Abstract
Induction of the breast cancer resistance protein (BCRP/ABCG2) expression has been found in various tissues and cell-types after exposure to chemicals including 17β-estradiol, rosiglitazone, imatinib, as well as aryl hydrocarbon receptor (AhR) activators such as 2,3,7,8-tetrachlorodibenzodioxin, 3-methylcholanthrene (3MC), and omeprazole. However, the mechanism(s) underlying AhR-related induction of ABCG2 is largely unknown. Here, we demonstrate the AhR-dependent induction of ABCG2 expression in human colon adenocarcinoma LS174T cells. Importantly, a novel distal AhR responsive element (AhRE5) located −2357/−2333 bp upstream of the ABCG2 transcriptional start site has been identified and characterized as a functional unit pivotal to 3MC-mediated induction of ABCG2. Cell-based reporter assays revealed that deletion of AhRE5 and 4 dramatically attenuated 3MC-induced activation of ABCG2 reporter activity, while further deletion of the proximal AhRE3 and 2 only moderately changed the luciferase activities. Notably, site-directed mutation of the AhRE5 in the BCRP-3.8kb reporter construct alone resulted in approximately 80% decrease in 3MC activation of the ABCG2 promoter; additional mutation of the AhRE4 site had negligible effect on the ABCG2 promoter activity. Moreover, chromatin immunoprecipitation assays demonstrated that treatment with 3MC significantly enhanced the recruitment of AhR to the AhRE5 occupied region, and mutation of the AhRE5 site clearly dissociated AhR protein from this promoter region. Together, these data show that the novel distal AhRE5 is critical for AhR-mediated transcriptional activation of ABCG2 gene expression in LS174T cells, and it may offer new strategies for early identification of ABCG2 inducers, which would be of benefit for preventing transporter-associated drug-drug interactions.
Keywords: BCRP, AhR, induction, regulation, transporter
1. Introduction
Initially identified as a mechanism of chemotherapeutic resistance in breast cancer cells, the breast cancer resistance protein (BCRP/ABCG2), a member of the ATP-binding cassette family of transporters, is now known to play a pivotal role in the efflux transport of xenobiotics from tissues such as intestine, liver, placenta, blood-brain barrier, as well as various stem cells [1–5]. Substrates for ABCG2-mediated transport include cholesterol, hormones (17β-estradiol), vitamins, drugs (mitoxantrone, methotrexate, topotecan) and environmental chemicals, such as benzo[a]pyrene-3-sulfate, and benzo[a]pyrene-3-glucuronide [6–8]. Perturbation of the expression and function of ABCG2 often leads to profound consequences in different tissues. For instance, inhibition of ABCG2 in tumor cells increases the intracellular drug concentrations and the efficacy of chemotherapeutics; whereas inhibition of ABCG2 in liver and intestine may attenuate its defense against environmental toxicants [7]. Compared with the extensively characterized ABCG2 expression in tumor cells, relatively little is known regarding the transcriptional regulation of ABCG2 in metabolic tissues such as the liver and intestines. In the liver, ABCG2 is located on the canalicular membrane of the hepatocyte, and is responsible for effluxing substrates out of the liver into the bile [9]. In the small intestine, ABCG2 is located on the brush border apical surface exposed to the gut lumen. Therefore, ABCG2 is strategically placed in the small intestine to be a critical determinant of oral drug absorption.
Computer-based analysis of the 5’-upstream region of ABCG2 reveals that the human ABCG2 gene lacks a canonical TATA box, yet possesses several SP1 binding sites in the proximal promoter upstream of its transcriptional start site with a CCAAT-box and several CpG islands in its downstream proximity [10]. Like other ABC transporters, up-regulation of ABCG2 seems to be mediated through a group of transcription factors, namely nuclear receptors (NRs). To date, several putative NR-binding sites have been identified in the promoter of ABCG2 gene. These elements include an estrogen responsive element (−188 to −177), three peroxisome proliferator-activated receptor gamma (PPARγ) binding sites (−3946 to −3796), and a hypoxia-inducible factor-1 (HIF-1) site (−116 to −112) [11–13]. Nonetheless, the expression of ABCG2 is also induced by a large number of xenobiotics that are not typically known to interact with estrogen receptor (ER), PPAR, or HIF-1. For example, ABCG2 was up-regulated by phenobarbital, rifampicin, and omeprazole (OMP) in human primary hepatocytes [14]; benzopyrene conjugates were reported to induce ABCG2 expression in Caco-2 cells [7]; and imatinib, a tyrosine kinase inhibitor and ABCG2 substrate, chronically but potently induced the expression of ABCG2 in Caco-2 cells [15]. Additionally, a number of cancer cells develop their drug-resistance during the course of treatment against chemotherapeutic compounds which up-regulate the expression of ABCG2 in these cells. However, the mechanisms underlying these observed inductions are largely unknown.
Recent accumulating evidence indicates that ABCG2 expression in Caco-2 and breast cancer cells can be induced in an aryl hydrocarbon receptor (AhR)-dependent fashion [7, 16, 17]. Prototypical activators of AhR such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and polycyclic aromatic hydrocarbons (PAHs) strongly induced the expression of ABCG2 in Caco-2 cells. Notably, transient transfection of AhR expression vector to the AhR-deficient MCF-7AHR200 cells substantially restored the ABCG2 expression and drug-resistance in these cells [16]. Since AhR can be activated by a broad array of xenobiotics including halogenated aromatic hydrocarbons and PAHs such as TCDD and 3-methylcholanthrene (3MC), as well as clinically-used drugs and endogenous chemicals such as omeprazole and bilirubin [18, 19], the potential for AhR-mediated drug-drug interactions (DDIs) through alteration of ABCG2 expression is theoretically high. Nevertheless, it is not fully understood whether and how AhR regulates the expression of ABCG2 at the transcriptional level. Here, we have identified a xenobiotic responsive element that specifically binds to AhR (AhRE) and mediates transcriptional regulation of ABCG2 in human colon cancer LS174T cells. Utilizing small interfering RNA (siRNA) knock-down, site-directed mutagenesis, chromatin immunoprecipitation (ChIP), and promoter serial deletion assays, we demonstrated that the AhRE5 located at −2357/−2333 in ABCG2 promoter is critical for AhR-mediated upregulation of ABCG2.
