Abstract
Background
Neutrophils (PMN) are the first cells recruited at the site of inflammation. They play a key role in the innate immune response by recognizing, ingesting and eliminating pathogens and participate in the orientation of the adaptive immune responses. However, in Inflammatory Bowel Disease (IBD), transepithelial neutrophil migration leads to an impaired epithelial barrier function, perpetuation of inflammation and tissue destruction via oxidative and proteolytic damage. Curcumin (diferulolylmethane) displays a protective role in mouse models of IBD and in human ulcerative colitis, a phenomenon consistently accompanied by a reduced mucosal neutrophil infiltration.
Methods
We investigated the effect of curcumin on mouse and human neutrophil polarization and motility in vitro and in vivo.
Results
Curcumin attenuated LPS-stimulated expression and secretion of MIP-2, IL-1β, KC and MIP-1α in colonic epithelial cells (CEC) and in macrophages. Curcumin significantly inhibited PMN chemotaxis against MIP-2, KC or against conditioned media from LPS-treated macrophages or CEC, a well as the IL-8-mediated chemotaxis of human neutrophils. At non-toxic concentrations, curcumin inhibited random neutrophil migration suggesting a direct effect on neutrophil chemokinesis. Curcumin-mediated inhibition of PMN motility could be attributed to a downregulation of PI3K activity, AKT phosphorylation and F-actin polymerization at the leading edge. The inhibitory effect of curcumin on neutrophil motility was further demonstrated in vivo in a model of aseptic peritonitis.
Conclusion
Our results indicate that curcumin interferes with colonic inflammation partly through inhibition of the chemokine expression and through direct inhibition of neutrophil chemotaxis and chemokinesis.
Keywords: PMN, inflammation, MIP-2, KC, IL-8, chemotaxis, chemokinesis
INTRODUCTION
Aberrant immune response has been proposed as the underlying cause of Inflammatory Bowel Disease (IBD), combining an early defect in the innate immune response followed by a dysregulated T cell response in the chronic stage of the disease. Impaired neutrophil recruitment has been hypothesized to contribute to the development of Crohn’s Disease (CD). It has been postulated, that neutrophil accumulation and associated bacterial clearance are impaired and favor the formation of granulomatous inflammation in CD (1). Although forms of typhlitis or enterocolitis have been reported in various neutropenias or genetic disorders with impaired granulocyte function (2), the hypothesis that inappropriate neutrophil recruitment or function may be at least in part responsible for the onset of CD remains controversial.
Initiating events differs from the complex mechanisms responsible for the disease progression and relapse, which are traditionally attributed to a hyperactive adaptive immune response to the bowel luminal contents and primarily linked to a T-cell dysfunction accompanied by an unabated neutrophil influx. The latter leads to the formation of crypt abscesses, a characteristic histologic lesion found particularly in patient with Ulcerative Colitis (UC). Disproportionate and persistent inflammatory process mediated by PMN leads to reduction of epithelial barrier function, perpetuation of inflammatory processes, and tissue destruction via the release of free radicals and proteases. Therefore in established disease, IBD severity seems to be directly correlated with the rate of transepithelial neutrophil migration, their accumulation in the intestinal lumen, and neutrophil fecal excretion (3). Neutrophils have also been proposed to be important pro-inflammatory effector cells capable of associating with lymphocytes to foster epithelial dysfunction and injury associated with IBD. Recently, neutrophil serine proteases have been demonstrated as regulators of cell signaling during inflammation by enzymatic processing of TNFα, IL-1β and IL-18 to their active forms, and even participate in activating the LPS receptor, TLR-4 (4).
Therefore, neutrophil recruitment and functions have become a target for pharmaceutical approaches aimed at reducing relapse rate in IBD patients. While major proinflammatory cytokines regulating T cell activation, such as TNFα, IFN-γ and IL-12/23p40 have been at the center of attention for pharmaceutical interventions (5), strategies interfering with neutrophil migration, particularly targeting major chemoattractant receptors such as CXCR2 and CXCR1, have also been considered (6). Such approaches could represent useful adjuncts to other therapeutic approaches using biologics that primarily target T cells or proinflammatory cytokines. Animal studies on the role of neutrophil recruitment in experimental colitis present somewhat inconsistent evidence. CXCR2−/− mice are protected from tissue damage in chronic DSS colitis (7). Similarly, antibody blockade of CXCR2 improves symptoms of DSS colitis (8). Moreover, neutrophil depletion or inhibition of CD11b/CD18 decreases the severity of DSS and TNBS colitis, respectively (9, 10). However other groups demonstrated that neutrophil depletion did not significantly improve acute colitis induced by acetic acid, TNBS/Ethanol and PMA (11, 12). In yet another report, blocking of neutrophil adhesion or neutrophil depletion aggravated colitis (13).
The effect of the dried rhizome of Curcuma longa Linn, also called turmeric, on the immune responses (both innate and adaptive) has generated considerable attention in the past decade (14–16). Curcumin has been identified as the most active constituent of turmeric and is also defined as an anti-inflammatory, anti-oxidant, pro-apoptotic, anti-proliferative and anti-infectious agent (17–19). The anti-inflammatory activity of curcumin has been attributed to its interference with the arachidonic acid cascade (20, 21), regulation of several enzymes via direct interaction (e.g COX2, 5LOX, iNOS), although its presumed primary mechanism of action has been ascribed to the inhibition of NF-κB (16). Curcumin was shown to be effective in both acute and chronic models of inflammation. The potential usefulness of curcumin in the treatment of IBD was suggested by studies in chemically induced rodent models of colitis (22–29), and in a well-controlled ulcerative colitis clinical trial (30). In experimental animals, curcumin significantly improves survival, colonic morphology, dampens local cytokine and chemokine production and consistently reduces mucosal neutrophil infiltration (26–28, 31, 32). The latter may be partially explained by the recently described pro-apoptotic in vitro effects of curcumin on human neutrophils via the activation of the p38 mitogen-activated protein kinase pathway (33), or by the curcumin-mediated reduction in IL-8-induced Ca2+ flux and reduced membrane expression of CXCR1 and CXCR2 via their sequestration in the vesicular compartments (34). However, the effects of curcumin on PMN migration have not been investigated, despite its potentially significant clinical implications.
In this report, we provide the evidence for the modulatory effects of curcumin on neutrophil motility. These effects are not only mediated through an inhibition of chemoattractant gradient formation, but also through modulation of PMN chemotaxis and chemokinesis in settings where no cytotoxic effects of curcumin are observed. The mechanisms of this modulation were traced to the reduction of PI3K activity, phosphatidylinositol (3,4,5)-trisphosphate [PI(3,4,5)P3] synthesis, and inhibition of AKT (protein kinase B) activation, leading to a potent inhibition of F-actin polymerization at the neutrophil leading edge. The in vitro findings were further corroborated in an in vivo model of neutrophil migration in aseptic peritonitis. These results provide further insight into the mechanisms of the protection curcumin affords in chronic mucosal inflammation.
MATERIALS AND METHODS
Curcumin
98.05% pure curcumin, free of contaminating curcuminoids (demethoxy-curcumin and bis-demethoxy-curcumin) was obtained from ChromaDex (Irvine, CA) and tested for its efficacy in vitro with LPS-treated RAW 264.7 mouse macrophages. Curcumin was dissolved in DMSO (Sigma Aldrich, Saint Louis, MO). In all cases, the final concentration of DMSO in cell culture media was 0.1%.
Experimental animals and Diets
Six-week-old male BALB/c mice were obtained from Harlan Laboratories (Indianapolis, IN) and maintained in a conventional animal facility at the University of Arizona Health Sciences Center. Sentinel mice were routinely monitored and determined as free from common murine pathogens (MHV, MPV, MVM, TMEV, Mycoplasma pulmonis, Sendai, EDIM, MNV, ecto- and endoparasites). Animal use protocols were approved by the University of Arizona Animal Care.
