Abstract
The homeostasis of Drosophila midgut is maintained by multipotent intestinal stem cells (ISCs), each of which gives rise to a new ISC and an immature daughter cell, enteroblast (EB), after one asymmetric cell division. In Drosophila, the Gal4-UAS system is widely used to manipulate gene expression in a tissue- or cell-specific manner, but in Drosophila midgut, there are no ISC- or EB-specific Gal4 lines available. Here we report the generation and characterization of Dl-Gal4 and Su(H)GBE-Gal4 lines, which are expressed specifically in the ISCs and EBs separately. Additionally, we demonstrate that Dl-Gal4 and Su(H)GBE-Gal4 are expressed in adult midgut progenitors (AMPs) and niche peripheral cells (PCs) separately in larval midgut. These two Gal4 lines will serve as invaluable tools for navigating ISC behaviors.
Keywords: Drosophila, intestinal stem cell, enteroblast, Gal4 lines
Introduction
The Drosophila adult midgut is replenished by multipotent intestinal stem cells (ISCs) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). After an asymmetric division, an ISC give rises to two cells: one that retains stem cell properties and one that becomes an immature daughter cell, enteroblast (EB), which can further differentiate into enterocytes (ECs) or enteroendocrine (ee) cells without dividing. Approximately ninety percent of the EBs will become EC cells, and ten percent will become ee cells (Ohlstein and Spradling, 2007). The ISC fate decision is primarily governed by Notch signaling pathway. ISCs modulate the Notch signaling pathway in their adjacent EBs in order to specify the differentiated lineages of their descendants. A strong Notch signal directs the fate of the EBs to the absorptive EC cells, whereas a weak Notch signal induces EBs into the secretory ee cells (Ohlstein and Spradling 2007). Recently, the transcriptional repression of Notch target genes by a Hairless-Suppressor of Hairless complex was found to be required for ISCs maintenance (Bardin et al., 2010). ISCs can be identified by the expression of Delta (Dl), a ligand for the Notch receptor, which activates the Notch signal in the neighboring EBs and can be detected using the Notch signal reporter Su(H)GBE-LacZ as an EB marker (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2007). Interestingly, a transient niche generated by the ISC progenitor cells via Notch signaling was recently found to regulate the specification of ISC through DPP signaling in larvae (Mathur et al., 2010). In addition, the anatomy and cell renewal in the Drosophila midgut are similar to those in the mouse small intestine (Wang and Hou, 2010) and Drosophila ISCs were used as a model to study stem cell behavior responding to some tissue damage and pathogenic stimulation (Amcheslavsky et al., 2009; Chatterjee and Ip, 2010). Studies in the adult Drosophila midgut indicate that tissue homeostasis following tissue damage caused by bacterial infection, directed cell ablation, or stress signaling is regulated by JAK/STAT signaling (Buchon et al., 2009; Jiang et al., 2009; Cronin et al., 2009), in addition to the requirement of JAK/STAT in midgut stem cell proliferation under steady-state conditions (Beebe et al., 2009; Liu et al., 2010; Lin et al., 2010).
The Gal4-UAS system (Brand and Perrimon, 1993) is widely used to both over-express and knock down the expression of target genes in specific cell types or tissues in a spatial and temporal manner at different stages of development. In Drosophila midgut, the Escargot (Esg)-Gal4 is the only Gal4 line widely used to manipulate gene expression in the ISCs and label the ISC. However, Esg-Gal4 is expressed not only in the ISCs but also in the EB cells (Micchelli and Perrimon, 2006); ISC- or EB-specific Gal4 lines are lacking. To facilitate ISC- or EB-specific gene manipulation, we generated and characterized an ISC-specific Gal4 line by converting the P{PZ}Dl05151 line into a strain containing a P[Gal4, w+] element and an EB-specific Gal4 line, Su(H)GBE-Gal4.
