Abstract
Mental motor imagery is subserved by the same cognitive systems that underlie action. In turn, action is informed by the anticipated sensory consequences of movement, including pain. In light of these considerations, one would predict that motor imagery would provide a useful measure pain-related functional interference. We report a study in which 19 patients with chronic musculoskeletal or radiculopathic arm or shoulder pain, 24 subjects with chronic pain not involving the arm/shoulder and 41 normal controls were asked to indicate if a line drawing was a right or left hand. Previous work demonstrated that this task is performed by mental rotation of the subject’s hand to match the stimulus. Relative to normal and pain control subjects, arm/shoulder pain subjects were significantly slower for stimuli that required greater amplitude rotations. For the arm/shoulder pain subjects only there was a correlation between degree of slowing and the rating of severity of pain with movement but not the non-specific pain rating. The hand laterality task may supplement the assessment of subjects with chronic arm/shoulder pain.
Keywords: pain assessment, motor imagery, musculoskeletal pain
Introduction
Motor imagery is the mental rehearsal of an action without movement (e.g. Jeannerod, 1995). Several lines of evidence suggest that motor imagery and action are mediated by the same brain circuits. For example, functional neuroimaging (Grafton et al., 1996; Grezes and Decety, 2001) and Transcranial Magnetic Stimulation (Ganis et al., 2000; Rossini et al., 1999) studies demonstrate that the same brain structures are involved in action and imagining the same action. Additionally, motor imagery obeys the same biomechanical constraints as action (Decety and Michel, 1989; Moseley, 2004).
The present investigation builds on a series of elegant studies by Parsons and colleagues demonstrating that motor imagery is employed when a subject judges the laterality of a pictured hand or foot (Parsons, 1987a, 1995). When shown a picture of the left hand in the palm up position and asked to indicate if the stimulus was a left or right hand, Parsons demonstrated that there is a consistent and principled relationship between the length of the trajectory through which the subject’s hand must be mentally rotated to match the stimulus and response time. Using Parson’s paradigm we demonstrated that subjects with Complex Regional Pain Syndrome (CRPS) involving one upper extremity were slower to respond to pictures of the painful hand (Schwoebel et al., 2001); this difference was eliminated after treatment that reduced the subjects’ pain (Schwoebel et al., 2002). Moseley found that CRPS subjects were slower to recognize the painful hand and that performance was best predicted by the degree of pain that subjects believed would be provoked by moving their hand to the depicted position (Moseley, 2004).
We report an investigation that differs from previous work on this topic in a number of important respects. First, unlike previous studies, we include a group with chronic pain not involving the arm/shoulder to control for the non-specific effects of pain and its treatment. Second, we explored the relationship between the degree of slowing and severity of pain with movement. Third, whereas most investigations have focused on subjects with CRPS (but see Moseley et al, 2008), a condition that differs in important ways from most other pain syndromes (Janig and Baron, 2002), we report data from subjects selected on the basis of pain location rather than etiology.
We predicted that subjects with arm/shoulder pain would be slower to respond to depictions of a painful hand. Thus, for subjects with unilateral arm/shoulder pain, subjects would be expected to be slower than controls for the painful hand; subjects with bilateral arm/shoulder pain, would be expected to be slower relative to controls for both hands. Importantly, as arm/shoulder pain is often exacerbated by movement, the slowing of response time would be expected to be most apparent for stimuli that require the greatest degree of mental rotation. Finally, as the predicted effects of pain are attributed to a slowing of mental rotation of a specific body part the effects would be expected only in subjects with pain in one or both arm/shoulder.
Methods
Subjects
Four groups of subjects were included. There were 19 subjects with musculoskeletal or radiculopathic pain involving the shoulder or arm; one group included 10 subjects with unilateral (4 left, 6 right) arm/shoulder pain and a second group included nine subjects with chronic pain of both upper extremities. There were two control groups. First, to control for the non-specific effects of pain and therapy for pain, a Pain Control group was recruited; this group included 24 subjects with chronic pain that did not involve the arm or shoulder. Finally, a normal control group consisting of 41 subjects with no history of significant neurologic disorders or chronic pain was recruited. The four groups of subjects did not differ significantly with respect to age (unilateral arm/shoulder Pain 47.5 ± 6.2, bilateral arm/shoulder Pain 55.8 ± 8.9, pain control 48.6 ± 11.1 and normal control 44.4 ± 16.8; all p>.05). All subjects with pain were asked to rate their pain at the time of testing and general pain with movement using a 0–10 point scale. The unilateral Arm/shoulder Pain, bilateral arm/shoulder pain and Pain Control subjects did not differ with respect to mean pain severity (unilateral Arm/shoulder Pain 6.5 ± 2.4, bilateral Arm/shoulder Pain 6.2 ± 2.3, and Pain Control 6.3 ± 2.6; all p>.40). Additional information regarding the subjects with pain is included in Table 1.
Table 1.
Subject information.