Model of aseptic peritonitis
Curcumin was dissolved in sterile olive oil and administered intraperitoneally (i.p.) at the dose of 100μg/g body weight in 80μl injection volume. BALB/c mice received 3 injections (i.p.) of olive oil (vehicle) with or without curcumin in 12h intervals. Four hours after the last injection, peritonitis was induced by i.p. injection of 500μl of 3% (wt/vol) sterile thioglycollate (Sigma). 3 hours later, peritoneal cavity was lavaged with 5mL of cold RPMI 1640 medium, and the number of cells was determined by Vi-Cell XR automated cell counter and viability analyzer (Beckman Coulter, Miami, FL). Cytospins of the lavaged cells were performed at 900 rpm for 5 min in Shandon Cytospin III centrifuge (Thermo Fisher Scientific). Giemsa staining confirmed that granulocytes represented >90% of the cells lavaged 3 hours after thioglycollate broth injection.
YAMC cell culture and treatment
Conditionally immortalized mouse colonic epithelial cells (YAMC) were obtained from Dr. Robert Whitehead (Vanderbilt University) (35) and were cultured under permissive conditions in RPMI 1640 with 2mM L-glutamine, 5% heat-inactivated fetal bovine serum (Hyclone, Fisher, Pittsburgh, PA), 1mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin, 6.25mg/L insulin and 5U/ml of recombinant murine IFNγ (Pierce, Rockford, IL) at 33°C and 5% CO2. If not indicated otherwise, media and other reagents used for cell culture were purchased from Invitrogen (Carlsbad, CA).
Cells were seeded in 24-well plates, 24 hours prior to the experiments, they were transferred into 37°C in the same culture medium but without IFNγ. Cells were treated with DMSO or 50μM of curcumin for 30 minutes prior to LPS from E. coli O55:B55 at 100ng/ml (Calbiochem/Merck Chemicals, Nottingham, UK) for 4 hours. Cell culture supernatant was collected and YAMC cells monolayer was frozen in liquid nitrogen and stored at −80°C until RNA extraction.
Intraperitoneal macrophages selection, culture and treatment
Macrophages were collected from the peritoneum of BALB/c mice by a lavage with 5mL of cold RPMI 1640. The number of macrophages was determined by counting adherent cells after a 1 hour attachment period on a hemocytometer glass surface at 37°C in 5% CO2. After collection, cells were seeded at 106 cells/well in 24-well plates and cultured overnight in RPMI 1640 supplemented with 2mM L-glutamine 10% heat-inactivated fetal bovine serum (Hyclone, Fisher), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C with 5% CO2. Then, intra-peritoneal macrophages were treated with or without curcumin at 50μM 30 minutes prior to stimulation with LPS from E. coli O55:B55 at 10ng/ml (Calbiochem) for 2 hours. Cell culture supernatants were collected and stored at −80°C until analysis. Macrophages were snap frozen in liquid nitrogen and stored at −80°C.
Real-time RT-PCR analysis
Real-time RT-PCR was used to evaluate colonic expression of MIP-2, keratinocyte chemoattractant (KC), MIP-1α and IL-1β mRNA. Total RNA was isolated from peritoneal macrophages or YAMC cells using TRIzol reagent (Invitrogen) and its integrity was confirmed by denaturing agarose gel electrophoresis and calculated densitometric 18S/28S ratio. 250ng of total RNA was reverse-transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Subsequently, 20 μL of the PCR reactions were set up in 96-well plates containing 10 μl 2x IQ Supermix (Bio-Rad), 1μL TaqMan® respective primer/probe set (Applied Biosystems, Foster City, CA), 2μL of the cDNA synthesis reaction (10% of RT reaction) and 7μL of nuclease-free water. Reactions were run and analyzed on a Bio-Rad iCycler iQ real–time PCR detection system. Cycling parameters were determined and resulting data were analyzed by using the comparative Ct method as means of relative quantification, normalized to an endogenous reference (TATA Box Bonding Protein, TBP) and relative to a calibrator (normalized Ct value obtained from control mice) and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin #2: Rev B “Relative Quantification of Gene Expression”).
Chemokine Assays
MIP-2 concentrations in the supernatants from peritoneal macrophages and YAMC cell cultures were measured by enzyme-linked immunosorbent assay (ELISA; eBioscience, San Diego, CA) according to the manufacturer’s protocols. KC, MIP-1α and IL-1β were determined using the Bio-Plex Cytokine Assay (Bio-Rad) on a Luminex-100 workstation (Liquichip; Qiagen) with MasterPlexCT software (MiraiBio, San Francisco, CA).
Neutrophil selection
Bone marrow derived neutrophils were obtained from BALB/c femurs and tibiae and filtered through a Falcon 100-μm nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ). After red cell lysis in a hypotonic buffer (Becton Dickinson), cells were washed and resuspended in complete medium (RPMI 1640; Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (Hyclone, Fisher), 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Ly-6G positive neutrophils were purified from the total population of bone marrow derived cells using automated magnetic separation (autoMACS™, Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions with the PosseS program. Cell purity was assessed by flow cytometry using FITC-conjugated rat anti-mouse Ly-6G and Ly-6C monoclonal antibody (BD, Pharmingen). After selection cells were washed and resuspended in RPMI 1640, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) and 0.1% BSA (globulin and fatty acid free, Sigma).
Curcumin cytotoxicity assay
Potential neutrophil cytotoxicity of curcumin was determined using the ToxiLight Non-destructive Cytotoxicity BioAssay Kit (Cambrex, Charles City, IA) according to the manufacturer’s instructions. This assay is based on the bioluminescent measurement of adenylate kinase (AK) release. A loss of cell membrane integrity results in the leakage of AK from the cytoplasm and the measurement of AK in the cell culture medium allows for an accurate and sensitive determination of cytotoxicity. Curcumin cytotoxicity was also examined by trypan blue dye exclusion assay using a Vi-Cell XR automated cell counter and viability analyzer (Beckman Coulter). PMNs were incubated for 2 hours (exceeding the maximal exposure time in our experimental settings) at 37°C with 5% CO2, with increasing concentration of curcumin from 5μM to 100μM.
Human neutrophil separation from peripheral blood
Peripheral blood was collected from healthy volunteers. Blood was diluted 1:1 in Hank’s balanced salt solution (without Ca+ or Mg+; Invitrogen) and carefully layered onto 3ml of Histopaque 1119 (density 1.119g/mL; Sigma) and Histopaque 1077 (density 1.077g/mL; Sigma). After centrifugation at 800g for 40 minutes, human PMN were collected from the interphase between Histopaque 1119 and 1077. Collected cells were washed in HBSS and resuspended in RPMI 1640 with 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) and 0.1% BSA (Sigma).
Chemotaxis and chemokinesis assay
Mouse and human-derived PMN were labeled with 5mM of the fluorescent indicator Calcein (CellTrace Calcein red-orange, AM, Molecular Probes) for 30 minutes at 37°C and then washed in RPMI 1640, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) and 0.1% BSA (Sigma). Twenty- nine microliters of chemotactic factor: MIP-2, KC or IL-8 (Peprotech, Rocky Hill, NJ), diluted in RPMI medium or conditioned media from YAMC cells or peritoneal macrophages were placed in the bottom compartment of a modified 96-well Boyden chamber fitted with polycarbonate track-etch (PCTE) membranes with 5μm pore size (ChemoTX System, Neuroprobe, Gaithersburg, MD). Calcein-stained PMN (250,000 cells/25μl) were placed directly onto the filter (top chamber). To study the effect of curcumin on chemokinesis, MIP-2 was placed on both sites of the membrane at the same concentration of 20ng/ml. In some experiments, PMNs were pretreated with 25 or 50μM of curcumin at 37°C for 30 min prior to the assay. The chamber was placed for 1 hour at 37°C, 5% CO2. Fluorescence, corresponding to calcein-stained PMN in the bottom part was monitored using a Fluoroskan Ascent plate fluorometer and Fluoroskan software (Labsystems, Thermo Fisher Scientific).