Results and Discussion
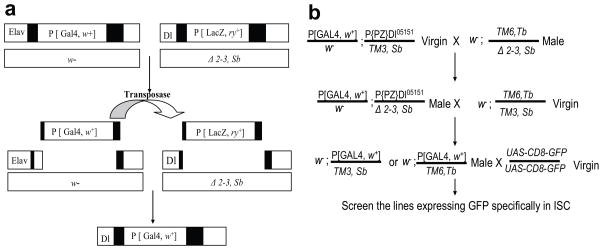
Drosophila adult midgut contains at least four types of cells: ISCs, EBs, ee cells, and ECs. Each of these cell types expresses a specific set of markers. ISCs can be identified by the expression of Delta (Dl), the ligand of the Notch signal pathway (Ohlstein and Spradling, 2007). A lacZ enhancer trap line, P{PZ}Dl05151 (Dl-LacZ), in which the P-element is inserted at the 5′ untranslated region (UTR) of the Delta genomic locus, was found to specifically label the ISCs in the Drosophila midgut (Jiang et al., 2009; Beebe et al., 2009). However, this insertion only allows for labeling the ISCs, but not for manipulating the gene expression specifically in ISCs through the GAL4-UAS system. We generated an ISC-specific Gal4 line (Dl-Gal4) via a targeted transposition strategy, shown in Figure 1a, in which we replaced the LacZ-expressing P-element P[ry+, PZ] with a Gal4-expressing P[Gal4, w+] element (Sepp and Auld, 1999). To induce targeted P-element replacement, the X-chromosomal insertion P[Gal4, w+]elavC155 was used as a donor line, and P{PZ}Dl05151 as a recipient line. Both of the P-element lines were combined with transposase Δ 2–3, and the flies were then out-crossed to remove the Δ 2–3 and the original P[GawB] chromosome. Subsequently, the flies with potential targeted transposition were crossed to a UAS-CD8-GFP carrying line, and the progeny was assayed for the GFP expression in the adult midgut ISCs. Figure 1b illustrates the general crossing scheme that was used to obtain flies with the desired genotype. Among the 106 potential targeted transposition lines thus obtained, three targeted transposition lines in which the P[ry+, PZ] P-element was replaced by the Gal4-expressing P-element P[Gal4, w+] were recovered and further tested. All three lines appear to have similar expression pattern, so we further characterized one of the candidate lines in detail and refer to it as Dl-Gal4.
Fig. 1.
Schematic diagrams of targeted P-element replacement and the crossing scheme for targeted transposition. (a) Target Dl-LacZ P-element excision on the third chromosome is induced by transposase Δ 2–3. This transposition results in a double-stranded DNA gap with remnants of P-element termini on either side, and the gap can be filled out by the donor Elavc155-Gal4 P-element by homologous recombination. (b) To induce targeted P-element replacement, both P-elements and a source of transposase Δ 2–3 were combined, and potential targeted transpositions were then out-crossed to a homozygous UAS-CD8-GFP. The progeny from the final cross was assayed for GFP expression in adult midgut ISCs.
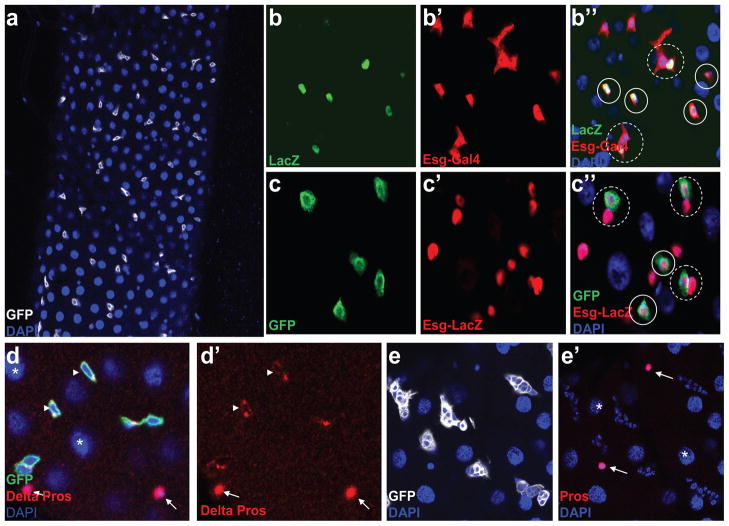
To further characterize the expression pattern of Dl-Gal4, Dl-Gal4 and UAS-CD8-GFP were recombined to the same chromosome to generate a stable line expressing GFP. GFP was found to express only in small diploid cells, as shown in Figure 2a, which is consistent with that ISCs are diploid cells. Both diploid ISCs and EBs can be labeled by Escargot (esg), a transcription factor that belongs to the conserved Snail/Slug family (Micchelli and Perrimon, 2006) and they appear to stay paired in some cases. Just like Dl-LacZ, which labels ISCs (Figure 2b, b′, b″), Dl-Gal4 is only expressed in some single Esg labeled or one of the paired Esg labeled diploid cells (Figure 2c, c′, c″). To further confirm that these GFP-labeled cells are ISCs, the guts were co-stained with an ISC-specific marker, Delta antibody, as shown in Figure 2d, d′. Most of the GFP-positive cells could be labeled by Delta antibody staining, which further confirms that, similar to the