2. Materials and Methods
2.1. Chemicals and Biological Reagents
Wy-14,643, clofibrate (CLF), OMP, resveratrol (Resv), troglitazone (TGZ), and 3MC were purchased from Sigma-Aldrich (St. Louis, MO). The ginkgo biloba extract (EGB 761) was from Schwabe Pharmaceuticals (Karlsruhe, Germany). The Dual-Luciferase Reporter Assay System was purchased from Promega (Madison, WI). Lipofectamine 2000 transfection reagent was from Invitrogen (Calsbad, CA). All primers were obtained from IDT (Coralville, IA). Other cell culture reagents were purchased from Invitrogen or Sigma-Aldrich.
2.2. Plasmid Constructs and Site-directed Mutagenesis
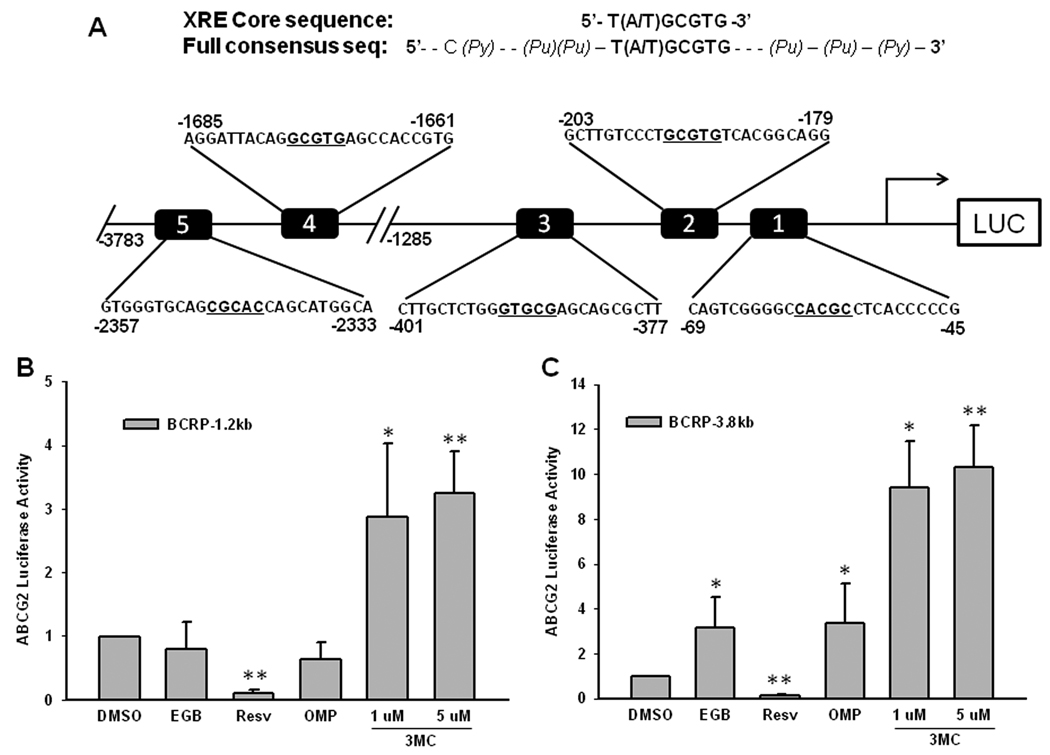
A series of pGL3-basic vectors containing the proximal ABCG2 promoter spanning −1285/+362, −628/+362, −312/+362, −243/+362, or −115/+362 in the multi-cloning site were generated as described previously [10]. A 3.8 kb PCR product spanning −3783/+977 was cloned into the NheI/HindIII sites of the pGL3-basic vector, termed BCRP-3.8kb. Utilizing “GCGTG” as the core of AhR binding site, computer-based search has resulted in five potential AhR binding sites in the first 3.8 kb of ABCG2 promoter (Figure 3A). Site-directed mutagenesis was accomplished using QuikChange Site-directed mutagenesis kit (Stratagene/Agilent Technologies, La Jolla, CA) with the following primers: AhRE1 (forward-5’CCCGGCAGTCGGGGCTTGAACTCACCCCCGCCCGC 3’, reverse-5’GCGGGCGGGGGTGAGTTCAAGCCCCGACTGCCGGG 3’); AhRE4 (forward-5’GGCCTCCCAAAGTACTAGGATTACAGCTTGAAGCCACCGTGCCTGGCC 3’, reverse-5’GGCCAGGCACGGTGGCTTCAAGCTGTAATCCTAGTACTTTGGGAGGCC 3’), and AhRE5 (forward-5’GCTAGATGACACGTTAGTGGGTGCAGTTGGACAGCATGGCACATGTATAC 3’, reverse-5’GTATACATGTGCCATGCTGTCCAACTGCACCCACTAACGTGTCATCTAGC 3’). The newly generated reporter construct and all mutants were confirmed by sequence analysis. The pRL-TK vector (Promega, Madison, WI) expressing renilla luciferase was used as transfection internal control.
Figure 3. Identification of AhR response elements in the ABCG2 promoter.
A) The schematic figure depicts the prototypical AhR response element (AhRE) sequences and five putative elements within the −3.8 kb of the ABCG2 promoter region. The core sequences were bolded and underlined. LS174T cells were transfected with B) the BCRP-1.2kb reporter construct (containing 3 putative AhREs) or C) the BCRP-3.8kb reporter construct (containing 5 putative AhREs), and treated with AhR agonists EGB (100 µg/ml), Resv (100 µM), OMP (50 µM) or 3MC at 1 µM and 5 µM. Luciferase activities were measured and data were presented as fold activation over DMSO control.
2.3. Transfection Assays in LS174T cells
LS174T cells (from American Type Culture Collection, Manassas, VA) were seeded in 24-well plates at a density of 1.8 × 105 cells per well in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were transfected with one of the ABCG2 promoter constructs (as depicted in Figure 3–4) and the pRL-TK by use of Lipofectamine 2000 following the manufacturer’s instruction. Twenty-four hours after transfection, cells were treated with vehicle control (0.1% DMSO, v/v), or 3MC at the concentration of 1 and 5 (µM) for another 24 hr. Cell lysates were harvested in 1X Reporter Lysis Buffer and assayed using Promega Dual Luciferase Reporter assay kit (Promega). Transfection efficiency was normalized to renilla luciferase and data were presented as mean ± SD of fold activation over control from three independent experiments.