Western blot analysis
Bone marrow derived neutrophils were pretreated with curcumin (10–50μM) for 5 minutes prior to 30-second stimulation with MIP-2 (20ng/ml). Proteins were extracted by cell lysis in boiling Laemmlisample buffer with 2 mM β-mercaptoethanol. Proteins were fractionated by 8% SDS-PAGE and blotted on a nitrocellulose membrane for immunoblot analysis. After overnight incubation in 1X PBS/0.1% Tween 20, primary antibody to phospho-AKT (Ser473, Cell Signaling, Danvers, MA) or to phospho-p44/42 MAPK (Thr202/Tyr204) (Cell Signaling) were added. Total AKT (Cell Signaling) or total p44/42 MAPK antibodies were used to confirm equal loading. HRP-conjugated anti-rabbit secondary antibody was used at 1:20,000 dilution (Amersham Bioscience, Piscataway, NJ). Western detection was performed with Super Signal West Pico (Pierce). Developed films were scanned and densitometric analysis was done using Biorad ChemiDoc XRS Imaging station and Quantity One 4.6 software (Biorad, Hercules, CA).
PI3K activity assay
Bone marrow derived neutrophils were pretreated with curcumin (10–50μM) for 5 minutes prior to 30-seconds stimulation with MIP-2 (20ng/ml). PI3K activity was determined by measuring the concentration of PI(3,4,5)P3 (PIP3) using a competitive mass ELISA (Echelon Biosciences, Salt Lake City, UT). PIP3 extraction as well as the ELISA was performed according to the manufacturer’s protocol.
Under agarose migration assay
Under agarose neutrophil migration was performed as described (36) with minor modifications. Glass cover slips were placed in Falcon 35mm × 10mm culture dishes and overlaid with 3ml of 1.6% agarose solution prepared with RPMI1640 culture medium with 20% heat-inactivated FCS. After the agarose solidified, five wells 3.5 mm in diameter and 2.4mm apart were cut using a sterile silicone template and bore. The gels were allowed to equilibrate for 1hour at 37°C, 5% CO2. For chemotaxis assay, the wells were loaded with either neutrophils or 20ng/ml of MIP-2. In separate experiment series, DMSO or curcumin-pretreated PMNs (1–50 μM for 30 minutes) were used. In another series, curcumin was added directly to the gel at various concentrations.
F-actin staining
Following under-agarose assay, cells were fixed overnight in absolute methanol at 4°C, agarose gel was then gently removed, and PMNs fixed on a glass-coverslip were permeabilized in 1X PBS, 0.1% Triton for 20 minutes. Alexa 647-conjugated phalloidin (Molecular Probes) was then added the cells (1:50 dilution in 1X PBS/0.1%Triton) and incubated on ice for 30 minutes. Cells were counterstained with Sytox green (Molecular probes) to image the nuclei. After mounting, phalloidin staining was imaged with a Bio-Rad MRC1024ES confocal microscope (Bio-Rad).
Statistical Analysis
Statistical significance was determined by the analysis of variance (ANOVA) followed by Fisher PLSD post-hoc test with StatView software package v.4.53 (SAS Institute, Cary, NC). Data are expressed as mean ± standard error of mean.
Ethical Considerations
Animal use and blood collection from healthy human volunteers were approved by the University of Arizona Institutional Animal Care and Use Committee, and Institutional Review Board approval, respectively.
RESULTS
Curcumin inhibits LPS-stimulated expression and secretion of MIP-2, KC, IL-1β and MIP-1α in macrophages and colonic epithelial cells
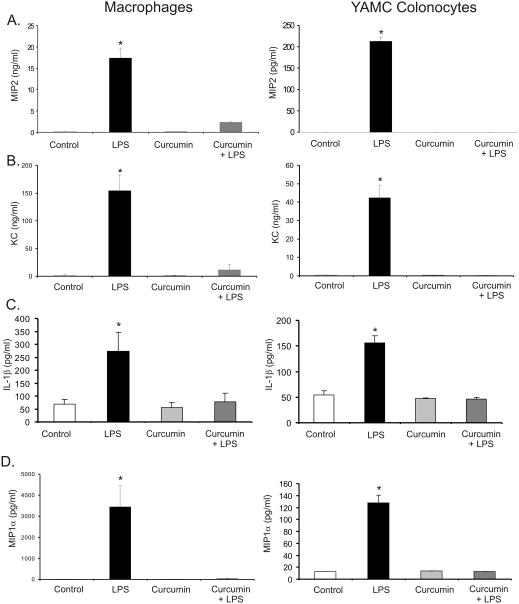
In IBD, chemokine production contributes to the exacerbation of the intestinal inflammation and has been considered as a potential therapeutic target (37, 38). Therefore, we investigated whether curcumin inhibits the formation of neutrophil chemoattractant gradient in vitro. MIP-2, KC, IL-1β and MIP-1α were chosen as key chemoattractants based on their involvement in neutrophil homing to the site of inflammation, as well as based on the previously reported microarray data (32). Epithelial YAMC cells and peritoneal macrophages were used as models of the two primary chemokine sources in the inflamed colonic mucosa. Both cell types were exposed to 50μM of curcumin 30 minutes prior to LPS treatment (100ng/ml for 4 hours in YAMC colonocytes and 10ng/ml for 2 hours in macrophages). Curcumin effectively reduced LPS-stimulated chemokine secretion in both peritoneal macrophages and in YAMC cells (Fig. 1). The same pattern was observed in real-time RT-PCR analysis (data not shown), thus suggesting transcriptional mechanism of inhibition.
Figure 1.
ELISA for secreted MIP-2 (A) and Bio-Plex analysis for secreted KC (B), IL-1β (C) and MIP-1α (D) in the cell culture medium of peritoneal macrophages or YAMC colonocytes pretreated with or without curcumin (50μM) for 30 minutes at 37°C LPS prior to 2 hour (10 ng/mL; macrophages) of 4 hour (100 ng/mL, YAMC) treatment E. coli. LPS. *Statistically significant differences (p≤0.05) between values from cells treated with LPS alone and other respective treatments (ANOVA followed by Fisher PLSD post-hoc test).
Curcumin has no cytotoxic effect on the bone marrow derived neutrophils
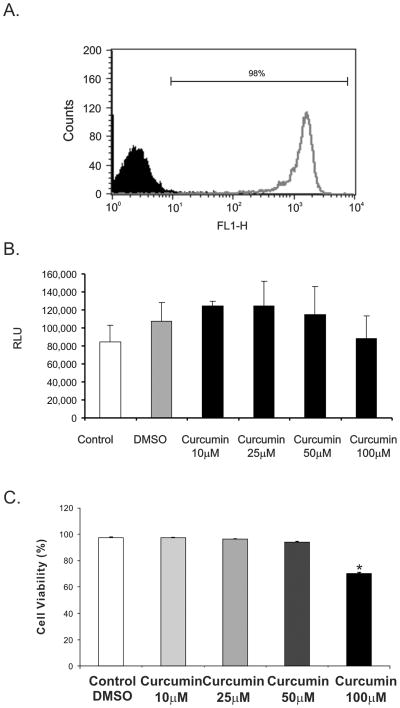
To investigate the indirect and direct effects of curcumin on neutrophils, we purified mouse PMNs from the bone marrow using anti-Ly6G microbeads (Miltenyi Biotech). This technique was superior to density gradient centrifugation and consistently yielded ≥95% pure neutrophil population as estimated by flow cytometry (Figure 2A). To investigate the potential harmful effects of curcumin on PMN reported in other publication (33), we analyzed the release of adenylate kinase and performed trypan blue dye exclusion assay as a measure of cytoxicity. Under experimental conditions used in or exceeding subsequent experiments, curcumin had no significant cytotoxic effects on PMN at the concentrations ranging between 10 to 100μM (Figure 2B–2C).