expression pattern of Dl-LacZ, Dl-Gal4 is also specifically expressed in the ISCs. In addition, from our immuno-staining data, we observed that a few of the GFP-positive ISCs could not be labeled by Delta antibody staining (Supplemental Figure S1). Our finding is consistent with previously published work that demonstrates that Delta antibody marks only a subset of ISCs in the midgut (Ohlstein and Spradling, 2007). Moreover, we further observed that there is no Prospero (ee cell marker) or Su(H)GBE-LacZ (EB marker) expression in these Delta-negative diploid cells (Supplemental Figure S1). Thus, Dl-Gal4 is a more sensitive ISC marker than Delta antibody. The adult midgut ISCs originate from adult midgut progenitors (AMPs) in the embryonic and larval stage, which can proliferate and form clusters, known as midgut imaginal islands, in larvae. Dl-LacZ has been previously reported to be expressed in the AMPs in the larval midgut (Mathur et al., 2010) (Supplemental Figure S2). Consistent with the previously published work, we also observed that Dl-Gal4 specifically labels the AMP clusters in the larval midgut (Figure 2e, e′).
Fig. 2.
Dl-Gal4 is specifically expressed in adult midgut ISCs and in the AMPs in larvae.
(a) GFP (white) is expressed in the diploid cell (as seen by DAPI staining the smaller diploid nucleus). (b,b′b″) ISCs are labeled by Dl-lacZ (b) and both of ISCs and EBs are labeled by Esg-Gal4,UAS-EGFP (b′). The solid circles in b″ point to a single ISC that co-expresses Dl-LacZ and Esg-Gal4,UAS-EGFP while the dotted circles indicate ISCs that are still paired with daughter EB cells. (c,c′,c″) Just like the expression pattern of Dl-lacZ in (b), Dl-Gal4,UAS-CD8-GFP (c) is expressed in some single (solid circle) Esg-lacZ labeled or one of the paired (dotted circle) Esg-lacZ labeled cells (c′), which we believe to be ISCs. (d,d′) The Dl-Gal, UAS-CD8-GFP (Green) positive diploid cells can be labeled by ISC specific marker Delta antibody (arrowhead, cytoplasmic red in d and d′). (e,e′) In the larval gut, GFP (white) is expressed in AMP clusters. EC cells are polyploid (asterisk), diploid ee cells contain nuclear Prospero (arrow, nuclear red) and Blue is DAPI. a–d represent samples from adult midgut while e and e′ are samples from the midgut of third instar wandering larvae.
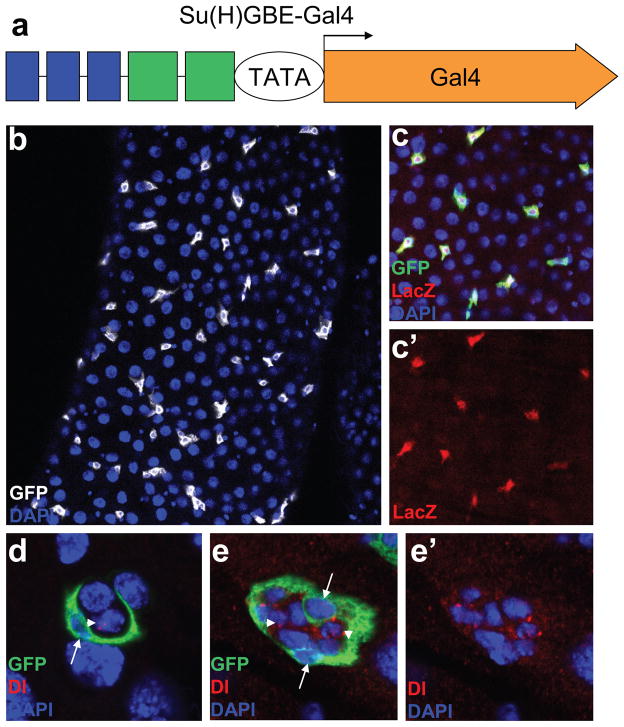
ISCs control daughter cell fate by modulating Notch signaling (Ohlstein and Spradling, 2007), and Notch is specifically activated in EBs. Notch signal reporter Su(H)GBE-LacZ (Furriols and Bray, 2001), which contains tandem binding sites for the transcription factors Grainyhead (GBE) and suppressor of Hairless [Su(H)], is widely used as a specific EB marker. We cloned this enhancer fragment combination (Figure 3a) in Su(H)GBE-LacZ into a Gal4 vector pPTGAL (Sharma et al., 2002) to generate an EB-specific Gal4 line, Su(H)GBE-Gal4. Su(H)GBE-Gal4 was recombined with UAS-CD8-GFP to generate a stable line expressing GFP, but GFP was found to be expressed only in Su(H)GBE-LacZ-labeled EBs (Figure 3b, c, c′). A Su(H)GBE-LacZ-labeled peripheral cell (PC), which functions as a niche to regulate the specification of the ISCs in Drosophila larvae, was recently identified (Mathur et al., 2010). A niche-specific Gal4 line will provide a potent tool for detecting the interaction between the niche and stem cells by manipulating the gene expression in the niche. We detected the expression of Su(H)GBE-Gal4 in the third instar larvae midgut and found that GFP specifically labeled the niche PCs, as expected (Fig. 3d, e, e′). As shown in Figure 3d, e, e′, the GFP labeled niche PC tightly encased the Delta labeled AMPs.