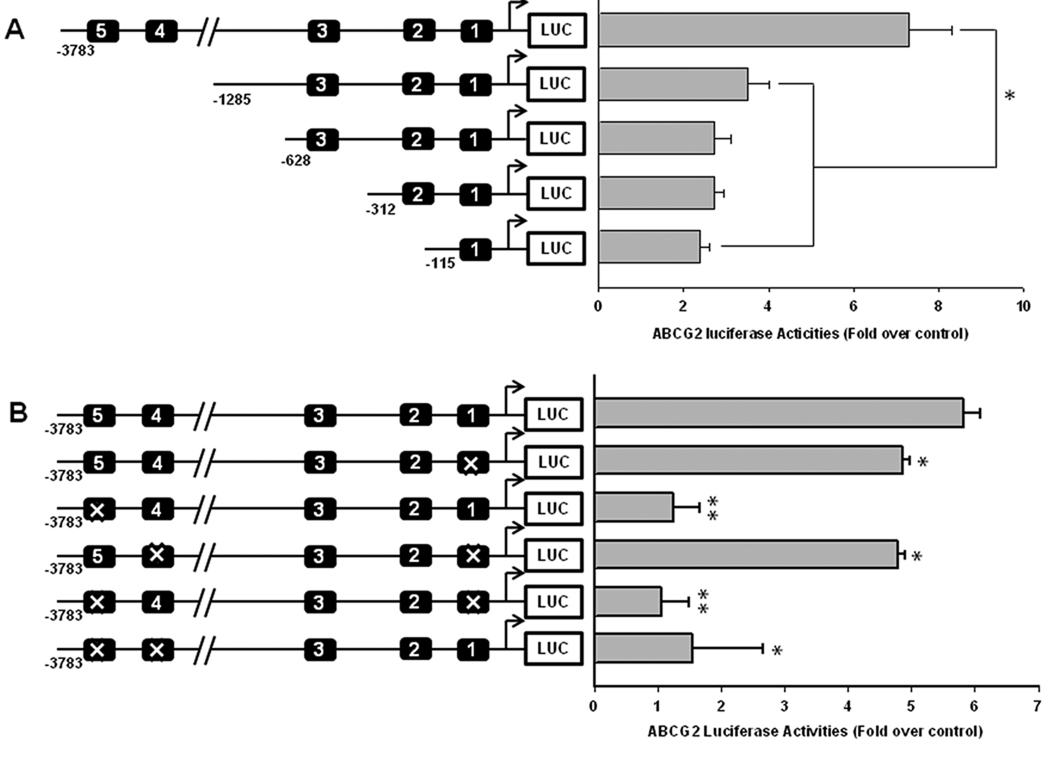
Figure 4. Activation of the putative AhREs of ABCG2 in LS174T cells.
A) LS174T cells were transfected with promoter deletion constructs and treated with 3MC (1µM) for 24 hr. The number of putative AhREs was illustrated in the promoter schematic to the left. B) LS174T cells were transfected with BCRP-3.8kb reporter constructs with site-directed mutagenesis of AhRE1, 4 and 5 in a number of configurations: BCRP-3.8-Δ1, BCRP-3.8-Δ5, BCRP-3.8-Δ1/4, BCRP-3.8-Δ1/5, and BCRP-3.8-Δ4/5. Transfected cells were then treated with 3MC (1µM) for 24 hr. In both A and B, luciferase activities were measured and presented as fold activation over DMSO control.
2.4. Real-time PCR Analysis
LS174T cells were seeded in 12-well plates at a density of 2.5 × 105 cells per well. After growing to approximately 80% confluence, cells were treated with vehicle control (0.1% DMSO) or test compounds at the concentrations as indicated in Figure 1 for 24 hr. Total RNA was isolated from treated cells using the RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed using the High Capacity cDNA Archive kit (Applied Biosystems Inc; Foster, CA) following the manufacturers’ instruction. SYBR green real-time PCR assays were performed in 96-well optical plates on an ABI 7000 Sequence Detection System (Applied Biosystems Inc; Foster City, CA) to measure GAPDH (forward: 5’ CCCATCACCATCTTCCAGGAG 3’, and reverse: 5’ GTTGTCATGGATGACCTTGGC 3’) and ABCG2 (forward: 5’-GCGACCTGCCAATTTCAAATG-3’, and reverse: 5’-GACCCTGTTAATCCGTTCGTTT-3’). Data were represented as fold induction calculated by the equation 2−ΔΔCt, where ΔΔCt is the relative change in threshold cycle between control and treated samples.
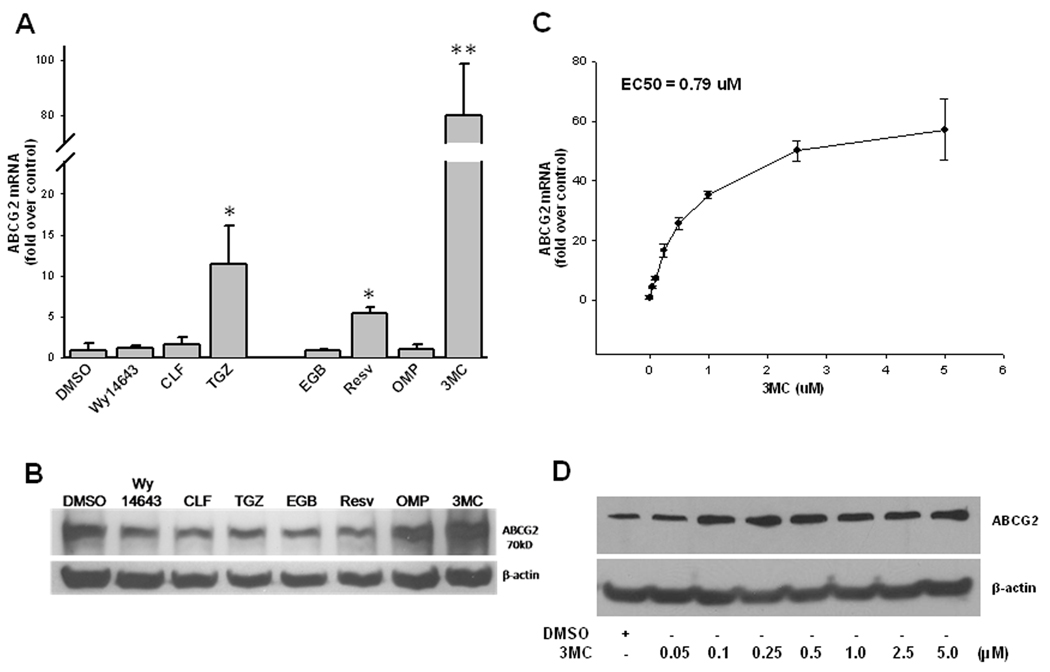
Figure 1. Induction of ABCG2 in LS174T cells.