Figure 2.
(A) Flow cytometry analysis of PMN after magnetic selection. (B) Cytotoxity assay with PMN treated with and without curcumin at different concentrations (5 to 100μM) for 2 hours evaluated by adenylate kinase release (RLU, relative light units). (C) Trypan blue dye exclusion assay with PMN treated with and without curcumin at different concentrations (5 to 100μM) for 2 hours.
Curcumin inhibits neutrophil chemotaxis toward conditioned media from LPS-treated macrophages and colonic epithelial cells
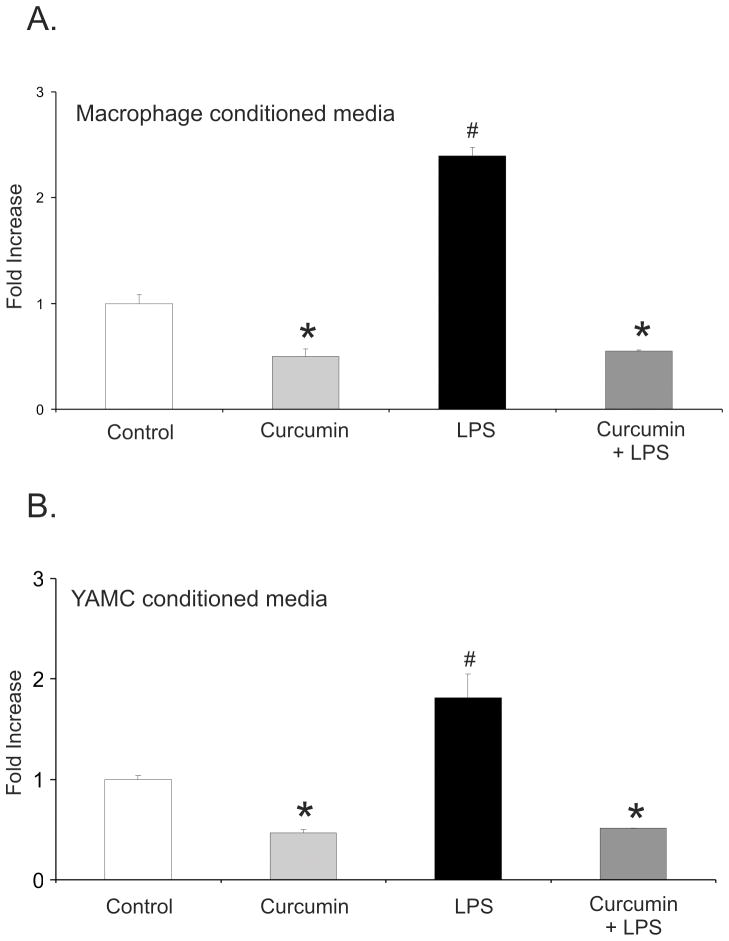
Since we analyzed macrophages and colonocytes only for the selected four chemokines, we verified the effects of curcumin on neutrophil chemotaxis using conditioned media from the two cell types from experiments analogous to those presented in Fig. 1. Conditioned media from both LPS-treated macrophages and colonic epithelial cells potently attracted neutrophils, while media from cells treated with curcumin alone or with curcumin and LPS were ineffective (Fig. 3A,B). The inhibition of chemotaxis toward conditioned media from cells treated with curcumin suggested that curcumin inhibited basal (unstimulated) chemokine secretion, or that trace amounts of the non-metabolized compound remaining in the media inhibited random neutrophil migration in the absence of chemoattractant gradient. Since the first hypothesis was not supported by the data from any of the four analyzed chemokines (Fig. 1), inhibition of chemokinesis appeared more likely and was tested in the subsequent experiments.
Figure 3.
Chemotaxis Assay with calcein red-orange AM stained bone marrow-derived mouse neutrophils against conditioned medium obtained from intraperitoneal macrophages (A) or YAMC cells (B) treated with DMSO (control), 50 μM curcumin, LPS (10 ng/mL), or LPS with curcumin (the same treatment conditions as described in Fig. 1). *Statistically significant differences (p≤0.05) between values from cells treated with curcumin or curcumin/LPS and control or LPS treatment; # statistically significant differences between values from cells treated with LPS alone and other respective treatments (ANOVA followed by Fisher PLSD post-hoc test).
Curcumin inhibits neutrophil chemotaxis in MIP-2 gradient
For the following experiments, MIP-2 was chosen as a functional mouse homologue of IL-8 and a chemoattractant of reference (39). Neutrophil chemotaxis was tested with increasing concentrations of MIP-2 (5–20 ng/mL) added to the bottom compartment with DMSO or curcumin (25 or 50 μM). Curcumin significantly inhibited neutrophil migration toward MIP-2, with no significant difference between the effects of 25 and 50 μM of the compound (Fig. 4A). Consistent with data from experiments with conditioned media, addition of curcumin alone to the bottom compartment also inhibited neutrophil migration, thus further suggesting that curcumin inhibits chemokine-independent neutrophil chemokinesis (Fig. 4B). To further confirm this observation, neutrophils were pre-treated with curcumin at 10–50μM prior to the migration assay, washed and applied in a uniform MIP-2 concentration (20 ng/mL in both compartments of the ChemoTX system). As anticipated, all tested concentrations of curcumin effectively reduced the random neutrophil migration in a homogenous chemoattractant concentration (Figure 4C), thus suggesting a direct effect on the neutrophil chemokinesis. To verify whether curcumin inhibition of neutrophil chemotaxis was to MIP-2, we also tested its effects in KC-driven chemotaxis. Similarly, curcumin significantly inhibited neutrophil migration in KC gradient (Figure 4D).
Figure 4.
(A) Chemotaxis of untreated, calcein-stained neutrophils against recombinant MIP-2 (5–20 ng/mL) without or with 25 or 50 μM curcumin in the lower chamber of the ChemoTx® Chemotaxis System. * p≤0.05 MIP-2 vs. control; # p≤0.05 curcumin vs. control or curcumin/MIP-2 vs. MIP-2 alone. (B) Chemotaxis of neutrophils pretreated with 10–50μM of curcumin (30 min prior to the assay) against recombinant MIP-2 (20ng/mL) * p≤0.05 MIP-2 vs. control; # p≤0.05 Curcumin 10μM/MIP-2 vs. MIP-2 alone; † Curcumin 25 or 50μM vs. Curcumin 10μM/MIP-2 or MIP-2 alone. (C) Chemokinesis assay of PMN with recombinant MIP-2 (20ng/mL) placed in the top and bottom chamber. PMNs were pretreated with curcumin (10, 25 or 50μM) 30 minutes prior to the assay. * p≤0.05 10–25μM curcumin vs. DMSO; # p≤0.05 50 μM curcumin vs. control or 10–20 μM curcumin (ANOVA followed by Fisher PLSD post-hoc test). (D) Chemotaxis of untreated, calcein-stained neutrophils against recombinant KC (75 ng/mL) without or with 25 or 50 μM curcumin in the lower chamber of the ChemoTx® Chemotaxis System. * p≤0.05 KC vs. control; # p≤0.05 KC/curcumin vs. KC; ** control vs. curcumin alone.
