DOI] [PubMed] [Google Scholar]
- 101.Seshadri M, Mazurchuk R, Spernyak JA, et al. Activity of the vascular-disrupting agent 5,6-dimethylxanthenone-4-acetic acid against human head and neck carcinoma xenografts. Neoplasia. 2006;8:534–542. doi: 10.1593/neo.06295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siemann DW, Mercer E, Lepler S, et al. Vascular targeting agents enhance chemotherapeutic agent activities in solid tumor therapy. Int J Cancer. 2002;99:1–6. doi: 10.1002/ijc.10316. [DOI] [PubMed] [Google Scholar]
- 103.Zwi L, Baguley B, Gavin J, et al. Correlation between immune and vascular activities of xanthenone acetic acid anti-tumor agents. Oncol Res. 1994;6:79–85. [PubMed] [Google Scholar]
- 104.Seshadri M, Toth K. Acute vascular disruption by 5,6-dimethylxanthenone-4-acetic Acid in an orthotopic model of human head and neck cancer. Transl Oncol. 2009;18:121–127. doi: 10.1593/tlo.09103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salmon BA, Salmon HW, Siemann DW. Monitoring the treatment efficacy of the vascular disrupting agent CA4P. Eur J Cancer. 2007;43:1622–1629. doi: 10.1016/j.ejca.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galbraith SM, Rustin GJ, Lodge MA, et al. Effects of 5,6-dimethylxanthenone-4-acetic acid on human tumor microcirculation assessed by dynamic contrast-enhanced magnetic resonance imaging. J Clin Oncol. 2002;20:3826–3840. doi: 10.1200/JCO.2002.09.144. [DOI] [PubMed] [Google Scholar]
- 107.Galbraith SM. Imaging the effects of vasculature-targeting agents. In: Siemann DW, editor. Vascular-Targeted Therapies in Oncology. London: WileyBlackwell; 2006. pp. 277–304. [Google Scholar]
- 108.Siemann DW, Chaplin DJ. An Update on the clinical development of drugs to disable tumour vasculature. Expert Opinion Drug Discovery. 2007;2:1–11. doi: 10.1517/17460441.2.10.1357. [DOI] [PubMed] [Google Scholar]
- 109.Siemann DW, Rojiani AM. Antitumor efficacy of conventional anticancer drugs is enhanced by the vascular targeting agent ZD6126. Int J Radiat Oncol Biol Phys. 2002;54:1512–1517. doi: 10.1016/s0360-3016(02)03919-6. [DOI] [PubMed] [Google Scholar]
- 110.Maitland ML, Kasza KE, Karrison T, et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15:6250–6257. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu S, Chen JJ, Kudelka A, et al. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 112.Avastin Safety Information. 2007 Available at: http://www.avastin.com.
- 113.Rustin GJ, Nathan PD, Boxhall J, et al. A Phase Ib trial of combretastatin A-4 phosphate (CA4P) in combination with carboplatin or paclitaxel chemotherapy in patients with advanced cancer. J Clin Oncol. 2005;23:3013. [Google Scholar]
- 114.Gould S, Westwood FR, Curwen JO, et al. Effect of pretreatment with atenolol and nifedipine on ZD6126-induced cardiac toxicity in rats. J Natl Cancer Inst. 2007;99:1724–1728. doi: 10.1093/jnci/djm202. [DOI] [PubMed] [Google Scholar]
- 115.Ke Q, Bodyak N, Rigor DL, et al. Pharmacological inhibition of the hypertensive response to combretastatin A-4 phosphate in rats. Vascul Pharmacol. 2009;51:337–343. doi: 10.1016/j.vph.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 116.Jameson MB, Thompson PI, Baguley BC, et al. Clinical aspects of a phase I trial of 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a novel antivascular agent. Br J Cancer. 2003;88:1844–1850. doi: 10.1038/sj.bjc.6600992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rustin GJ, Galbraith SM, Anderson H, et al. Phase I clinical trial of weekly combretastatin A4 phosphate: clinical and pharmacokinetic results. J Clin Oncol. 2003;21:2815–2822. doi: 10.1200/JCO.2003.05.185. [DOI] [PubMed] [Google Scholar]