| Subject #| | Group | Age | Pain severity |
Pain with movement |
Pain location | Medication |
|---|---|---|---|---|---|---|
| 56 | Bilateral arm | 8 | 5 | Shoulders, arms, hands | Gabapentin | |
| 66 | Bilateral arm | 50 | 5 | 1 | Hands | Gabapentin |
| 73 | Bilateral arm | 50 | 10 | 10 | Arms | Gabapentin |
| 141 | Bilateral arm | 41 | 3 | 2 | Arms | Pregabalin, Tizanidine, Clonazepam |
| 142 | Bilateral arm | 54 | 5 | No data | Arms | Oxycodone, Gabapentin, |
| 147 | Bilateral arm | 52 | 4 | 5 | Arms | Amitriptyline, Codeine |
| 159 | Bilateral arm | 66 | 8 | 1 | Arms | Clonazepam, Methadone |
| 164 | Bilateral arm | 64 | 5 | 9 | Arms | Methadone |
| 151 | Bilateral arm | 58 | 8 | No data | Arms | None |
| 32 | Unilateral arm | 42 | 6 | 10 | L. Shoulder | Oxycodone, Lidocaine Patch, Duloxetine |
| 93 | Unilateral arm | 54 | 3 | 8 | L. shoulder, arm | Gabapentin |
| 94 | Unilateral arm | 54 | 7 | 9 | L. Shoulder | None |
| 171 | Unilateral arm | 47 | 4 | No data | Left hand, arm, shoulder | Hydromorphone, Gabapentin, Ibuprofen |
| 46 | Unilateral arm | 42 | 5 | 8 | R. shoulder, arm | Naproxen |
| 109 | Unilateral arm | 48 | 10 | 10 | R. shoulder | Oxycodone, Acetaminophen and Ibuprofen |
| 118 | Unilateral arm | 51 | 7 | 9 | R. shoulder | Cyclobenzaprine, Clonazepam |
| 150 | Unilateral arm | 53 | 10 | 10 | Right shoulder, arm, hand | Oxycodone,Acetaminophen, Ibuprofen, Gabapentin |
| 155 | Unilateral arm | 35 | 5 | 7 | Right shoulder, arm | Clonazepam, Tramadol |
| 165 | Unilateral arm | 49 | 8 | 9 | Right hand | Gabapentin, Methadone |
| 41 | Pain Control | 57 | 6 | 4 | Low back, arthritis, lumbar/sacral | Naproxen |
| 43 | Pain Control | 48 | 5 | 6 | Belly, abdomen | Methadone |
| 47 | Pain Control | 36 | 2 | 3 | Low back, left knee | Ibuprofen |
| 55 | Pain Control | 44 | 6 | 10 | Low back, left leg | Ibuprofen, Naproxen |
| 57 | Pain Control | 62 | 8 | 6 | Low back and neck | Gabapentin, Oxcarbazepine |
| 61 | Pain Control | 49 | 9 | 8 | Low back | Methadone |
| 65 | Pain Control | 41 | 4 | 9 | Low back, cervical spine | Oxycodone |
| 67 | Pain Control | 34 | 8 | 6 | Abdomen | Meperidine, Oxycodone |
| 69 | Pain Control | 27 | 5 | 9 | Back | Methadone |
| 76 | Pain Control | 41 | 8 | 9 | Abdomen | Hydromorphone |
| 78 | Pain Control | 44 | 5 | 8 | All joints | Oxycodone, Gabapentin, |
| 92 | Pain Control | 53 | 8 | 9 | Neck, back joints | Oxycodone, Gabapentin, Ibuprofen |
| 99 | Pain Control | 41 | 7 | 9 | Upper and mid back | Lidocaine, prednisone |
| 100 | Pain Control | 42 | 9 | 10 | Lower back | Oxycodone and Acetaminophen |
| 106 | Pain Control | 49 | 1 | 1 | Upper and lower back | Hydrocodone Bitartrate and Acetaminophen, Cyclobenzaprine |
| 120 | Pain Control | 55 | 9 | 10 | Lumbar, cervical | Methadone |
| 123 | Pain Control | 69 | 9 | 7 | Sciatic nerve | Gabapentin |
| 129 | Pain Control | 72 | 7 | 8 | Mid and low back | Oxycodone, Acetaminophen |
| 146 | Pain Control | 46 | 4 | 10 | Low back, legs, right knee | Oxycodone, Acetaminophen, Gabapentin, Paroxetine, Quetiapine |
| 149 | Pain Control | 42 | 7 | 2 | Back | None |
| 167 | Pain Control | 45 | 9 | 9 | Migraines, low back | Gabapentin, Bupropion |
| 170 | Pain Control | 50 | 4 | 10 | Back and hips | Ibuprofen |
| 172 | Pain Control | 52 | 10 | 0 | Back | None |
| 62 | Pain Control | 68 | 1 | 8 | Mouth | Carbamazepine |
| 2 | Control | 39 | 0 | n/a | n/a | n/a |
| 6 | Control | 26 | 0 | n/a | n/a | n/a |
| 8 | Control | 35 | 0 | n/a | n/a | n/a |
| 11 | Control | 25 | 0 | n/a | n/a | n/a |
| 20 | Control | 28 | 0 | n/a | n/a | n/a |
| 21 | Control | 41 | 0 | n/a | n/a | n/a |
| 25 | Control | 36 | 0 | n/a | n/a | n/a |
| 26 | Control | 36 | 0 | n/a | n/a | n/a |
| 30 | Control | 28 | 0 | n/a | n/a | n/a |
| 37 | Control | 53 | 0 | n/a | n/a | n/a |
| 45 | Control | 23 | 0 | n/a | n/a | n/a |
| 48 | Control | 57 | 0 | n/a | n/a | n/a |
| 68 | Control | 30 | 0 | n/a | n/a | n/a |
| 77 | Control | 48 | 0 | n/a | n/a | n/a |
| 80 | Control | 48 | 0 | n/a | n/a | n/a |
| 85 | Control | 32 | 0 | n/a | n/a | n/a |
| 105 | Control | 31 | 0 | n/a | n/a | n/a |
| 107 | Control | 56 | 0 | n/a | n/a | n/a |
| 110 | Control | 59 | 0 | n/a | n/a | n/a |
| 112 | Control | 37 | 0 | n/a | n/a | n/a |
| 113 | Control | 48 | 0 | n/a | n/a | n/a |
| 115 | Control | 65 | 0 | n/a | n/a | n/a |
| 122 | Control | 53 | 0 | n/a | n/a | n/a |
| 12,108 | Control | 51 | 0 | n/a | n/a | n/a |
| 12,121 | Control | 67 | 0 | n/a | n/a | n/a |
| 12,161 | Control | 49 | 0 | n/a | n/a | n/a |
| 12,179 | Control | 73 | 0 | n/a | n/a | n/a |
| 12,181 | Control | 59 | 0 | n/a | n/a | n/a |
| 12,191 | Control | 81 | 0 | n/a | n/a | n/a |
| 31,343 | Control | 65 | 0 | n/a | n/a | n/a |
| 6001 | Control | 59 | 0 | n/a | n/a | n/a |
| 6002 | Control | 58 | 0 | n/a | n/a | n/a |
| 6003 | Control | 62 | 0 | n/a | n/a | n/a |
| 6004 | Control | 64 | 0 | n/a | n/a | n/a |
| 6005 | Control | 63 | 0 | n/a | n/a | n/a |
| 3 | Control | 23 | 0 | n/a | n/a | n/a |
| 10 | Control | 23 | 0 | n/a | n/a | n/a |
| 13 | Control | 23 | 0 | n/a | n/a | n/a |
| 59 | Control | 23 | 0 | n/a | n/a | n/a |
| 111 | Control | 22 | 0 | n/a | n/a | n/a |
| 130 | Control | 22 | 0 | n/a | n/a | n/a |
Subjects with arm/shoulder pain and most controls were recruited from the Pain Center at the University of Pennsylvania. All subjects with chronic pain were tested at the time of a regularly scheduled appointment and were following their usual medical regimen. Subjects were paid for their participation. Consent was obtained according to the Declaration of Helsinki; the project was approved by the University of Pennsylvania Institutional Review Board.