Fig. 3.
Specific expression of Su(H)GBE-Gal4 in adult midgut EBs and in the larval PC niche. (a) Schematic representation of the Su(H)GBE-Gal4 construct. The combination of 3 copies of GBE (blue) and 2 copies of Su(H)m8 (green) was inserted into the Gal4 reporter vector pPTGAL to generate Su(H)GBE-Gal4 construct. Su(H)GBE-Gal4 was recombined to UAS-CD8-GFP. (b) GFP (white) is expressed in diploid cells (smaller nuclei as seen by DAPI).
(c,c′) GFP (green in c) is only expressed in Su(H)GBE-LacZ labeled diploid EB cells (red in c,c′). (d,e,e′) In the third instar larval gut, GFP (green) labeled the niche PCs (arrow), which encases the Delta (red) antibody–labeled AMPs (arrowhead). There is one PC in d and two PCs in e,e′. Blue is DAPI. b,c,c′ are samples from the adult midgut while d,e,e′ represent tissues from the third instar larvae.
To further determine the expression pattern of Dl-Gal4 and Su(H)GBE-Gal4 during development and to exploit these lines more efficiently for RNAi based experiments, we immuno-stained the wing and the eye imaginal discs from the wandering third instar larvae. Our results indicated that Dl-Gal4 and Su(H)GBE-Gal4 expression is also detected in the larval wing and eye imaginal discs. As shown in supplemental Figure S3, Dl-Gal4 is expressed throughout the wing imaginal disc epithelium, and appears to be stronger in the dorsal and ventral cells along the dorsal-ventral boundary (Figure S3 a, a′, a″). However, in the eye imaginal disc, Dl-gal4 is expressed in all cell types and appears to be predominantly expressed in the morphogenetic furrow, which is consistent with the expression pattern of Delta (Figure S3 b, b′, b″). As expected, Su(H)GBE-Gal4 is predominantly expressed in the Dorsal-ventral boundary in the wing imaginal disc, which is consistent with the expression pattern of Su(H)GBE-LacZ (Figure S3 c, c′, c″), while it is expressed in all cell types in the eye imaginal disc (Figure S3 d, d′, d″)
The Drosophila ISC is emerging as an excellent model system to investigate stem cell behaviors. The ISC-specific Dl-Gal4 and EB/niche-specific Su(H)GBE-Gal4 described here could be a potent tool for manipulating a gene’s expression as well as overexpression or RNAi knock-down in an ISC- or EB/niche-specific manner, and for labeling the ISCs or EBs in living tissue.
Materials and Methods
Fly Genetics
All flies were maintained at 25°C. UAS-CD8-GFP and ElavC155 were obtained from Bloomington stock center, and P{PZ}Dl05151 was kindly provided by Dr. Bruce A Edgar. The P{PZ}Dl05151 insertion was replaced by a donor Gal4 from ElavC155 at X-chromosome, according to the scheme provided in Figure 1b. Three Dl-Gal4 lines were recovered among 106 potential targeted transposition lines.