A) LS174T cells were treated for 24 hr with ligands for PPARα [Wy 14643 (10 µM), CLF (10 µM)], PPARγ [TGZ (10 µM)] and activators for AhR [EGB (100 µg/ml), Resv (100 µM), OMP (50 µM), 3MC (1 µM)]. ABCG2 expression was measured by real-time RT-PCR, normalized by GAPDH expression and presented as fold induction over DMSO (0.1%) control. B) LS174T cells were treated with the same batch of chemicals as described in A for 72 hr. Whole cell lysates (45 µg each) were loaded for Western immunoblot analysis of ABCG2 as described under Materials and Methods. C) LS174T cells were treated with 3MC at the concentrations ranging from 0.05 µM to 5 µM for 24 hr before ABCG2 mRNA expression was detected as outlined in A. The nonlinear regression analysis was carried out to obtain estimate of the EC50 value for 3MC. D) LS174T cells treated with 3MC at concentrations from 0.05 µM to 5 µM for 72 hr. Whole cell lysate (45 µg each) were subjected to immunoblot analysis of ABCG2 as described in Materials and Methods.
2.5. Western Blot Analysis
LS174T cell cultures were treated for 72 hr with indicated compounds and harvested in lysis buffer [20 mM Tris, 100 mM NaCl, 0.5% NP-40, 0.5 mM PMSF and 1X cocktail of protease inhibitors (Roche; Basel, Switzerland)]. Protein samples (45 µg) were separated on 10% polyacrylamide gel under denaturing conditions, and electropheretically transferred to PVDF membranes. Subsequently, membranes were incubated with ABCG2 antibody (BXP-21; Abcam, Cambridge, MA) at 1:1000. β-actin (Sigma-Aldrich) was used for normalization of protein loading. After incubating with horseradish peroxidase goat anti-rabbit IgG antibody (1:4000), membranes were developed using SuperSignal enhanced chemiluminescence reagent (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
2.6. siRNA Knockdown of the AhR in LS174T Cells
The siRNA specific for AhR and a non-target siRNA were obtained from Dharmacon. LS174T cells seeded in 12-well plates were transfected with siRNA-AhR (40 pmol/well) or siRNA-NT (40 pmol/well) using Lipofectamine 2000 (Invitrogen) as described previously [20]. Twenty four hours after the siRNA transfection, cells were treated with DMSO (0.1%, v/v) or 3MC (1 µM) for another 24 hr before harvest. Total RNA isolated from the transfected cells was reverse-transcribed into cDNA, and subjected to real-time PCR analysis of AhR mRNA expression as described above.
2.7. Chromatin Immunoprecipation (ChIP) assays
Experiments were performed using a ChIP assay kit according to the manufacturer’s protocol (Millipore, Bedford, MA). Briefly, 2 × 106 LS174T, or Hepa-1 cells were seeded in 10 cm dishes, grown to full confluence, and treated with 3MC (5 µM) or DMSO (0.1% v/v) for 1.5 hr. Cells were crosslinked with 1% formaldehyde for 10 min at 37°C, washed with ice-cold phosphate-buffered saline containing a protease inhibitor cocktail, and sonicated.
Immunoprecipitation was performed overnight at 4°C using rabbit anti-human AhR polyclonal antibody (sc-5579) or isotype control IgG followed by precipitation using protein A coupled to agarose beads. After de-crosslink, immunoprecipitated DNA fragments were recovered by QIAquick PCR purification kit (Qiagen). Quantitative PCR was performed using specific sets of primers (AhRE1: forward: 5’-CGGGAGTGTTTGGCTTGT -3’ and reverse: 5’-CAGGTCGGGGTTCGCG -3’; AhRE4: forward: 5’-ACCATGCCCGGCTAATTTT -3’ and reverse: 5’-GGCACTCAGGAGGAGACTG -3’; AhRE5: forward: 5’-TCACACTCTGGGGACTGTG -3’ and reverse: 5’-GTATACATGTGCCATGCTGG -3’). PCR products were also resolved on a 1.5% agarose gel and visualized by ethidium bromide staining. In a parallel experiment, Hepa-1 cells were transfected with BCRP-3.8kb or BCRP-3.8kb-Δ5 mutation construct. Twenty four hours after transfection, cells were treated with 3MC (5 µM) or DMSO (0.1% v/v) for 1.5 hr. Subsequently, ChIP experiments were performed as described above.
2.8. Statistical Analysis
All reporter assay data represent at least three independent experiments and are expressed as the mean ± S.D. Statistical comparisons were made using Student’s t-test. Statistical significance was set at *: p <0.05 and **: p <0.01. Nonlinear regression estimation of EC50 was carried out using the WinNonlin software (Pharsight, Cary, NC).
3. Results
3.1. Induction of ABCG2 in LS174T cells
As mentioned previously, ABCG2 is known to be regulated by a variety of nuclear receptor pathways in a number of distinct tissues. Ebert et al. specifically identified the involvement of AhR in colon-derived cell culture [16]. However, ABCG2 expression was found to be extremely variable in Caco-2 cells, dependent on passage number and differentiation status; therefore colon adenocarcinoma-derived LS174T cells expressing a full complement of NRs were chosen as a model system. In the current study, AhR ligands or activators were chosen to represent the natural product (EGB761, Resv), pharmaceutical compound (OMP), and the prototypical agonist (3MC). As demonstrated in Figure 1A, significant induction of ABCG2 mRNA expression by 12- and 5-fold was observed after treated with troglitazone (TGZ) and Resv, respectively; however, this induction was minimal in comparison to the approximately 80-fold induction mediated by 3MC (1 µM). Analysis of protein expression showed only 3MC treatment was capable of increasing both RNA and protein expression of ABCG2. Up-regulated mRNA levels following TGZ and Resv treatment were not translated to changes in ABCG2 protein, but OMP up-regulated ABCG2 protein without showing a change in mRNA levels (Figure 1B). Treatment of LS174T cells with a full range of 3MC concentrations (from 0.05 to 5 µM) demonstrated increasing mRNA expression in response to increasing dose (Figure 1C). Nonlinear regression analysis of this data generated a dose-response curve, with the Emax achieved at the concentration of 5 µM and an EC50 value of 0.79 µM. ABCG2 protein expression was also increased after 3 days treatment with 3MC at concentrations (0.05 µM to 5 µM) (Figure 1D). However, the protein induction observed is clearly not dose-dependent under the current experimental conditions. Treatment of LS174T cells with 3CM at concentrations greater than 5 µM resulted in significant cyctotoxicity (data not shown).