Task
Subjects sat in a comfortable chair in front of a computer with their arms and hands resting comfortably on a table with their right index finger over the “m” key and the left index finger over the “z” key. A series of line drawings of either the right or left hand was presented, and subjects were instructed to depress the “z” key when seeing a picture of the left hand or the “m” key when seeing a right hand. Subjects were instructed to respond as quickly and accurately as possible; no subject reported pain related to responding. Each trial began with the presentation of a fixation cross that persisted for one second before being replaced by a line drawing of a hand. The trial was terminated by depressing the “z” key for a left hand or the “m” key for a right hand. A new fixation cross was presented one second after the subject’s response. No feedback regarding accuracy or reaction time (RT) was provided. Subjects were instructed not to move their hands to match the position of the stimulus.
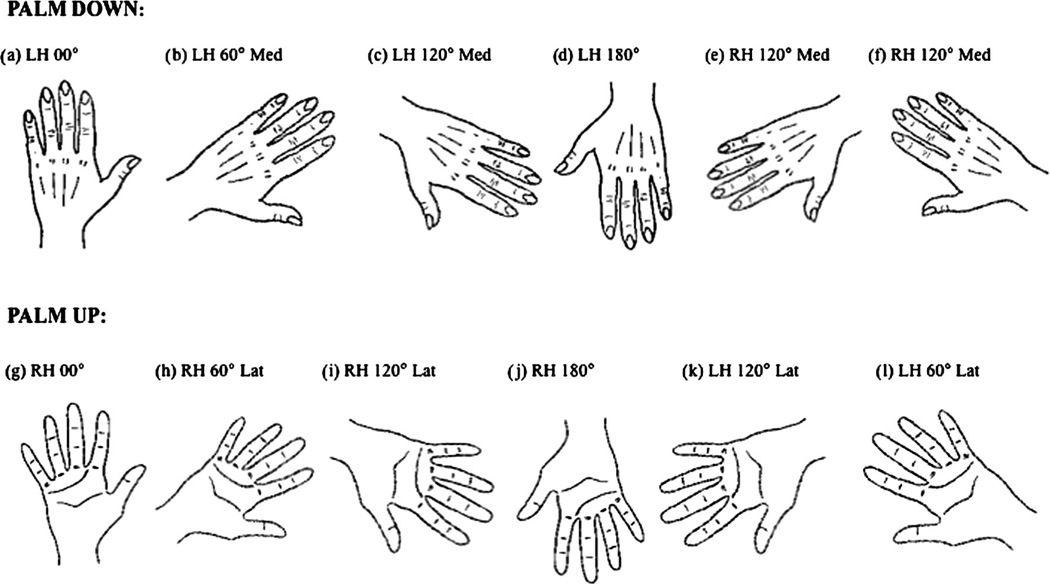
Stimuli included 12 drawings of the right and left hand. For each hand, six drawings depicted the hand in the palm up and six in the palm down configuration. For both the palm up and palm down stimuli there were drawings of six different angular rotations: 0°, 60° medial rotation, 120° medial rotation, 180°, 120° lateral rotation and 60° lateral rotation (See Figure 1).
Figure 1.
Depictions of a representative group of stimuli.
There were eight trials with each of the 24 stimuli (six palm down left, six palm up left, six palm down right, six palm up right) presented in random sequence. On average, the task lasted approximately 10–12 minutes. The experiment was programmed in E-Prime and run on a PC. All statistical analyses were performed with SPSS16. For analyses where Mauchly’s test of sphericity was significant, the Huynh-Feldt correction was applied.
Data Analysis
Accuracy and RT were recorded for each trial; mean RT and accuracy were calculated for each of the 24 stimuli for each subject. For the RT analysis, trials on which the RT differed from the subject’s mean for that stimulus by more than 2.5 standard deviations were discarded. Additionally, those trials for which the subject responded incorrectly were eliminated from the RT analysis. Finally, in order to exclude subjects who performed poorly because of factors such as failure to engage in the task, inability to understand the task, inability to maintain set, or poor right/left discrimination, we assessed each subject’s performance on those trials that require minimal rotation of the hand (0° for the right and left hands in the palm up and palm down conditions).
Results
Collapsing across subjects, 3.0% of responses of normal subjects, 2.4% of responses of Pain Control and 2.3% of responses of subjects with arm/shoulder pain responses were excluded. Additionally, three subjects (one bilateral arm/shoulder pain and 2 pain controls) were excluded from the analysis because they performed at chance on the minimal rotation conditions. Mean correct for the minimal rotation condition was greater than 95% for all groups; the four groups did not differ in this condition (all p>.30).
In order to demonstrate that our task generated results consistent with previous reports, data from the 41 normal controls were analyzed first. A repeated measures ANOVA was performed on the RT data in which within-subject factors included hand, rotation (0°, 60° medial, 120° internal, 180°, 120° lateral and 60° lateral), and palm up/palm down. There were significant main effects of hand (F[1,38]=6.33, p=.016), rotation (F[5,190]=44.0, p<.001), and view (palm up, palm down) (F[1,38]=12.68, p=.001). As in previous studies (Fiorio et al., 2006; Parsons, 1987a, 1987b), RTs were faster for the right hand (1924 ± 123 vs. 2045 ± 132 ms) and in the palm down condition (1831 ± 119 vs. 2138 ± 145 ms). There was also a view by rotation interaction (F[14.71, p<.001). As noted by previous investigators, this difference reflects the fact that biomechanical constraints differ in the palm up and palm down conditions (Parsons, 1987a, 1987b; Parsons and Fox, 1998; Parsons et al., 1998).