Curcumin inhibits human neutrophil chemotaxis toward IL-8
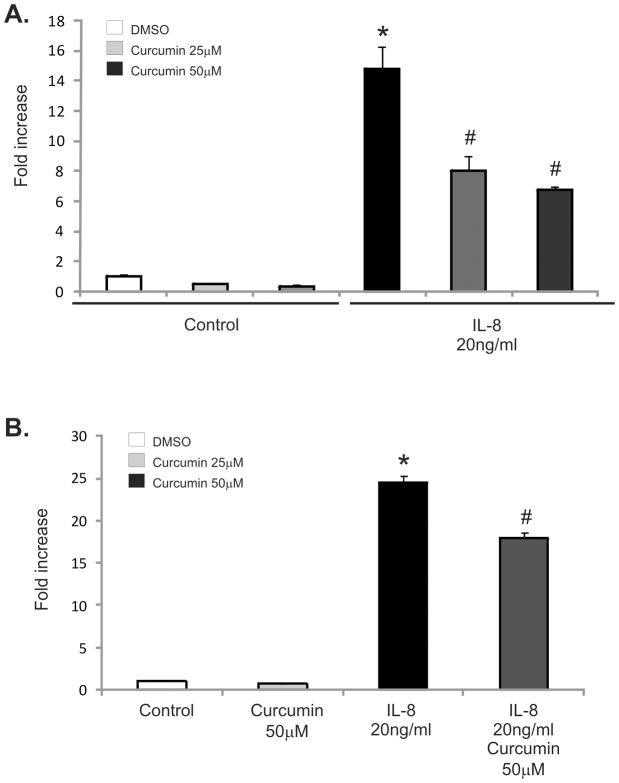
The inhibitory effect of curcumin on neutrophil chemotaxis and chemokinesis was also demonstrated using human-blood derived neutrophils and IL-8 as a reference chemoattractant. Consistent with our previous results, addition of curcumin in the bottom compartment of the chemotaxis chamber inhibited neutrophil migration toward IL-8 (Figure 5A). In addition, human-neutrophil pretreated with 50μM of curcumin 30min prior to the migration assay demonstrated reduce migration toward IL-8, albeit to a somewhat lesser extent (Figure 5B). Human neutrophil migration in a homogenous chemoattractant concentration was also inhibited by curcumin pretreatment (25 and 50 μM) (Figure 5C).
Figure 5.
(A) Chemotaxis of untreated, calcein-stained human- neutrophils against recombinant IL-8 (20 ng/mL) without or with 25 or 50 μM curcumin in the lower chamber of the ChemoTx® Chemotaxis System. * p≤0.05 IL-8 vs. control; # p≤0.05 IL-8/curcumin vs. control or curcumin/IL-8 vs. IL-8 alone. (B) Chemotaxis of neutrophils pretreated with 50μM of curcumin (30 min prior to the assay) against recombinant IL-8 (20ng/mL) * p≤0.05 IL-8 vs. control; # p≤0.05 Curcumin 50μM/IL-8 vs. IL-8 alone or IL-8/curcumin vs. control. (C) Chemokinesis assay of PMN with recombinant IL-8 (20ng/mL) placed in the top and bottom chamber. PMNs were pretreated with curcumin (25 or 50μM) 30 minutes prior to the assay. * p≤0.05 10–20μM curcumin vs. DMSO; # p≤0.05 50 μM curcumin vs. control or 10–25 μM curcumin (ANOVA followed by Fisher PLSD post-hoc test)
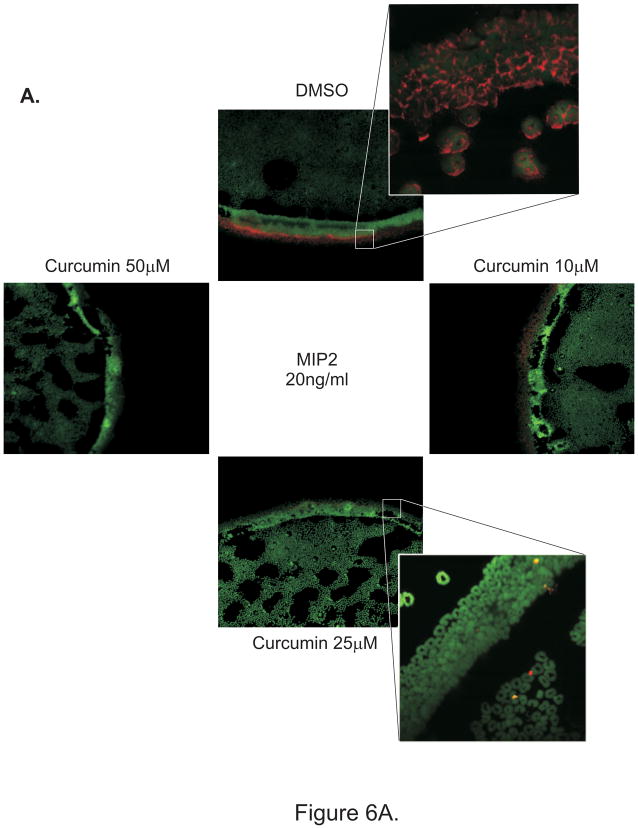
Curcumin inhibits MIP-2-induced F-actin polymerization in mouse neutrophils
Polymerization of actin cytoskeleton is an essential element of PMNs motility, with F-actin formation at the leading edge guiding cell locomotion. Chemoattractants stimulate F-actin polymerization and allow cells to migrate directionally in a concentration gradient. We hypothesized that curcumin may exert its effects at least partially through inhibiting F-actin polymerization. To test this hypothesis, we employed the under-agarose migration assay to study the effect of curcumin (pretreatment with 10, 25 or 50μM) on neutrophils migration in a MIP-2 gradient. This well established technique allows the analysis of the behavior of cell population rather than of individual cells (36). As shown by phalloidin staining in Fig. 6A–B, curcumin dose dependently inhibited F-actin polymerization and reduced neutrophil migration. To test whether prolonged exposure (30 min) to curcumin is a prerequisite to observe this phenomenon, under-agarose migration assay was also performed with untreated neutrophils added to a well cut in agarose polymerized with addition of DMSO or 25–50μM of curcumin (solid phase curcumin). Fig. 6C depicts a representative phalloidin staining (red) demonstrating on-contact inhibition of F-actin polymerization in neutrophils as they attempt to migrate toward MIP-2 gradient. These observations suggest a rapid mechanism of inhibition of neutrophil migration, unlikely secondary to the previously reported effects of curcumin on cell apoptosis or endocytic retention of CXCR2 (34).
Figure 6.
Under agarose neutrophil migration assay: F-actin was labeled after migration with Alexa-647 conjugated phalloidin and nuclei were counterstained with sytox green and cells were imaged under a confocal laser scanning microscope. (A) Control DMSO-treated PMN or PMN pretreated with 10–50 μM of curcumin for 30 min prior to the assay were loaded into different wells cut in 1.6% agarose gel. Recombinant MIP-2 (20ng/ml) was placed in the central well. Each well was placed at equal distance from each other. High magnification images from PMN treated with DMSO or 25 μM curcumin are depicted as crop-outs. (B) Curcumin was homogeneously added to the gel at 25 or 50 μM. PMN or recombinant MIP-2 (20ng/ml) were loaded into adjacent, evenly spaced wells.