3.2. AhR mediates 3MC induction of ABCG2 expression
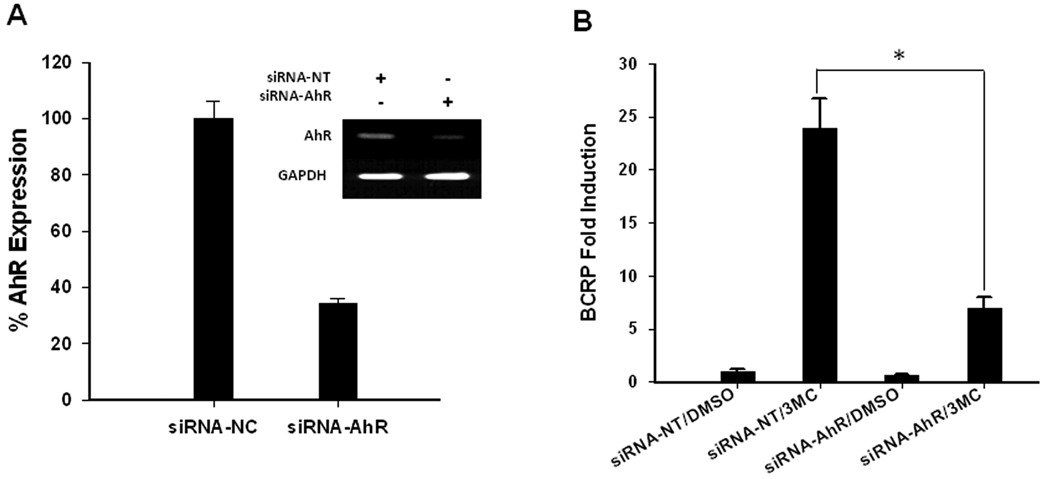
To confirm the involvement of AhR in 3MC-mediated ABCG2 up-regulation, the endogenous AhR expression in LS174T cells was knocked down using siRNA. Forty-eight hours after transfection of siRNA specific to AhR, the expression of AhR mRNA in LS174T cells was decreased by 65% compared with the control group transfected with non-targeting siRNA (Figure 2A). In parallel experiments, the knockdown of AhR expression in LS174T cells significantly attenuated 3MC-mediated induction of ABCG2 gene expression, where the induction of ABCG2 in siRNA-AhR transfected cells only accounts for less than 25% of the induction seen in the 3MC treated non-specific siRNA control (Figure 2B).
Figure 2. AhR is required for 3MC-mediated induction of ABCG2 in LS174T cells.
A) LS174T cells were transfected with non-specific siRNA (siRNA-NC) or AhR-specific siRNA (siRNA-AhR). Forty-eight hours after transfection down-regulation of ABCG2 was measured by real-time RT-PCR. In addition to the bar figure, the RT-PCR products of AhR knock down were also visualized on agarose gel. B) In a parallel experiment, LS174T cells transfected with siRNA-NC or siRNA-AhR were treated with DMSO control or 3MC (1 µM) for 24 hr. ABCG2 expression was determined by real-time RT-PCR, and expressed as fold over siRNA-NC DMSO control.
3.3. Identification of AhR response elements (AhREs) in the ABCG2 promoter
As a ligand-activated receptor, AhR responds like all other nuclear receptors by interacting directly with the promoter region of responsive target genes. The necessity of AhR involvement in 3MC-mediated ABCG2 expression suggests the presence of AhR-responsive elements in the ABCG2 promoter. A 4 kb upstream region of the ABCG2 promoter was analyzed for the putative AhR response element containing the core sequence 5’-GCGTG-3’ (Figure 3A). Computer-based analysis revealed five such elements within the first 4 kb of ABCG2 promoter (Figure 3A). In addition to the core sequence, a consensus sequence of 14 residues across 22 nucleotides has been identified as the full sequence of an AhR element. To address the functional relevance of these predicted AhREs, the BCRP-3.8kb construct containing all five AhREs and the BCRP-1.2kb containing three AhREs were initially tested in cell-based reporter assays. As indicated in Figure 3, the BCRP-1.2kb promoter was activated 2.8- and 3.2-fold above control levels by 3MC treatment at 1 and 5 µM, respectively, whereas the BCRP-3.8kb promoter was activated 9.3- to 10.3-fold over control by 3MC, and 3-fold by the treatment of EGB761 (100 µg/ml) or OMP (50 µM). Resv (100 µM) on the other hand, demonstrated an ability to inhibit the activity of both promoter constructs.
3.4. Serial deletion and site-directed mutation of the AhREs in the ABCG2 promoter
To further delineate the role of the five identified AhREs in 3MC-mediated activation of ABCG2 promoter, a number of reporter constructs containing serial deletion of the ABCG2 promoter as described in Material and Methods were utilized in the following LS174T cell reporter experiments. As illustrated in Figure 4A, deletion of the distal AhRE4 and AhRE5 has resulted in a dramatic decrease in 3MC-mediated activation of the ABCG2 promoter from 7.3- fold (BCRP-3.8kb) to 3.5-fold (BCRP-1.2kb). Nevertheless, further deletion of the AhRE2 and AhRE3 only led to relatively moderate decreases of ABCG2 reporter activities, and the AhRE1 alone was responsible for more than 2-fold activation after 3MC treatment. Together with the observations in Figure 3B and 3C, the significant loss in activity between the −3.8 kb promoter and the −1.2 kb promoter suggests an important role for AhRE4 and 5, and a lesser role for AhRE1 in mediating ABCG2 transcription.
Site-directed mutagenesis of AhRE1, 4 and 5 would allow for evaluation of their relative contributions to the 3MC-mediated ABCG2 promoter activations. The BCRP-3.8kb promoter was engineered to contain mutations in AhRE1 (BCRP-3.8-Δ1), AhRE5 (BCRP-3.8-Δ5), AhRE1 and 4 (BCRP-3.8-Δ1/4), AhRE 1 and 5 (BCRP-3.8-Δ1/5), and AhRE4 and 5 (BCRP-3.8-Δ4/5). Transfection assays in LS174T cells showed that mutation of AhRE1 caused a relatively moderate decrease in 3MC-mediated activation (Figure 4B), whereas disruption of AhRE5 resulted in almost a complete loss of activation. The additional loss of AhRE4 only has negligible effects on the promoter activation of the Δ1 and Δ5 mutants, as illustrated by the Δ1/4 and the Δ4/5 mutants. Notably, all mutants missing AhRE5 (BCRP-3.8-Δ5, -Δ1/5, and -Δ4/5) demonstrated significant losses of ABCG2 promoter activation. These data suggest that AhRE5 is essential for the maximal upregulation of ABCG2 expression in response to 3MC treatment.