A similar analysis was performed for the accuracy data. There was a significant main effect of rotation (F[5,200]=16.77, p<.001) and no hand effect (F[1,40]=1.51, p=.226). Pairwise comparisons demonstrated that subjects were significantly less accurate in the 180° condition than in all other conditions; subjects were also significantly less accurate with 120° lateral stimuli than all other conditions except 180° stimuli. There was no effect of view (F[1,40]=.129, p=.721). Finally, there was a significant interaction between view and rotation (F[5,200]=3.64, p=.013). The fact that controls are faster and less accurate with the right hand could represent a speed-accuracy trade-off with respect to hand; note, however, that the effects of rotation and view do not reflect such a trade-off as the stimuli for which subjects were slowest were also the least accurate.
Next, RT data for the 10 subjects with unilateral arm/shoulder pain was analyzed to determine if subjects with right and left arm/shoulder pain differed. There were no effects of group (left, right) on ANOVAs for the RT and accuracy data ([F[1,8]=.865, p=.379, F[1,8]=.149, P=.710, respectively). In light of this finding, data from the right and left hand unilateral pain groups were combined in the analyses below.
In order to compare the performance of subjects with arm/shoulder pain and controls, an omnibus ANOVA was performed in which group (unilateral pain, bilateral pain, normal controls, pain controls) served as a between subject factor and hand (right, left), rotation, and view served as within subjects factors. In order to normalize data, RTs were subjected to a reciprocal transformation prior to the ANOVA. For ease of interpretation, means are expressed in milliseconds and percent correct whereas the F and p values were generated in the ANOVA with the transformed data.
There were four significant main effects. Subjects responded faster to images of right as compared to left hands (2413 ± 115 vs 2504 ± 126 ms; F(1,77)=15.99, p<.001). Subjects were faster for palm down as compared to palm up stimuli (2332 ± 123 vs. 2586 ± 126 ms; F(1,77)=29.99, p<.001). There was a robust effect of rotation (F(5,385)=98.16; p<.001); as illustrated in Figure 3, subjects were slower for stimuli involving larger degrees of angular rotation. Post-hoc tests (LSD) demonstrated that all conditions differed significantly from each other except 0° and 60° medial and 120° and 60° lateral (all other ps<.001). Finally, there was a main effect of group (F(3,77)=3.59, p=.017); post-hoc tests revealed that controls (1985 ± 144 ms) were significantly faster than subjects with bilateral arm/shoulder pain (2956 ± 298 ms, p=.03) and subjects with unilateral arm/shoulder pain (2742 ± 283 ms, p=.007) but did not differ from pain control subjects (2153 ± 187 ms, p=.541); pain control subjects differed from unilateral arm/shoulder subjects (p=.035). There was also a view by rotation interaction (F[5,385]=23.43, p<.001); reflecting biomechanical constraints, as they were sitting with their palms down, subjects were faster to respond to palm down (2332 ± 123 vs 2586 ± 126) as compared to palm up stimuli (F=30.0, p<.0001).
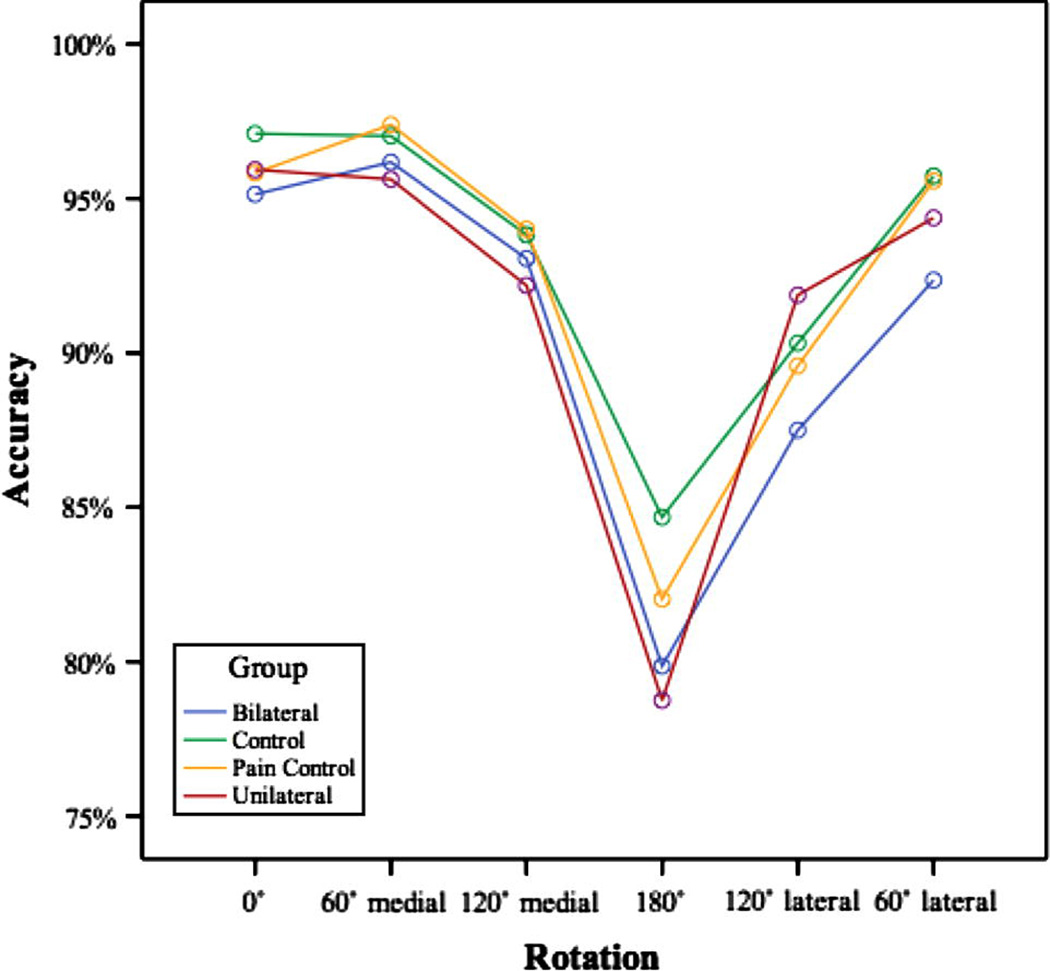
Figure 3.
Accuracy data for the unilateral arm/shoulder pain group, bilateral arm/shoulder pain group, pain controls and normal controls collapsed across the palm up/palm down condition.