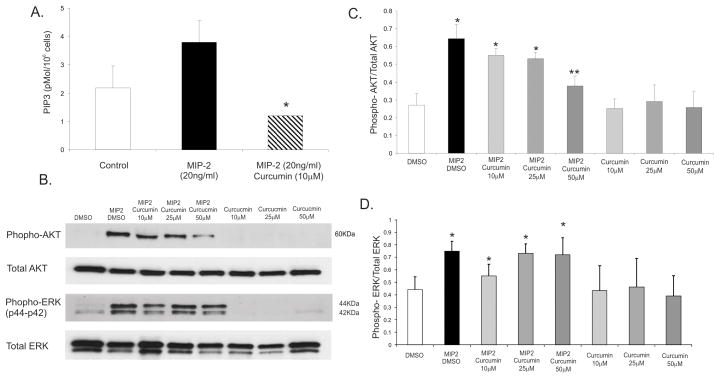
Curcumin inhibits MIP-2 induced PI3K and AKT pathway but not p44/42 ERK phosphorylation in mouse neutrophils
It has been shown previously that the response to intermediate chemokines such as MIP-2, cellular signaling guiding F-actin polymerization and leading edge formation is governed primarily by PI3K/AKT pathway (40). PI3Kγ has been implicated as a major effector of neutrophil chemotaxis (41, 42) and was reported as one of curcumin’s targets in another model (43). To gain a mechanistic insight into the inhibitory effect of curcumin observed with ChemoTX system and under-agarose assay, we tested the effects of curcumin on MIP-2- induced PI3K activation and its downstream target, protein kinase B (AKT). We therefore investigated the effect of curcumin on MIP-2-stimulated production of PIP3 utilizing a competitive ELISA assay. As depicted in Fig. 6A, MIP-2 treatment resulted in noticeable (although not statistically significant; p=0.06) increase in total cellular PIP3 concentration, which was reversed by pretreatment with 10μM of curcumin and reduced below the basal level (Fig. 6A). Consistent with this observation, MIP-2 significantly induced AKT activation (as measured by Ser473 phosphorylation status), which was dose-dependently reduced by 10–50μM of curcumin (Fig. 7B–C). MIP-2 also activated the MAPK signaling cascade as measured by ERK p42/p44 activation. This pathway, however, remained unaffected in curcumin-pretreated neutrophils, thus demonstrating specificity toward PI3K/AKT pathway (Fig. 7B–C). Curcumin alone did not affect the baseline levels of phospho-AKT or phospho-ERK.
Figure 7.
(A) ELISA for PIP3 concentration (pMol/106 cells) in bone marrow derived-neutrophil treated with 10–50μM of curcumin 5 minutes prior to a 30-second stimulation with MIP-2 (20ng/ml). (B) Western blot analysis of phospho-AKT (Ser473) or to phospho-p44/42 MAPK (Thr202/Tyr204) in PMNs treated with 10–50μM of curcumin 5 minutes prior to a 30-second stimulation with MIP-2 (20ng/ml). Total AKT and total ERK were evaluated as loading control. * p≤0.05 curcumin/MIP-2 vs. control or MIP-2 alone (ANOVA followed by Fisher PLSD post-hoc test). (C) Densitometric analysis of AKT and (D) ERK phosphorylation in response to MIP-2 with or without 10–50μM curcumin.
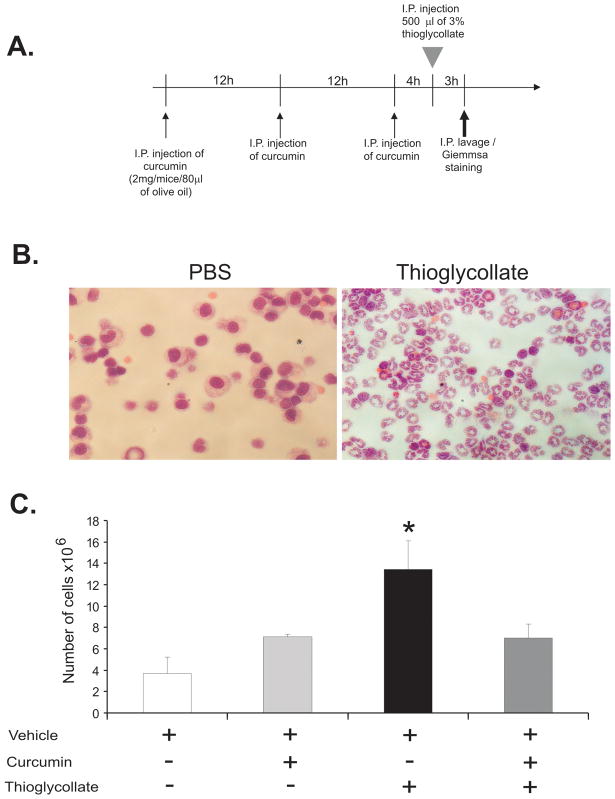
Curcumin inhibits neutrophil migration in a model of aseptic peritonitis
To further confirm the effects of curcumin in PMN recruitment to an active site of inflammation, we utilized a commonly accepted model of aseptic peritonitis. BALB/c mice were injected intra-peritoneally with curcumin or vehicle prior to i.p. injection of sterile thioglycollate broth (Figure 8A). We confirmed that in PBS-injected mice, the primary cells lavaged from the peritoneum were resident macrophages, while three hours following thioglycollate broth injection, the lavaged cell number increased 3–4 fold and was represented primarily (>90%) by PMNs (Fig. 8B–C). Administration of curcumin prior to thioglycollate broth injection significantly reduced peritoneal recruitment of neutrophils (Figure 8C).
Figure 8.
Effect of curcumin on neutrophil recruitment in thioglycollate induced aseptic peritonitis. (A) BALB/c mice received three i.p. injections of curcumin or vehicle alone every 12 hours in a total volume of 80μl. 4 hours after the last i.p. injection of curcumin or vehicle, peritonitis was induced by i.p. injection of 500μl of 3% thioglycollate (wt/vol; Sigma). 3 hours later, intra-peritoneal cells lavaged with 5mL of cold RPMI 1640 and the number of cells was determined using the Vi-Cell XR automatic cell counter and viability analyzer (Beckman-Coulter). (B) Giemsa staining of the lavaged peritoneal cells in PBS or thioglycolate injected mice. (C) The effect of curcumin on peritoneal neutrophil recovery in aseptic peritonitis. * p≤0.05 curcumin/thioglycollate vs. all other treatments (ANOVA followed by Fisher PLSD post-hoc test).
DISCUSSION
Recruitment and activation of neutrophils to the site of injury is one of the hallmarks of active IBD. Disproportionate and persistent inflammatory process mediated by the transepithelial migration of neutrophils leads to reduction of epithelial barrier function, perpetuation of inflammatory processes and tissue destruction via oxidative damage and the release of proteases.
Curcumin has been identified as the most active constituent of turmeric and is also defined as an anti-inflammatory, anti-oxidant, pro-apoptotic, anti-proliferative and anti-infectious agent. The anti-inflammatory activity of curcumin has been investigated in a variety of in vitro and in vivo settings (16, 19). Based on these observations, curcumin has been extensively tested for it efficacy in the prevention and treatment of active disease in multiple models of colitis and other autoimmune disorders such as rheumatoid arthritis (44, 45). In rheumatoid arthritis, turmeric was shown to down-regulate chemokine production in the acute and chronic phase of the disease, and affected expression of adhesion molecules required for the recruitment of inflammatory cells such as PMNs to the joint (44). In our previous studies, curcumin effectively attenuated TNBS-induced colitis in BALB/c mice (32). In this report we provided an overview of the effects of curcumin on colonic transcriptome using genome-wide microarray analysis of gene transcription (32). Reiteration of this analysis focused on genes involved in neutrophil function indicated numerous genes affected by inflammation and normalized by dietary curcumin. These include some potent neutrophil chemoattractants: chemokines (C-X-C) ligand 1 (KC), chemokines (C-X-C) ligand 2 (MIP-2), chemokines (C-X-C) ligand 5 (LIX), IL-1β and their associated receptors, as well as small cytoplasmic proteins, calgranulin A (S100A8) and B (S100A9). The latter two are not only released by neutrophils during inflammatory reaction, but also act as potent chemoattractants, and their inhibition effectively inhibits neutrophil migration (46). Additionally, we identified genes involved in neutrophils diapedesis including CD43 (leukosialin) which play an essential role in maintaining neutrophils in their “non-sticky” state (47) and was increased in curcumin-treated animals. Several other genes associated with neutrophil migration and actin-based cytoskeleton rearrangement such as Cdc42, Arp protein complex, WASP (Wiskott-Aldrich syndrome protein), were also downregulated by dietary curcumin.