3.5. Binding of AhR to AhREs in the ABCG2 promoter
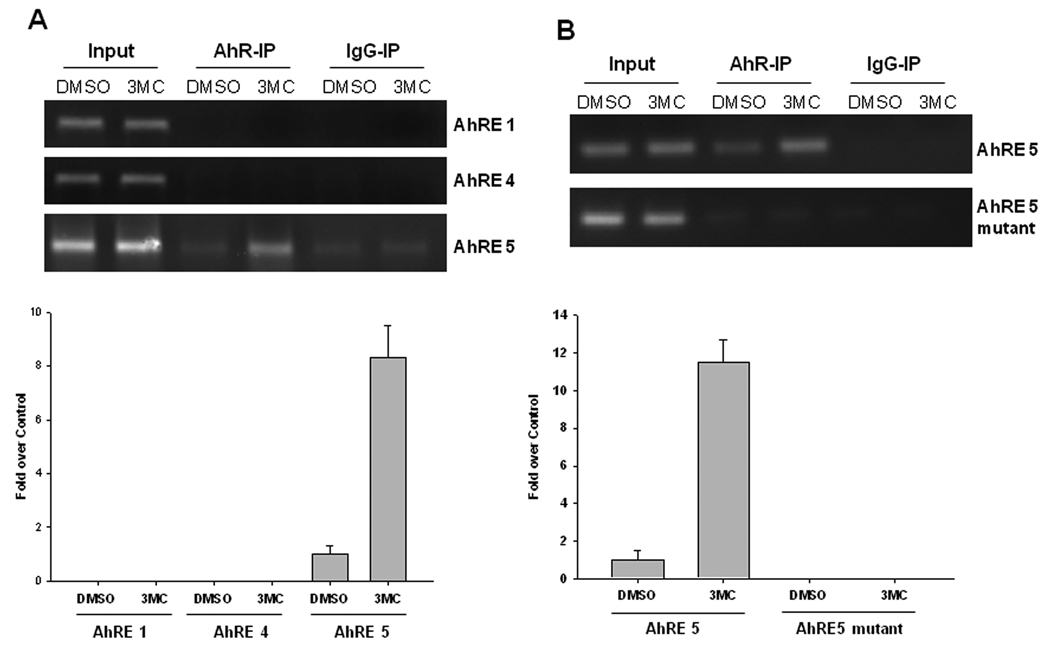
Predicted elements AhRE1, AhRE4 and AhRE5 were further evaluated for their ability to bind AhR in the physiologically relevant cellular environment. Chromatin immunoprecipitation experiments in LS174T cells showed that AhR protein was recruited to the AhRE5- but not AhRE1- and AhRE4-containing regions of ABCG2 promoter, and the interaction was significantly increased after 3MC treatment (Figure 5A). To further illustrate the exact role of AhRE5 in 3MC-mediated AhR binding, a modified ChIP assay was conducted in Hepa-1 cells, an immortalized mouse hepatoma cell line, transfected with the BCRP-3.8kb or the BCRP-3.8- Δ5 construct as outlined in Materials and Methods. Since the primers used in the ChIP assay are specific to the human ABCG2, the potential interference of mouse abcg2 could be avoided in these cells (data not shown). Using this unique system, we observed that 3MC treatment clearly increased the recruitment of AhR to the AhRE5 region, while mutation of the AhRE5 essentially abolished the interaction between AhR and the AhRE5 containing region (Figure 5B). These results indicate that AhR activates the ABCG2 promoter through direct interaction with the AhRE5 in the presence of 3MC in vivo.
Figure 5. Recruitment of AhR to the ABCG2 promoter.
A) Chromatin immunoprecipitation assays were carried out in LS174T cells treated with DMSO control or 3MC as described in Materials and Methods. Chromatin was precipitated with antibodies to IgG (non-specific) or AhR. PCR primers were specific to the regions containing AhRE1, 4 or 5. B) Mouse Hepa-1 cells were transfected with BCRP-3.8kb or BCRP-3.8-Δ5 promoter construct, following the treatment of DMSO or 3MC. After precipitation with IgG or AhR antibody, AhRE5 region was amplified by real-time PCR.
4. Discussion
Xenobiotic-induced expression of efflux transporters represents one of the adaptive and protective mechanisms to maintain physiological homeostasis after exposure of the body to foreign chemicals. Induction of ABCG2 expression has been found in a tissue and cell-type specific manner after exposure to a number of chemicals including 17β-estradiol, rosiglitazone, imatinib, as well as AhR activators such as TCDD, 3MC, OMP, and chrysin [12, 13, 15, 16]. Nevertheless, the mechanisms underlying these inductions are not well established. In particular, whether ABCG2 represents a direct target gene of AhR is largely unknown. In the current study, we demonstrate that 3MC induces the expression of ABCG2 in LS174T cells in an AhR-dependent fashion. More importantly, a novel distal responsive element of AhR located −2357/−2333 upstream of the ABCG2 transcriptional start site (AhRE5) has been identified and characterized as a functional unit pivotal to 3MC-mediated induction of ABCG2, which offers new insights into the molecular mechanism of ABCG2 regulation in the intestines.
Small intestine is a prime site of absorption, metabolism and efflux for any ingested compound – nutrient, vitamin or drug. Compounds absorbed across the small intestine are then transported to the liver for further processing, which makes the small intestine critical in determining drug bioavailability [21, 22]. Based on existing limitations, colon-derived cells lines are the best and only option for an in vitro system resembling small intestine. The human colon adenocarcinoma-derived LS174T cells, expressing a full complement of nuclear receptors, are chosen here for ABCG2 induction studies. Our initial experiment with common nuclear receptor ligands successfully confirmed the involvement of PPARγ and AhR in the regulation of ABCG2 in a small intestine-specific context. AhR activators were previously identified as ABCG2 inducers in Caco-2 cells, while PPARγ regulation was characterized in lymphocytes [13, 16]. Notably, Resv, a known AhR antagonist induced ABCG2 mRNA expression in LS174T cells, which was consistent with the earlier observation by Ebert et al. in Caco-2 cells [16]. In contrast, our AhR reporter assays indicated that treatment with Resv significantly decreased the luciferase activity of both BCRP-1.2kb and BCRP-3.8kb constructs. Given that Resv also functions as an agonist of ER and activator of the nuclear factor-like 2 (Nrf2) signaling pathway [23, 24], one possible explanation would be that Resv-induced expression of ABCG2 in LS174T cells was AhR-independent.