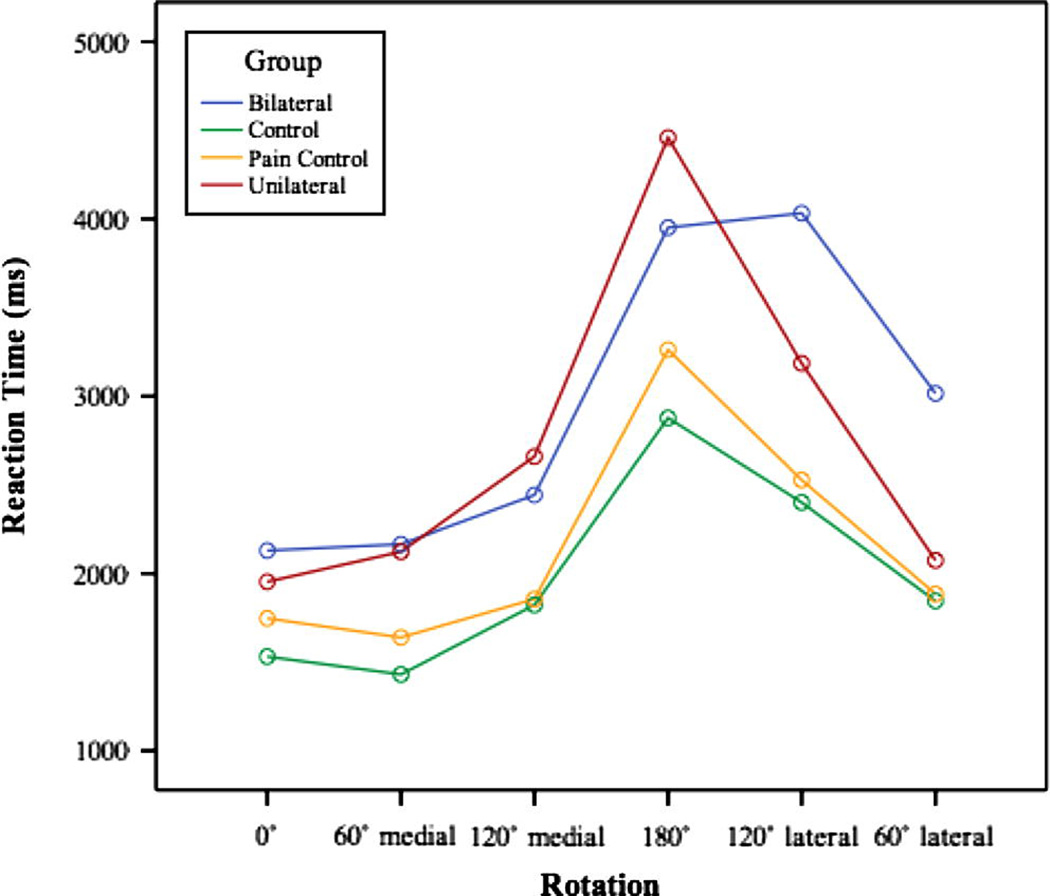
Of greatest significance was a group by rotation interaction (F(5,385)=2.06, p=.019); whereas there was a substantial RT cost associated with mental rotation of the hands for all groups, as demonstrated in Figure 2, this cost was greater for subjects with hand pain than for normal subjects.
Figure 2.
RT data for the unilateral arm/shoulder pain group, bilateral arm/shoulder pain group, pain controls and normal controls collapsed across the palm up/palm down condition.
Importantly, we predicted that there would be a greater cost with increasing magnitudes of mental rotation for arm/shoulder pain subjects than controls and that this would be manifested as a group by rotation interaction. Therefore, we explored this effect in a series of planned contrasts in which subjects with unilateral and bilateral hand pain were compared to normal controls. As we, like a number of previous investigators (e.g, Parsons, 1987a; Fiorio et al, 2006), do not find robust accuracy differences on this task, subsequent analyses are restricted to RT data. Comparing subjects with unilateral pain to normal controls, we found a significant group by rotation interaction (F(5,235)=2.6, p=.038); subjects with hand pain exhibited a greater cost from larger angular rotations than normal subjects. Furthermore, we predicted that this effect would be evident when comparing RTs from the easiest stimuli (0° conditions) to the most difficult stimuli (180° condition), and used the difference in reaction times between these two conditions as our metric.
We found a significant difference between RTs for the 180° and 0° conditions for unilateral pain subjects (2505) compared to controls (1287 ms; t=3.41 p=.004). Although there was a trend, the comparison of bilateral pain subjects to controls did not yield a significant group by rotation interaction (F[5, 230]=1.37, p=.097).
An additional set of analyses was performed to explore the effects of severity of arm/shoulder pain. First, we performed correlations for subjects with arm/shoulder pain between RTs differences for the 180° and 0° conditions and non-specific pain severity ratings and pain with movement ratings. Data for the latter measure were not available for two subjects. We found a significant correlation between pain with movement and difference in RT (Pearson r=.576, p=.01) but not between non-specific pain severity and difference in RT (r=.210, p=.217). There was a significant correlation between pain with movement and non-specific pain score (r=.444, p=.042). In order to control for the non-specific effects of pain and its treatment, similar analyses were performed for the pain control group. Correlations between both non-specific pain and pain with movement were not significant (r=−.130, p=.278 and r=.051, p=.409 respectively).
We also performed an omnibus ANOVA on the accuracy data, in which group (unilateral pain, bilateral pain, normal controls, pain controls) served as a between subject factor and hand (right, left), rotation, and view served as within subject factors. We observed significant effects of rotation (F[5,400]=32.05, p<.001) and hand (F[1,80]=4.15, p=.045). Pairwise comparisons demonstrated that the 180° and 120° lateral conditions differed from all other conditions (ps<.01). Performance with right hand stimuli was slightly less accurate than with left hand stimuli (91.2% vs. 92.6%). There were no effects of view (F[1,80]=.207, p=.650) or group (F[3,80]=.403, p=.751). There was a view by rotation interaction (F[5,400]=4.73, p=.003) as previously described in the normal control data.