- 118.McKeage MJ, Reck M, Jameson MB, et al. Phase II study of ASA404 (vadimezan, 5,6-dimethylxanthenone-4-acetic acid/DMXAA) 1800mg/m2 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Lung Cancer. 2009;65:192–197. doi: 10.1016/j.lungcan.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 119.McKeage MJ, von Pawel J, Reck M, et al. Randomised phase II study of ASA404 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Br J Cancer. 2008;99:2006–2012. doi: 10.1038/sj.bjc.6604808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilson WR, Li AE, Cowan DS, et al. Enhancement of tumor radiation response by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid. Int J Radiat Oncol Biol Phys. 1998;42:905–908. doi: 10.1016/s0360-3016(98)00358-7. [DOI] [PubMed] [Google Scholar]
- 121.Liu JJ, Ching LM, Goldthorpe M, et al. Antitumour action of 5,6-dimethylxanthenone-4-acetic acid in rats bearing chemically induced primary mammary tumours. Cancer Chemother Pharmacol. 2007;59:661–669. doi: 10.1007/s00280-006-0321-7. [DOI] [PubMed] [Google Scholar]
- 122.Baguley BC, Wilson WR. Potential of DMXAA combination therapy for solid tumors. Exp Rev Anticancer Ther. 2002;2:593–603. doi: 10.1586/14737140.2.5.593. [DOI] [PubMed] [Google Scholar]
- 123.Wankhede M, deDeugd C, Siemann DW, et al. In vivo functional differences in microvascular response of 4T1 and Caki-1 tumors after treatment with OXi4503. Oncol Rep. 2010;23:685–692. doi: 10.3892/or_00000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li L, Rojiani AM, Siemann DW. Preclinical evaluations of therapies combining the vascular targeting agent combretastatin A-4 disodium phosphate and conventional anticancer therapies in the treatment of Kaposi’s sarcoma. Acta Oncol. 2002;41:91–97. doi: 10.1080/028418602317314127. [DOI] [PubMed] [Google Scholar]
- 125.Gray L, Conger A, Ebert M, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 126.Murata R, Siemann DW, Overgaard J, et al. Improved tumor response by combining radiation and the vascular-damaging drug 5,6-dimethylxanthenone-4-acetic acid. Radiat Res. 2001;156:503–509. doi: 10.1667/0033-7587(2001)156[0503:itrbcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 127.Jorgensen TJ, Tian H, Joseph IB, et al. Chemosensitization and radiosensitization of human lung and colon cancers by antimitotic agent, ABT-751, in athymic murine xenograft models of subcutaneous tumor growth. Cancer Chemother Pharmacol. 2007;59:725–732. doi: 10.1007/s00280-006-0326-2. [DOI] [PubMed] [Google Scholar]
- 128.Akashi Y, Okamoto I, Suzuki M, et al. The novel microtubule-interfering agent TZT-1027 enhances the anticancer effect of radiation in vitro and in vivo. Br J Cancer. 2007;96:1532–1539. doi: 10.1038/sj.bjc.6603769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murata R, Siemann DW, Overgaard J, et al. Interaction between combretastatin A-4 disodium phosphate and radiation in murine tumours. Radiother Oncol. 2001;60:155–161. doi: 10.1016/s0167-8140(01)00384-x. [DOI] [PubMed] [Google Scholar]
- 130.Horsman MR, Murata R, Overgaard J. Combination studies with combretastatin and radiation: effects in early and late responding tissues. Radiother Oncol. 2002;63 (Suppl 1):S50. [Google Scholar]
- 131.Kelland LR. Targeting established tumour vasculature: A novel approach to cancer treatment. Curr Cancer Ther Rev. 2005;1:1–9. [Google Scholar]
- 132.Siim BG, Lee AE, Shalal-Zwain S, et al. Marked potentiation of the antitumour activity of chemotherapeutic drugs by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) Cancer Chemother Pharmacol. 2003;51:43–52. doi: 10.1007/s00280-002-0529-0. [DOI] [PubMed] [Google Scholar]
- 133.Green C, Griffiths-Johnson D, Dunmore K. Marked potentiation of the in vivo antitumor activity of docetaxel in a human prostate cancer xenograft by the vascular targeting agent 5,6 dimethyl xanthenone acetic acid, DMXAA. Proc Amer Assoc Cancer Res. 2005;46 abstract 2990. [Google Scholar]
- 134.McKeage MJ. The potential of DMXAA (ASA404) in combination with docetaxel in advanced prostate cancer. Expert Opin Investig Drugs. 2008;17:23–29. doi: 10.1517/13543784.17.1.23. [DOI] [PubMed] [Google Scholar]
- 135.Grosios K, Loadman P, Swaine D, et al. Combination chemotherapy with combretastatin A-4 phosphate and 5-fluorouracil in an experimental murine colon adenocarcinoma. Anticancer Res. 2000;20:229–233. [PubMed] [Google Scholar]