Su(H)GBE-Gal4 Construct and Transgene
The DNA oligo of the combination of three copies of GBE(CTTGGAAACCGGTTATGCGAG) and two copies of Su(H)m8 (AAACTTACTTTCAGCTCGGTTCCCACGCCAC) were synthesized and cloned into pPTGAL (Sharma et al., 2002) at XbaI and KpnI sites. The purified plasmid was injected into the w1118 embryo for transformation using standard techniques.
Immunofluorescence Staining
Immunofluorescence was performed as previously described (Zeng et al., 2007). Briefly, Drosophila guts were dissected in PBS and fixed for 30 min in 4% formaldehyde. After three washes with PBT (PBS plus 0.1% Triton X-100), the samples were preabsorbed overnight at 4°C in 5% normal goat serum. The guts were then incubated in primary antibody diluted in PBT for 2 h at room temperature. After four washes with PBT, the guts were incubated with secondary antibody diluted in PBT for 1 h at room temperature. After four washes with PBT, the guts were mounted in Vectashield mounting medium with DAPI. Confocal images were obtained using a Zeiss LSM510 system, and processed using Adobe Illustrator CS4.
The following antibodies were used: chicken polyclonal anti-GFP antibody (1:3000; Abcam); rabbit anti-β-Gal antibody (1:1000; Cappel); mouse monoclonal anti-β-Gal antibody (1:200; Clontech); mouse monoclonal anti-Delta 1:20 (DSHB); mouse monoclonal anti-Prospero 1:50 (DSHB). Secondary Abs were goat anti-chicken IgG and goat anti-mouse conjugated to Alexa 488 or Alexa 568 (1:500; Molecular Probes).
Supplementary Material
Supplemental Fig. S1. ISCs can be labeled by Dl-Gal4. (a,a′,a″,a‴,a″″) Dl-Gal4, UAS-CD8-GFP positive cells (green) can also be stained with Dl antibody (arrow in a′), cytoplasmic red. However, a few Dl-Gal4, UAS-CD8-GFP positive cells (solid circle in a) are negative for Dl antibody staining. These GFP labeled but Delta negative diploid cells could neither be labeled by Prospero antibody (arrowhead in a″, nuclear red), an ee cell marker, or by Su(H)GBE-LacZ (asterisk in a″, purple), an enteroblast marker. Blue in a‴ is DAPI.
Supplemental Fig. S2. Expression of Dl-LacZ in larval AMPs. (a, a′) Dl-LacZ (green) is expressed in the AMPs (arrow in a′). Polyploid EC cells have larger nuclei (asterisk in a′), and diploid ee cells (arrowhead in a′) express nuclear Prospero (nuclear red). Blue is DAPI.
Supplemental Fig. S3. The expression pattern of Dl-Gal4 and Su(H)GBE-Gal4 in the wing and eye imaginal disc of the third wandering instar larvae.
(a) a third instar wing imaginal disc shows the expression of Dl-Gal4 on all epithelial cells with stronger signal near the dorsal ventral boundary. (a′) Delta (red) stains both the epithelial cells and the cells at the dorsal-ventral boundary as indicated by the white arrow. (a″) Dl-Gal4 (green) and Dl-GAL4 (red) show co-localization throughout the wing disc epithelium, and some dorsal and ventral cells along the dorsal-ventral boundary.
(b) a third instar eye imaginal disc shows the expression of Dl-Gal4 in all epithelial cells with a stronger signal along the morphogenetic furrow (arrowhead). (b′) Delta (red) shows a similar staining pattern as (b). (b″) Dl-Gal4 (green) and Delta (red) show co-localization throughout the eye imaginal disc epithelium, especially along the morphogentic furrow.
(c) a third instar wing imaginal disc shows the expression of Su(H)GBE-Gal4 is concentrated at the dorsal ventral boundary. (c′) Su(H)GBE-LacZ (red) is expressed in the dorsal-ventral boundary as indicated by the arrow. (c″) Su(H)GBE-GFP (green) and Su(H)GBE-LacZ (red) show co-localization throughout the dorsal-ventral boundary.
(d) a third instar eye imaginal disc shows the expression of Su(H)GBE-Gal4 in all epithelial cells. (d′) Su(H)GBE-LacZ (red) shows a similar staining pattern as (d). (d″) Su(H)GBE-GFP (green) and Su(H)GBE-LacZ (red) show co-localization throughout the eye imaginal disc epithelium.