Discussion
We demonstrate that a simple motor imagery task - discriminating between right and left hands - distinguishes subjects with arm/shoulder pain from controls and subjects with chronic pain involving other parts of the body. Not only are subjects with arm/shoulder pain generally slower than control subjects but, consistent with the predictions outlined in the Introduction, they exhibit a significantly greater cost for stimuli that require a larger trajectory of mental rotation (e.g., the 180° stimuli). This interaction between group and rotation is important as it strongly argues that the differences between controls and arm/shoulder pain subjects are not simply a non-specific effect of pain or its treatment. Whereas differences in pain severity would represent a potential explanation for overall RT differences between controls and arm/shoulder pain subjects, they are unlikely to cause a selective impairment for those stimuli that require the most substantial degree of mental rotation. Rather, the selective nature of the impairments strongly suggests that arm/shoulder pain produces deficits in mental rotation that, at least in part, reflect an impairment specific to the movements that are likely to be associated with pain (Moseley, 2004). It is also relevant to note in this context that there were no group effects in accuracy. The fact that arm/shoulder pain subjects did not differ from controls with respect to accuracy is important as it demonstrates that subjects with arm/shoulder pain were able to reliably perform the task.
We believe that these data are important for several reasons. First, the data confirm and extend the limited literature documenting that chronic pain is associated with a specific impairment of motor imagery. Second, by including a pain control group that was matched to the arm/shoulder subjects with respect to pain severity and systematically exploring a wide range of stimuli, the study demonstrates that arm/shoulder pain is associated with a specific impairment in motor imagery. Third, by including subjects with arm/shoulder pain secondary to musculoskeletal causes or radiculopathy, the study demonstrates that the association between pain and selective slowing of motor imagery is not specific to patients with CRPS, thereby greatly expanding its potential applications.
We suggest that there are several reasons to believe that the hand laterality task described here may have significant clinical utility. First, the task is easily administered and quick; on average, subjects completed the task in approximately 10–12 minutes. No subject complained of discomfort during the task or failed to understand the task; in fact, no subject failed to complete the task for any reason. Second, the task does not require specialized or costly equipment but can be run with a PC. Finally, the task provides a measure of pain without explicitly demanding a pain rating. Informal de-briefing after the task indicated that most subjects were unaware of the purpose of the task.
Although formal data are lacking, the demonstration that pain ratings and performance on hand (Schwoebel et al, 2001, 2002; Moseley, 2004) and foot (Coslett et al, In press) laterality tasks are related suggests that the hand laterality test may also be useful in the assessment of malingering or factitious pain disorders. The effect angular rotation on RT is highly reliable in group analyses and is apparent when individual data are inspected. For example, 38 of 41 (92.6%) controls 19 of 19 subjects with arm/shoulder pain demonstrated at least a 25% increase in RT for stimuli in the 180° of rotation as compared to 0° rotation. In light of this consistency, the absence of an effect of the angular rotation of the stimulus would suggest that the subject was not performing the task in the normal fashion.
There is precedent for the claim that a deviation from the highly consistent (and non-intuitive) pattern of performance exhibited by normal subjects on motor imagery tests may indicate psychological confounders present in patients with chronic pain. For example, Maruff and Velakoulis reported an investigation in which normal subjects were asked to feign weakness while performing a motor imagery task (Maruff and Velakoulis, 2000). Whereas subjects performed more slowly while feigning weakness, they did not demonstrate the normal, highly predictable pattern of performance on the task. Similarly, a subject diagnosed with “conversion disorder” failed to demonstrate the normal inverse relationship between movement time and target size.
In this and previous investigations subjects did not report a change in their pain after performing the task (Schwoebel et al., 2001; Schwoebel et al., 2002). This finding contrasts with the report of Moseley et al (2008) that mental motor imagery alone increased pain and swelling in subjects with arm pain. One potential explanation for the discrepancy appeals to the distinction between implicit and explicit motor imagery. In our task, subjects were asked to simply determine if the stimulus depicted a right or left hand; there was no explicit instruction to imagine moving their hands and most subjects do not report mentally rotating their hand. In contrast, Moseley et al (2008) instructed subjects to imagine themselves producing the movement that would be required to make their hand match the position of the pictured hand twice before responding. As we have previously reported substantial differences in performance on tasks involving explicit and implicit motor imagery tasks in a study of 70 subjects with unilateral stroke, one possible reason for the discrepancy between our findings and those of Moseley et al (2008) is that implicit and explicit motor imagery tasks tap representations of the body that are, at least in part, dependent on different neural structures (Schwoebel and Coslett, 2005). Furthermore, the distinction between implicit and explicit mental rotation may have clinical implications. Moseley et al (2006) reported that subjects with pathologic pain benefited from a treatment protocol involving explicit mental imagery (see Daly and Bialocerkowski, 2009 for review).
Another difference between our findings and previous reports of motor imagery in subjects with unilateral arm/shoulder pain secondary to CRPS is that our subjects do not exhibit a difference in performance between their symptomatic and asymptomatic hands (Schwoebel et al., 2001; Schwoebel et al., 2002). We predicted that subjects would be exhibit a greater slowing for stimuli depicting the painful hand and have no compelling account for the lack of asymmetry in this study. We note that some other investigators have also failed to find lateralized abnormalities in subjects with unilateral symptoms Fiorio et al, for example, reported that dystonic subjects performed abnormally on RT (but not accuracy) measures for both the symptomatic and asymptomatic hand (Fiorio et al., 2006). In a study using a foot laterality task in subjects with chronic leg pain that was in many respects similar to the present study, we also failed to find a difference between the symptomatic and asymptomatic extremity. One possible explanation appeals to differences in the underlying cause of the pain. For example, CRPS may be more discrete with respect to the affected area than the disorders included in our heterogeneous group; indeed, the subjects included in the Schwoebel et al (2001, 2002) studies were selected in part on the basis of pain that was clearly restricted to one arm/shoulder. Musculoskeletal pain may be less discrete, with the result that subjects are more likely generalize the anticipated pain across both extremities. Alternatively, the discrepancy might reflect random differences across relatively small samples or subtle differences in the methods employed in the different studies.
Consistent with the fact that subjects with CRPS exhibit many of the features of the neglect syndrome (Frettloh et al., 2006; Galer and Jensen, 1999), Moseley and colleagues have argued that the impaired performance of these patients on motor imagery tasks similar to that reported here is attributable to inattention to the painful hand (Moseley et al., 2009). Although “neglect-like” phenomena that might be influenced by attention have been reported in subjects with CRPS (Gener and Jensen, 1999; Förderreuther et al, 2004, Frettloh et al, 2006, Lewis et al, 2007) and attention to a painful hand influences the performance of normal subjects with experimentally induced pain (Hudson et al, 2006), we believe attentional effects to be an unlikely explanation for our subjects performance for several reasons. First, we are unaware of reports that musculoskeletal and radiculopathic pain are associated with inattention to the painful hand. Second, while inattention to the painful hand could explain a general slowing of RT for stimuli depicting the painful hand, it is not clear that inattention could explain the finding that subjects with arm/shoulder pain were not only slower to respond to stimuli depicting the painful hand but were disproportionately slower for stimuli requiring larger amplitude mental rotations.