Computer based analysis of the −4 kb upstream region of the ABCG2 promoter resulted in the identification of five putative AhR responsive elements (AhREs) each contained the core (5’-NGCGTG-3’) sequence required for AhR/ARNT interaction [25]. Serial deletion of the ABCG2 promoter revealed that the deletion of the distal AhRE4 and 5 dramatically decreased the 3MC-activated ABCG2 reporter activity, while further deletion of the AhRE2 and 3 only resulted in moderate decreases of the reporter activity. This indicates that AhRE1 may play a major role in the activation of BCRP-1.2kb following 3MC treatment (Figure 4A). Indeed, the role of AhRE1 in 3MC-mediated activation of BCRP promoter was also confirmed in site-directed mutagenesis assays, though to a much smaller extent than AhRE 5 (Figure 4B). While this manuscript was in preparation, Tan et al., reported the identification of a proximal dioxin response element (DRE) located −194/−190 upstream of ABCG2 promoter by functional analysis of the first 1285 bp of the ABCG2 promoter [26]. Intriguingly, this DRE represents the binding site that we named AhRE2 (Figure 3A). However, since the deletion of AhRE2 in our reporter assays only led to a moderate reduction of the 3MC induced ABCG2 activation, our further investigations were centered on the distal AhRE4 and 5 as well as the proximal AhRE1.
Site-directed mutagenesis of the AhRE5 and AhRE1 in the BCRP-3.8kb construct reduced the 3MC-induced ABCG2 reporter activation by approximately 80% and 20%, respectively, while additional mutation of AhRE4 only has negligible effects on the promoter activation. These results indicate that the distal AhRE5 alone appears to be able to confer the majority of 3MC-mediated ABCG2 reporter activation. More importantly, our ChIP assays demonstrated that recruitment of AhR to the AhRE5 but not AhRE4 or AhRE1 was clearly increased in a cellular environment after 3MC treatment. Furthermore, a modified ChIP experiment using mouse Hepa-1 cells transfected with the BCRP-3.8kb or BCRP-3.8kb-Δ5 construct showed clearly that mutation of AhRE5 resulted in dissociation of the AhR protein. Although these results were obtained from a hybrid cellular system, this is the first study to demonstrate that the distal AhRE5 is required for 3MC induced AhR binding under physiologically relevant conditions.
Originally identified as the aryl hydrocarbon or dioxin receptor, AhR is a ligand-activated transcription factor of the bHLH protein of the PAS family [27]. As a xenobiotic sensor, AhR can be activated by a large number of halogenated aromatic hydrocarbons, PAHs, such as TCDD and 3MC, as well as clinically used drugs. Although the contribution of AhR to basic pathways of health and disease is largely unknown, it mediates the toxicity and carcinogenesis of many drugs and environmental chemicals by regulating a number of metabolism/detoxification genes including the cytochrome P450, CYP1A1, CYP1A2 and CYP1B1, the conjugative UDP-glucuronosyltransferases, UGT1A1, UGT1A3/1A4, UGT1A6, and now the efflux transporter ABCG2 [19, 28, 29]. Among these genes, CYP1As are the primary targets of AhR that almost exclusively regulated by AhR alone, while UGT1A1 represents a shared target of AhR and other xenobiotic receptors such as the pregnane X receptor (PXR) and the constitutive androstane receptor (CAR) [30, 31]. To date, investigations of ABCG2 regulation have discovered the involvement of ER, progesterone receptor (PR), PPARγ, HIF-1, and AhR by tissue and cell line specific mechanisms. For instance, treatment with estradiol enhanced the transcription of ABCG2 in ER-positive T47D:A18 cells but downregulated its expression in ER-positive MCF-7 cells [12, 32], whereas Resv induced the expression of ABCG2 in MCF-7 but not MCF-7AHR200 cells in an AhR-dependent manner even though Resv is a well established AhR antagonist [33, 34]. Additionally, AhR can crosstalk with ER through a competitive binding mechanism, and shares a number of common target genes with Nrf2 – a critical xenobiotic receptor for oxidative stress [35, 36]. Therefore, eventually the induction of ABCG2 by a particular compound could be defined by the interplay of an array of transcription factors and co-factors in a tissue specific manner.
In summary, we have identified and characterized a novel distal AhR responsive element located −2357/−2333 bp upstream of the ABCG2 promoter that is involved in AhR-mediated transcriptional activation of ABCG2 gene expression in LS174T cells. These data provide new insights into the transcriptional regulation of ABCG2 expression, and may offer a novel strategy for in vitro identification of chemicals as potential ABCG2 inducers. Given the fact that mammalian genomes often contain multiple dispersed enhancer elements, future studies to functionally analyze more distal promoter elements upstream of ABCG2 would shed light on the molecular mechanisms for the up-regulation of ABCG2 by multiple transcription factors.
Acknowledgements
This work was partly supported by the National Institutes of Health, National Institute of Diabetes Digestive and Kidney Diseases [Grant DK061652] (HW), the University of Maryland School of Pharmacy, and by a VA Merit Review grant (DDR).