In the current study, we confirmed the role of curcumin in inhibiting the expression and production of chemoattractant molecules; MIP-2, KC, MIP-1α and IL-1β by peritoneal macrophages and colonic epithelial cells. Moreover, in addition to altering the chemoattractant gradient formation, curcumin directly affected neutrophil chemotaxis and chemokinesis stimulated by MIP-2, a CXCL chemokine, which along with KC is considered to be a mouse functional homologue of IL-8. MIP-2 signals through CXCR2, a G-protein–coupled chemotactic receptor directing directional F-actin polymerization in the lamella region of a migrating neutrophils (48). Downstream from the CXCR2, the regulation of F-actin polymerization depends on PI3K activation and PtdIns(3,4,5)P3 production, and involves the activation of protein kinase B (Akt/PKB) and the guanosine triphosphatases (GTPases) Cdc42 and Rac2. Cdc42 and Rac2 form complexes with the Wiskott-Aldrich syndrome protein (WASp) family proteins and actin-related protein 2/3 (Arp2/3) to promote the formation of free barbed ends, which in turn initiate cytoskeletal F-actin polymerization in the lamella region. Curcumin inhibited F-actin formation at the leading edge, an event that could be attributed to the attenuation PI3K activity, PIP3 synthesis, and AKT phosphorylation. This pathways has been reported as curcumin target by several other reports, exploring the chemopreventive role of the compound in controlling cancer cell proliferation (43). Interestingly, contrary to some reports, curcumin did not affect MIP-2 stimulated ERK phosphorylation, demonstrating a degree of specificity in mouse neutrophils, and suggesting that initial stages MIP-2/CXCR2 signaling were not targeted by the compound. This finding, along with “on-contact” inhibition of F-actin polymerization in under-agarose migration assays, also argues that our findings were not mediated through reduction of surface availability of CXCR2 recently reported by Takahashi et al. (34).
Neutrophil contribution to the pathogenesis of IBD is not definitive, with data from animal studies often yielding contradictory results. Recent studies with CD patients suggest a defect in the recruitment and functions of the PMNs as a causative mechanism of disease initiation. Emerging picture, although far from being conclusive, is that neutrophils play different roles in different phases of the disease process: (i) in the initiation phase, neutrophils play a role in bacteria clearance limiting a possible overactive adaptive immune response; (ii) in the active part of the disease, neutrophils play a significant pro-inflammatory and destructive role; (iii) in the resolution phase, neutrophils may contribute to wound healing and tissue repair. Considering the dual role of neutrophil depending on the stage of the disease, clinical strategies based only on the inhibition of neutrophil function may need to be used with caution in CD patients. To date, there have been no well-designed clinical trials with CD patients involving curcumin treatment, while our own studies with mouse models of CD (TNBS colitis in SJL/J mice or microbially-induced colitis in IL-10−/− mice) demonstrated no or limited effects of curcumin (32, 49). On the other hand, in BALB/c mice (32) and other less immunologically defined models of colitis (50), as well as in UC patients (51), curcumin demonstrated a promising efficacy. It is conceivable, that part of the protective effects of curcumin in UC is due to neutrophil inhibition. Current understanding of the pathogenic role of neutrophils in UC appears less controversial. Functionally, compared to controls, neutrophils from UC patients have exacerbated response to inflammatory stimuli, such as phorbol ester, fMLP, or zymosan (52). Moreover, inflammatory mediators in the colon of patients with active UC stimulate the migration, activation, and survival of neutrophils (53). IL-8, MPO, and neutrophil-related luminol-dependent fluorescence are associated with the degree of inflammation in UC mucosa (54), and leukocytapheresis with selective removal of granulocytes and monocytes/macrophages gains recognition as a very safe and effective alternative to biological therapies for patients with refractory ulcerative colitis (55).
The presented results strongly suggest that curcumin reduces neutrophil recruitment to the inflammatory sites by affecting chemokine gradient formation as well as by the direct effect of the compound on neutrophil polarization, chemotaxis and chemokinesis. These mechanisms likely significantly contribute to the described protective and potent effect of curcumin in ulcerative colitis.
Acknowledgments
Investigation Supported by: NIDDK 5R01DK067286 and ABRC RFP 07-100 (to P.R. Kiela)
References
- 1.Marks DJ, Segal AW. Innate immunity in inflammatory bowel disease: a disease hypothesis. J Pathol. 2008;214:260–266. doi: 10.1002/path.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman FZ, Marks DJ, Hayee BH, et al. Phagocyte dysfunction and inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1443–1452. doi: 10.1002/ibd.20449. [DOI] [PubMed] [Google Scholar]
- 3.Saverymuttu SH, Peters AM, Lavender JP, et al. Quantitative fecal indium 111-labeled leukocyte excretion in the assessment of disease in Crohn’s disease. Gastroenterology. 1983;85:1333–1339. [PubMed] [Google Scholar]
- 4.Wiedow O, Meyer-Hoffert U. Neutrophil serine proteases: potential key regulators of cell signalling during inflammation. J Intern Med. 2005;257:319–328. doi: 10.1111/j.1365-2796.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122:1592–1608. doi: 10.1053/gast.2002.33426. [DOI] [PubMed] [Google Scholar]
- 6.Busch-Petersen J. Small molecule antagonists of the CXCR2 and CXCR1 chemokine receptors as therapeutic agents for the treatment of inflammatory diseases. Curr Top Med Chem. 2006;6:1345–1352. doi: 10.2174/15680266106061345. [DOI] [PubMed] [Google Scholar]
- 7.Buanne P, Di Carlo E, Caputi L, et al. Crucial pathophysiological role of CXCR2 in experimental ulcerative colitis in mice. J Leukoc Biol. 2007;82:1239–1246. doi: 10.1189/jlb.0207118. [DOI] [PubMed] [Google Scholar]
- 8.Farooq SM, Stillie R, Svensson M, et al. Therapeutic effect of blocking CXCR2 on neutrophil recruitment and dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther. 2009;329:123–129. doi: 10.1124/jpet.108.145862. [DOI] [PubMed] [Google Scholar]
- 9.Natsui M, Kawasaki K, Takizawa H, et al. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. J Gastroenterol Hepatol. 1997;12:801–808. doi: 10.1111/j.1440-1746.1997.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 10.Palmen MJ, Dijkstra CD, van der Ende MB, et al. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin Exp Immunol. 1995;101:351–356. doi: 10.1111/j.1365-2249.1995.tb08363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buell MG, Berin MC. Neutrophil-independence of the initiation of colonic injury. Comparison of results from three models of experimental colitis in the rat. Dig Dis Sci. 1994;39:2575–2588. doi: 10.1007/BF02087693. [DOI] [PubMed] [Google Scholar]
- 12.Yamada T, Zimmerman BJ, Specian RD, et al. Role of neutrophils in acetic acid-induced colitis in rats. Inflammation. 1991;15:399–411. doi: 10.1007/BF00917356. [DOI] [PubMed] [Google Scholar]
- 13.Kuhl AA, Kakirman H, Janotta M, et al. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology. 2007;133:1882–1892. doi: 10.1053/j.gastro.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 14.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 16.Jagetia GC, Aggarwal BB. Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim GY, Kim KH, Lee SH, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174:8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 18.Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 2000;483:78–82. doi: 10.1016/s0014-5793(00)02089-5. [DOI] [PubMed] [Google Scholar]