Another potential account of our findings is that subjects with arm pain are slower with the painful hand because the response itself causes pain. This possibility seems unlikely for several reasons. First, as noted above, such an account would predict a general slowing of RTs for the painful hand but not disproportionate slowing for stimuli requiring larger amplitude mental rotations; pain associated with keypress would not be expected to be influenced by the magnitude of mental rotations. Second, this account does not explain the findings of the bilateral arm pain subjects. Finally, we have recently demonstrated similar findings on a foot laterality task in subjects with leg/foot pain (Coslett et al, In press); as subjects responded with their (painfree) hands, the fact that RTs were proportional to the degree of mental rotation in these subjects cannot be explained by pain associated with responding. While these data do not definitively exclude the possibility that pain with movement influences the performance of subjects with unilateral arm/shoulder pain, collectively they suggest that this factor does not contribute substantially to our findings.
We suggest that imagined movements are mediated by a “forward model” that not only specifies the timing and force of muscle contractions but also anticipates the sensory consequences of that action (Blakemore et al., 1999; Desmurget and Grafton, 2000). On this account, subjects are slower to respond to stimuli that would require large amplitude rotations because those movements are likely to be associated with greater pain (Moseley, 2004). Additionally, the fact that subjects exhibit a significant correlation between slowing for large amplitude movements and ratings of pain with movement but not non-specific pain is consistent with the hypothesis that the anticipation of movement related pain underlies the effects reported here.
Finally, although we focus on the utility of the hand laterality task in the assessment of pain, the task has proven to be useful in subjects with neglect (Coslett, 1998; Sirigu et al., 1996), focal dystonia (Fiorio et al., 2006), torticollis (Fiorio et al., 2007) and limb amputation. We suggest that the task may provide important insights into the brain representations of the body that can lead to a deeper understanding of pain and other somatic disorders (Nico et al., 2004).
Table 2.
Control.
| Palm down | Palm up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | ||||||||||||
| Rotation | 0° | 60° medial |
120° medial |
180° | 120° lateral |
60° lateral |
0° | 60° medial |
120° medial |
180° | 120° lateral |
60° lateral |
| Reaction time |
1455.58 | 1432.63 | 2179.78 | 3062.69 | 1985.85 | 1595.85 | 1770.19 | 1572.92 | 1804.23 | 2736.91 | 2730.25 | 2172.10 |
| Accuracy (%) | 97.26 | 98.48 | 91.16 | 82.93 | 91.77 | 97.26 | 98.78 | 96.95 | 95.12 | 90.55 | 89.63 | 93.29 |
| Right | ||||||||||||
| Rotation | 0° | 60° medial |
120° medial |
180° | 120° lateral |
60° lateral |
0° | 60° medial |
120° medial |
180° | 120° lateral |
60° lateral |
| Reaction Time |
1292.49 | 1215.94 | 1522.66 | 2943.55 | 1914.38 | 1379.93 | 1622.59 | 1450.98 | 1708.17 | 2573.44 | 3065.46 | 2190.12 |
| Accuracy (%) | 97.87 | 98.48 | 94.51 | 80.18 | 92.07 | 98.17 | 94.51 | 94.21 | 94.51 | 85.06 | 87.80 | 94.21 |
Acknowledgments
This work was supported by the National Institutes of Health (RO1 NS046049 to HBC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blakemore SJ, Frith CD, Wolpert DM. Spatio-temporal prediction modulates the perception of self-produced stimuli. J Cogn Neurosci. 1999 Sep;11(5):551–559. doi: 10.1162/089892999563607. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, Pandya KJ. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330(9):592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- Coslett HB. Evidence for a disturbance of the body schema in neglect. Brain Cogn. 1998 Aug;37(3):527–544. doi: 10.1006/brcg.1998.1011. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Medina J, Kliot D, Burkey A. Mental Motor Imagery and Chronic Pain: The Foot Laterality Task. Journal of the International Neuropsychological Society. doi: 10.1017/S1355617710000299. In press. [DOI] [PubMed] [Google Scholar]
- Daly AE, Bialocerkowski AE. Does evidence support physiotherapy management of adult Complex Regional Pain Syndrome Type One? A systematic review. European Journal of Pain. 2009;13:339–353. doi: 10.1016/j.ejpain.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Decety J, Michel F. Comparative analysis of actual and mental movement times in two graphic tasks. Brain Cogn. 1989;11(1):87–97. doi: 10.1016/0278-2626(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000 Nov 1;4(11):423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Duncombe ME, Bradshaw JL, Iansek R, Phillips JG. Parkinsonian patents without dementia or depression do not suffer from bradyphrenia as indexed by performance in mental rotation tasks with and without advance information. Neuropsychologia. 1994 Nov;32(11):1383–1396. doi: 10.1016/0028-3932(94)00071-9. [DOI] [PubMed] [Google Scholar]
- Fiorio M, Tinazzi M, Aglioti SM. Selective impairment of hand mental rotation in patients with focal hand dystonia. Brain. 2006;129(1):47–54. doi: 10.1093/brain/awh630. [DOI] [PubMed] [Google Scholar]
- Fiorio M, Tinazzi M, Ionta S, Fiaschi A, Moretto G, Edwards MJ, Bhatia KP, Aglioti SM. Mental rotation of body parts and non-corporeal objects in patients with idiopathic cervical dystonia. Neuropsychologia. 2007;45(10):2346–2354. doi: 10.1016/j.neuropsychologia.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Frettloh J, Huppe M, Maier C. Severity and specificity of neglect-like symptoms in patients with complex regional pain syndrome (CRPS) compared to chronic limb pain of other origins. Pain. 2006;124:184–189. doi: 10.1016/j.pain.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Förderreuther S, Sailer U, Straube A. Impaired self-perception of a hand in complex regional pain syndrome (CRPS) Pain. 2004;110:756–761. doi: 10.1016/j.pain.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Galer BS, Jensen M. Neglect-Like Symptoms in Complex Regional Pain Syndrome: Results of a Self-Administered Survey. J Pain Symptom Manage. 1999;18(3):213–217. doi: 10.1016/s0885-3924(99)00076-7. [DOI] [PubMed] [Google Scholar]