- 136.Yeung S, She M, Yang H, et al. Combination chemotherapy including combretastatin A4 phosphate and paclitaxel is effective against anaplastic thyroid cancer in a nude mouse xenograft model. J Clin Endocrinol Metab. 2007;92:2902–2909. doi: 10.1210/jc.2007-0027. [DOI] [PubMed] [Google Scholar]
- 137.Morinaga Y, Suga Y, Ehara S, et al. Combination effect of AC-7700, a novel combretastatin A-4 derivative, and cisplatin against murine and human tumors in vivo. Cancer Sci. 2003;94:200–204. doi: 10.1111/j.1349-7006.2003.tb01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pruijn FB, van Daalen M, Holford NH, et al. Mechanisms of enhancement of the antitumour activity of melphalan by the tumour-blood-flow inhibitor 5,6-dimethylxanthenone-4-acetic acid. Cancer Chemother Pharmacol. 1997;39:541–546. doi: 10.1007/s002800050611. [DOI] [PubMed] [Google Scholar]
- 139.Cliffe S, Taylor ML, Rutland M, et al. Combining bioreductive drugs (SR 4233 or SN 23862) with the vasoactive agents flavone acetic acid or 5,6-dimethylxanthenone acetic acid. Int J Radiat Oncol Biol Phys. 1994;29:373–377. doi: 10.1016/0360-3016(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 140.Martinelli M, Bonezzi K, Riccardi E, et al. Sequence dependent antitumour efficacy of the vascular disrupting agent ZD6126 in combination with paclitaxel. Br J Cancer. 2007;97:888–894. doi: 10.1038/sj.bjc.6603969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Davis P, Tozer G, Naylor M, et al. Enhancement of vascular targeting by inhibitors of nitric oxide synthase. Int J Radiat Oncol Biol Phys. 2002;54:1532–1536. doi: 10.1016/s0360-3016(02)03925-1. [DOI] [PubMed] [Google Scholar]
- 142.Tozer G, Prise V, Lewis G, et al. Nitric oxide synthase inhibition enhances the tumor vascular-damaging effects of combretastatin A-4–3-O-phosphate at clinically relevant doses. Clin Cancer Res. 2009;15:3781–3790. doi: 10.1158/1078-0432.CCR-08-2906. [DOI] [PubMed] [Google Scholar]
- 143.Shi W, Siemann DW. Targeting the tumor vasculature: enhancing antitumor efficacy through combination treatment with ZD6126 and ZD6474. In Vivo. 2005;19:1045–1050. [PubMed] [Google Scholar]
- 144.Siemann DW, Shi W. Efficacy of combined antiangiogenic and vascular disrupting agents in treatment of solid tumors. Int J Radiat Oncol Biol Phys. 2004;60:1233–1240. doi: 10.1016/j.ijrobp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 145.Siemann D, Shi W. Dual targeting of tumor vasculature: Combining avastin and vascular disrupting agents (CA4P or OXi4503) Anticancer Res. 2008;28:2027–2032. [PMC free article] [PubMed] [Google Scholar]
- 146.Djeha H, Green C, Ireson C, et al. Synergistic in vivo antitumor activity in lung and colon cancer xenografts with the vascular disrupting agent DMXAA combined with bevacizumab. Proc Amer Assoc Cancer Res. 2006;47 abstract 233. [Google Scholar]
- 147.Djeha H, Shah K, McGeever G, et al. Combination of the vascular disrupting agent DMXAA (AS1404) with bevacizumab and paclitaxel produces synergistic antitumor activity in lung cancer xenografts. Proc Amer Assoc Cancer Res. 2007;48 abstract 4642. [Google Scholar]
- 148.Rocha S, Adams R. Molecular differentiation and specialization of vascular beds. Angiogenesis. 2009;12:139–147. doi: 10.1007/s10456-009-9132-x. [DOI] [PubMed] [Google Scholar]
- 149.McKeage MJ, Baguley BC. Disrupting established tumor blood vessels: an emerging therapeutic strategy for cancer. Cancer. 2010;116 doi: 10.1002/cncr.24975. [DOI] [PubMed] [Google Scholar]
- 150.Rustin G, Jayson G, Reed N, et al. Fosbretabulin (Combretastatin A-4 Phosphate [CA4P]) Carboplatin and Paclitaxel is active in patients with platinum resistant ovarian cancer. International Gynecologic Cancer Society Meeting; Bangkok, Thailand. 23–28 Oct 2008; abstract 315. [Google Scholar]
- 151.Siemann DW, Chaplin DJ, Walicke PA. A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P) Expert Opin Investig Drugs. 2009;18:189–197. doi: 10.1517/13543780802691068. [DOI] [PMC free article] [PubMed] [Google Scholar]