- 19.Yadav VS, Mishra KP, Singh DP, et al. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol. 2005;27:485–497. doi: 10.1080/08923970500242244. [DOI] [PubMed] [Google Scholar]
- 20.Cuendet M, Pezzuto JM. The role of cyclooxygenase and lipoxygenase in cancer chemoprevention. Drug Metabol Drug Interact. 2000;17:109–157. doi: 10.1515/dmdi.2000.17.1-4.109. [DOI] [PubMed] [Google Scholar]
- 21.Ammon HP, Anazodo MI, Safayhi H, et al. Curcumin: a potent inhibitor of leukotriene B4 formation in rat peritoneal polymorphonuclear neutrophils (PMNL) Planta Med. 1992;58:226. doi: 10.1055/s-2006-961438. [DOI] [PubMed] [Google Scholar]
- 22.Camacho-Barquero L, Villegas I, Sanchez-Calvo JM, et al. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol. 2007;7:333–342. doi: 10.1016/j.intimp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Deng C, Zheng J, et al. Curcumin inhibits trinitrobenzene sulphonic acid-induced colitis in rats by activation of peroxisome proliferator-activated receptor gamma. Int Immunopharmacol. 2006;6:1233–1242. doi: 10.1016/j.intimp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Deng CS, Zhang M, et al. Curcumin-attenuated trinitrobenzene sulphonic acid induces chronic colitis by inhibiting expression of cyclooxygenase-2. World J Gastroenterol. 2006;12:3848–3853. doi: 10.3748/wjg.v12.i24.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M, Deng CS, Zheng JJ, et al. Curcumin regulated shift from Th1 to Th2 in trinitrobenzene sulphonic acid-induced chronic colitis. Acta Pharmacol Sin. 2006;27:1071–1077. doi: 10.1111/j.1745-7254.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 26.Ukil A, Maity S, Karmakar S, et al. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol. 2003;139:209–218. doi: 10.1038/sj.bjp.0705241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salh B, Assi K, Templeman V, et al. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G235–243. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- 28.Sugimoto K, Hanai H, Tozawa K, et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123:1912–1922. doi: 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- 29.Venkataranganna MV, Rafiq M, Gopumadhavan S, et al. NCB-02 (standardized Curcumin preparation) protects dinitrochlorobenzene- induced colitis through down-regulation of NFkappa-B and iNOS. World J Gastroenterol. 2007;13:1103–1107. doi: 10.3748/wjg.v13.i7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss AC. Curcumin for maintenance therapy in ulcerative colitis. Clin Gastroenterol Hepatol. 2007;5:642. doi: 10.1016/j.cgh.2007.03.002. author reply 642. [DOI] [PubMed] [Google Scholar]
- 31.Jian YT, Mai GF, Wang JD, et al. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol. 2005;11:1747–1752. doi: 10.3748/wjg.v11.i12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billerey-Larmonier C, Uno JK, Larmonier N, et al. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis. 2008;14:780–793. doi: 10.1002/ibd.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu M, Du Q, Vancurova I, et al. Proapoptotic effect of curcumin on human neutrophils: activation of the p38 mitogen-activated protein kinase pathway. Crit Care Med. 2005;33:2571–2578. doi: 10.1097/01.ccm.0000186760.20502.c7. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi M, Ishiko T, Kamohara H, et al. Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) blocks the chemotaxis of neutrophils by inhibiting signal transduction through IL-8 receptors. Mediators Inflamm. 2007;2007:10767. doi: 10.1155/2007/10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitehead RH, VanEeden PE, Noble MD, et al. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci U S A. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heit B, Kubes P. Measuring chemotaxis and chemokinesis: the under-agarose cell migration assay. Sci STKE. 2003;2003:PL5. doi: 10.1126/stke.2003.170.pl5. [DOI] [PubMed] [Google Scholar]
- 37.MacDermott RP. Chemokines in the inflammatory bowel diseases. J Clin Immunol. 1999;19:266–272. doi: 10.1023/a:1020583306627. [DOI] [PubMed] [Google Scholar]
- 38.Pender SL, Chance V, Whiting CV, et al. Systemic administration of the chemokine macrophage inflammatory protein 1alpha exacerbates inflammatory bowel disease in a mouse model. Gut. 2005;54:1114–1120. doi: 10.1136/gut.2004.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohtsuka Y, Lee J, Stamm DS, et al. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut. 2001;49:526–533. doi: 10.1136/gut.49.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heit B, Tavener S, Raharjo E, et al. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrandi C, Ardissone V, Ferro P, et al. Phosphoinositide 3-kinase gamma inhibition plays a crucial role in early steps of inflammation by blocking neutrophil recruitment. J Pharmacol Exp Ther. 2007;322:923–930. doi: 10.1124/jpet.107.123026. [DOI] [PubMed] [Google Scholar]
- 42.Hannigan MO, Huang CK, Wu DQ. Roles of PI3K in neutrophil function. Curr Top Microbiol Immunol. 2004;282:165–175. doi: 10.1007/978-3-642-18805-3_6. [DOI] [PubMed] [Google Scholar]
- 43.Lin JK. Molecular targets of curcumin. Adv Exp Med Biol. 2007;595:227–243. doi: 10.1007/978-0-387-46401-5_10. [DOI] [PubMed] [Google Scholar]
- 44.Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452–3464. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]
- 45.Funk JL, Oyarzo JN, Frye JB, et al. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandal K, Rouleau P, Boivin A, et al. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol. 2003;171:2602–2609. doi: 10.4049/jimmunol.171.5.2602. [DOI] [PubMed] [Google Scholar]
- 47.Lopez S, Seveau S, Lesavre P, et al. CD43 (sialophorin, leukosialin) shedding is an initial event during neutrophil migration, which could be closely related to the spreading of adherent cells. Cell Adhes Commun. 1998;5:151–160. doi: 10.3109/15419069809040288. [DOI] [PubMed] [Google Scholar]
- 48.Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 49.Larmonier CB, Uno JK, Lee KM, et al. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1079–1091. doi: 10.1152/ajpgi.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanai H, Sugimoto K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr Pharm Des. 2009;15:2087–2094. doi: 10.2174/138161209788489177. [DOI] [PubMed] [Google Scholar]
- 51.Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 52.D’Odorico A, D’Inca R, Mestriner C, et al. Influence of disease site and activity on peripheral neutrophil function in inflammatory bowel disease. Dig Dis Sci. 2000;45:1594–1600. doi: 10.1023/a:1005521212948. [DOI] [PubMed] [Google Scholar]
- 53.Lampinen M, Sangfelt P, Taha Y, et al. Accumulation, activation, and survival of neutrophils in ulcerative colitis: regulation by locally produced factors in the colon and impact of steroid treatment. Int J Colorectal Dis. 2008;23:939–946. doi: 10.1007/s00384-008-0509-x. [DOI] [PubMed] [Google Scholar]
- 54.Anezaki K, Asakura H, Honma T, et al. Correlations between interleukin-8, and myeloperoxidase or luminol-dependent chemiluminescence in inflamed mucosa of ulcerative colitis. Intern Med. 1998;37:253–258. doi: 10.2169/internalmedicine.37.253. [DOI] [PubMed] [Google Scholar]
- 55.Arseneau KO, Cominelli F. Leukocytapheresis in ulcerative colitis: a possible alternative to biological therapy? Dig Liver Dis. 2009;41:551–552. doi: 10.1016/j.dld.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]