Acknowledgments
We thank B.A. Edgar and the Bloomington stock centers for fly stocks, the Developmental Study Hybridoma Bank for antibodies, and Drosophila Genomics Resource Center for the pPTGAL vector. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
References
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2009;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Ip YT. Pathogenic stimulation of intestinal stem cell response in Drosophila. J Cell Physiol. 2009;220:664–671. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, Simoes RD, Gruber S, Puc U, Ebersberger I, Zoranovic T, Neely GG, von Haeseler A, Ferrandon D, Penninger JM. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furriols M, Bray SJ. A model Notch response element detects Suppressor of Hairless dependent molecular switch. Curr Biol. 2001;11:60–64. doi: 10.1016/s0960-9822(00)00044-0. [DOI] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/JAK/STAT signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of Drosophila intestinal stem cells. J Mol Cell Biol. 2010;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- Liu W, Singh SR, Hou SX. JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem. 2010;109:992–999. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ. Conversion of lacZ enhancer trap lines to GAL4 lines using targeted transposition in Drosophila melanogaster. Genetics. 1999;151:1093–1101. doi: 10.1093/genetics/151.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Y, Cheung U, Larsen EW, Eberl DF. PPTGAL, a convenient Gal4 P-element vector for testing expression of enhancer fragments in Drosophila. Genesis. 2002;34:115–118. doi: 10.1002/gene.10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Hou SX. Regulation of intestinal stem cells in mammals and Drosophila. J Cell Physiol. 2010;222:33–37. doi: 10.1002/jcp.21928. [DOI] [PubMed] [Google Scholar]
- Zeng X, Sun M, Liu L, Chen F, Wei L, Xie W. Neurexin-1 is required for synapse formation and larvae associative learning in Drosophila. FEBS Lett. 2007;581:2509–2516. doi: 10.1016/j.febslet.2007.04.068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1. ISCs can be labeled by Dl-Gal4. (a,a′,a″,a‴,a″″) Dl-Gal4, UAS-CD8-GFP positive cells (green) can also be stained with Dl antibody (arrow in a′), cytoplasmic red. However, a few Dl-Gal4, UAS-CD8-GFP positive cells (solid circle in a) are negative for Dl antibody staining. These GFP labeled but Delta negative diploid cells could neither be labeled by Prospero antibody (arrowhead in a″, nuclear red), an ee cell marker, or by Su(H)GBE-LacZ (asterisk in a″, purple), an enteroblast marker. Blue in a‴ is DAPI.
Supplemental Fig. S2. Expression of Dl-LacZ in larval AMPs. (a, a′) Dl-LacZ (green) is expressed in the AMPs (arrow in a′). Polyploid EC cells have larger nuclei (asterisk in a′), and diploid ee cells (arrowhead in a′) express nuclear Prospero (nuclear red). Blue is DAPI.
Supplemental Fig. S3. The expression pattern of Dl-Gal4 and Su(H)GBE-Gal4 in the wing and eye imaginal disc of the third wandering instar larvae.
(a) a third instar wing imaginal disc shows the expression of Dl-Gal4 on all epithelial cells with stronger signal near the dorsal ventral boundary. (a′) Delta (red) stains both the epithelial cells and the cells at the dorsal-ventral boundary as indicated by the white arrow. (a″) Dl-Gal4 (green) and Dl-GAL4 (red) show co-localization throughout the wing disc epithelium, and some dorsal and ventral cells along the dorsal-ventral boundary.
(b) a third instar eye imaginal disc shows the expression of Dl-Gal4 in all epithelial cells with a stronger signal along the morphogenetic furrow (arrowhead). (b′) Delta (red) shows a similar staining pattern as (b). (b″) Dl-Gal4 (green) and Delta (red) show co-localization throughout the eye imaginal disc epithelium, especially along the morphogentic furrow.
(c) a third instar wing imaginal disc shows the expression of Su(H)GBE-Gal4 is concentrated at the dorsal ventral boundary. (c′) Su(H)GBE-LacZ (red) is expressed in the dorsal-ventral boundary as indicated by the arrow. (c″) Su(H)GBE-GFP (green) and Su(H)GBE-LacZ (red) show co-localization throughout the dorsal-ventral boundary.
(d) a third instar eye imaginal disc shows the expression of Su(H)GBE-Gal4 in all epithelial cells. (d′) Su(H)GBE-LacZ (red) shows a similar staining pattern as (d). (d″) Su(H)GBE-GFP (green) and Su(H)GBE-LacZ (red) show co-localization throughout the eye imaginal disc epithelium.