ABBREVIATIONS
- AhR
aryl hydrocarbon receptor
- AhRE
AhR response element
- BCRP
breast cancer resistance protein
- ChIP
chromatin immunoprecipitation
- CLO
clofibrate
- DDI
drug-drug interactions
- ER
estrogen receptor
- HIF-1
hypoxia-inducible factor-1
- 3MC
3-methylcholanthrene
- Nrf2
nuclear factor-like 2
- NR
nuclear receptor
- OMP
omeprazole
- PPARγ
peroxisome proliferator-activated receptor gamma
- Resv
resveratrol
- TGZ
troglitazone
- TCDD
2,3,7,8-tetrachlorodibenzodioxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 2.Hardwick LJ, Velamakanni S, van Veen HW. The emerging pharmacotherapeutic significance of the breast cancer resistance protein (ABCG2) Br J Pharmacol. 2007;151:163–174. doi: 10.1038/sj.bjp.0707218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg ACLM, Schinkel AH, et al. Subcellular Localization and Distribution of the Breast Cancer Resistance Protein Transporter in Normal Human Tissues. Cancer research. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 4.Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells. 2002;20:11–20. doi: 10.1002/stem.200011. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, et al. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer research. 2003;63:4048–4054. [PubMed] [Google Scholar]
- 7.Ebert B, Seidel A, Lampen A. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis. 2005;26:1754–1763. doi: 10.1093/carcin/bgi139. [DOI] [PubMed] [Google Scholar]
- 8.Kruijtzer CMF, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC, et al. Increased Oral Bioavailability of Topotecan in Combination With the Breast Cancer Resistance Protein and P-Glycoprotein Inhibitor GF120918. J Clin Oncol. 2002;20:2943–2950. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- 9.Ejendal KF, Hrycyna CA. Multidrug resistance and cancer: the role of the human ABC transporter ABCG2. Current protein & peptide science. 2002;3:503–511. doi: 10.2174/1389203023380521. [DOI] [PubMed] [Google Scholar]
- 10.Bailey-Dell KJ, Hassel B, Doyle LA, Ross DD. Promoter characterization and genomic organization of the human breast cancer resistance protein (ATP-binding cassette transporter G2) gene. Biochim Biophys Acta. 2001;1520:234–241. doi: 10.1016/s0167-4781(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 11.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. The Journal of biological chemistry. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 12.Ee PLR, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT. Identification of a Novel Estrogen Response Element in the Breast Cancer Resistance Protein (ABCG2) Gene. Cancer research. 2004;64:1247–1251. doi: 10.1158/0008-5472.can-03-3583. [DOI] [PubMed] [Google Scholar]
- 13.Szatmari I, Vamosi G, Brazda P, Balint BL, Benko S, Szeles L, et al. Peroxisome Proliferator-activated Receptor {gamma}-regulated ABCG2 Expression Confers Cytoprotection to Human Dendritic Cells. J Biol Chem. 2006;281:23812–23823. doi: 10.1074/jbc.M604890200. [DOI] [PubMed] [Google Scholar]
- 14.Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos. 2006;34:1756–1763. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- 15.Burger H, van Tol H, Brok M, Wiemer EA, de Bruijn EA, Guetens G, et al. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther. 2005;4:747–752. doi: 10.4161/cbt.4.7.1826. [DOI] [PubMed] [Google Scholar]
- 16.Ebert B, Seidel A, Lampen A. Phytochemicals Induce Breast Cancer Resistance Protein in Caco-2 Cells and Enhance the Transport of Benzo[a]pyrene-3-sulfate. Toxicol Sci. 2007;96:227–236. doi: 10.1093/toxsci/kfl147. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Zhou L, Gupta A, Vethanayagam RR, Zhang Y, Unadkat JD, et al. Regulation of BCRP/ABCG2 expression by progesterone and 17beta-estradiol in human placental BeWo cells. Am J Physiol Endocrinol Metab. 2006;290:E798–E807. doi: 10.1152/ajpendo.00397.2005. [DOI] [PubMed] [Google Scholar]
- 18.Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Archives of biochemistry and biophysics. 1998;357:155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- 19.Quattrochi LC, Tukey RH. Nuclear uptake of the Ah (dioxin) receptor in response to omeprazole: transcriptional activation of the human CYP1A1 gene. Molecular pharmacology. 1993;43:504–508. [PubMed] [Google Scholar]
- 20.Li L, Stanton JD, Tolson AH, Luo Y, Wang H. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharmaceutical research. 2009;26:872–882. doi: 10.1007/s11095-008-9788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight B, Troutman M, Thakker DR. Deconvoluting the effects of P-glycoprotein on intestinal CYP3A: a major challenge. Current opinion in pharmacology. 2006;6:528–532. doi: 10.1016/j.coph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Benet LZ. The drug transporter-metabolism alliance: uncovering and defining the interplay. Molecular pharmaceutics. 2009;6:1631–1643. doi: 10.1021/mp900253n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochemical and biophysical research communications. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 25.Hankinson O. The aryl hydrocarbon receptor complex. Annual review of pharmacology and toxicology. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 26.Tan KP, Wang B, Yang M, Boutros PC, Macaulay J, Xu H, et al. Aryl hydrocarbon receptor is a transcriptional activator of the human breast cancer resistance protein (BCRP/ABCG2) Molecular pharmacology. 2010;78:175–185. doi: 10.1124/mol.110.065078. [DOI] [PubMed] [Google Scholar]
- 27.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yueh MF, Huang YH, Hiller A, Chen S, Nguyen N, Tukey RH. Involvement of the xenobiotic response element (XRE) in Ah receptor-mediated induction of human UDP-glucuronosyltransferase 1A1. The Journal of biological chemistry. 2003;278:15001–15006. doi: 10.1074/jbc.M300645200. [DOI] [PubMed] [Google Scholar]
- 29.Erichsen TJ, Ehmer U, Kalthoff S, Lankisch TO, Muller TM, Munzel PA, et al. Genetic variability of aryl hydrocarbon receptor (AhR)-mediated regulation of the human UDP glucuronosyltransferase (UGT) 1A4 gene. Toxicology and applied pharmacology. 2008;230:252–260. doi: 10.1016/j.taap.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Sugatani J, Kojima H, Ueda A, Kakizaki S, Yoshinari K, Gong QH, et al. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology (Baltimore, Md. 2001;33:1232–1238. doi: 10.1053/jhep.2001.24172. [DOI] [PubMed] [Google Scholar]
- 31.Xie W, Yeuh MF, Radominska-Pandya A, Saini SP, Negishi Y, Bottroff BS, et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4150–4155. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai Y, Ishikawa E, Asada S, Sugimoto Y. Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2. Cancer research. 2005;65:596–604. [PubMed] [Google Scholar]
- 33.Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer research. 1998;58:5707–5712. [PubMed] [Google Scholar]
- 34.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, et al. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Molecular pharmacology. 1999;56:784–790. [PubMed] [Google Scholar]
- 35.Klinge CM, Kaur K, Swanson HI. The aryl hydrocarbon receptor interacts with estrogen receptor alpha and orphan receptors COUP-TFI and ERRalpha1. Archives of biochemistry and biophysics. 2000;373:163–174. doi: 10.1006/abbi.1999.1552. [DOI] [PubMed] [Google Scholar]
- 36.Yeager RL, Reisman SA, Aleksunes LM, Klaassen CD. Introducing the "TCDD-inducible AhR-Nrf2 gene battery". Toxicol Sci. 2009;111:238–246. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]