- Ganis G, Keenan JP, Kosslyn SM, Pascual-Leone A. Transcranial Magnetic Stimulation of Primary Motor Cortex Affects Mental Rotation. Cereb Cortex. 2000 Feb;10(2):175–180. doi: 10.1093/cercor/10.2.175. [DOI] [PubMed] [Google Scholar]
- Gracely RH. Pain Measurement. Acta Anaesthesiol Scand. 1999;43(9):897–908. doi: 10.1034/j.1399-6576.1999.430907.x. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Woods RP, Arbib MA. Functional anatomy of pointing and grasping in humans. Cereb Cortex. 1996;6:226–237. doi: 10.1093/cercor/6.2.226. [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum Brain Map. 2001 Jan;12(1):1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson ML, McCormick K, Zalucki N, Moseley GL. Expectation of pain replicates the effect of pain in a hand laterality recognition task: Bias in information processing toward the painful side? European Journal of Pain. 2006:219–224. doi: 10.1016/j.ejpain.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Janig W, Baron R. Complex regional pain syndrome is a disease of the central nervous system. Clin Autonom Res. 2002;12:150–164. doi: 10.1007/s10286-002-0022-1. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Mental imagery in the motor context. Neuropsychologia. 1995 Nov;33(11):1419. doi: 10.1016/0028-3932(95)00073-c. 32. [DOI] [PubMed] [Google Scholar]
- Krams M, Rushworth MF, Deiber MP, Frackowiak RS, Passingham RE. The preparation, execution and suppression of copied movements in the human brain. Exp Brain Res. 1998 Jun;120(3):386–398. doi: 10.1007/s002210050412. [DOI] [PubMed] [Google Scholar]
- Lewis JS, Kersten P, McCabe CS, McPherson KM, Blake DR. Body perception disturbance: A contribution to pain in complex regional pain syndrome (CRPS) Pain. 2007;133:111–119. doi: 10.1016/j.pain.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Maruff P, Velakoulis D. The voluntary control of motor imagery. Imagined movements in individuals with feigned motor impairment and conversion disorder. Neuropsychologia. 2000;38(9):1251–1260. doi: 10.1016/s0028-3932(00)00031-2. [DOI] [PubMed] [Google Scholar]
- Moseley GL. Why do people with complex regional pain syndrome take longer to recognize their affected hand? Neurology. 2004;62:2182–2186. doi: 10.1212/01.wnl.0000130156.05828.43. [DOI] [PubMed] [Google Scholar]
- Moseley GL. Graded motor imagery for pathologic pain: A randomized controlled trial. Neurology. 2006;67:2129–2134. doi: 10.1212/01.wnl.0000249112.56935.32. [DOI] [PubMed] [Google Scholar]
- Moseley Gl, Zalucki N, Birklein F, Marinus J, van Hilten JJ, Luomajoki H. Thinking about movement hurts: The effect of motor imagery on pain and swelling in people with chronic arm pain. Arthritis and Rheumatism. 2008;59:623–631. doi: 10.1002/art.23580. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Gallace A, Spence C. Space-based, but not arm-based, shift in tactile processing in complex regional pain syndrome and its relationship to cooling of the affected limb. Brain. 2009;132:3142–3151. doi: 10.1093/brain/awp224. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Parsons TJ, Spence C. Visual distortion of a limb modulates the pain and swelling evoked by movement. Curr Biol. 2008 Nov 25;18(22):R1047–R1048. doi: 10.1016/j.cub.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Nico D, Daprati E, Rigal F, Parsons L, Sirigu A. Left and right hand recognition in upper limb amputees. Brain. 2004 Jan;127(1):120–132. doi: 10.1093/brain/awh006. [DOI] [PubMed] [Google Scholar]
- Parsons LM. Imagined spatial transformations of one's hands and feet. Cogn Psychol. 1987a Apr;19(2):178–241. doi: 10.1016/0010-0285(87)90011-9. [DOI] [PubMed] [Google Scholar]
- Parsons LM. Imagined spatial transformation of one's body. J Exp Psychol Gen. 1987b;116(2):172–191. doi: 10.1037//0096-3445.116.2.172. [DOI] [PubMed] [Google Scholar]
- Parsons LM. Temporal and kinematic properties of motor behavior reflected in mentally simulated action. J Exp Psychol Percept Perform. 1994;20(4):709–730. doi: 10.1037//0096-1523.20.4.709. [DOI] [PubMed] [Google Scholar]
- Parsons LM. Inability to reason about an object’s orientation using an axis and angle of rotation. J Exp Psychol Hum Percept Perform. 1995;21:1259–1277. [Google Scholar]
- Parsons LM, Fox PT. The neural basis of implicit movements used in recognizing hand shape. Cogn Neuropsychol. 1998;15:583–615. [Google Scholar]
- Parsons LM, Gabrieli GDE, Phelps EA, Gazzaniga MS. Cerebrally-lateralized mental representations of hand shape and movement. J Neurosci. 1998;18:6539–6548. doi: 10.1523/JNEUROSCI.18-16-06539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Pasqualetti P, Tecchio F. Corticospinal excitability modulation to hand muscles during movement imagery. Cereb Cortex. 1999 Mar;9(2):161–167. doi: 10.1093/cercor/9.2.161. [DOI] [PubMed] [Google Scholar]
- Schwoebel J, Coslett HB, Bradt J, Friedman R, Dileo C. Pain and the body schema: effects of pain severity on mental representations of movement. Neurology. 2002 Sep 10;59(5):775–777. doi: 10.1212/wnl.59.5.775. [DOI] [PubMed] [Google Scholar]
- Schwoebel J, Friedman R, Duda N, Coslett HB. Pain and the body schema: evidence for peripheral effects on mental representations of movement. Brain. 2001;124:2098–2104. doi: 10.1093/brain/124.10.2098. [DOI] [PubMed] [Google Scholar]
- Schwoebel J, Coslett HB. Evidence for Multiple, Distinct Representations of the Human Body. J Cogn Neurosci. 2005;17:543–553. doi: 10.1162/0898929053467587. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The Mental Representation of Hand Movements After Parietal Cortex Damage. Science. 1996 Sept;13:1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]