Abstract
Naïve CD4+ T cells differentiate into diverse effector and regulatory lineages to orchestrate immunity and tolerance. The differentiation of pro-inflammatory TH1 and anti-inflammatory Foxp3+ regulatory T cells (Treg) was reciprocally regulated by S1P1, a receptor for the bioactive lipid sphingosine-1-phosphate. S1P1 inhibited extrathymic and natural Treg generation while driving TH1 cell development in a reciprocal manner and disrupted immune homeostasis. S1P1 signaled through mTOR and antagonized TGF-β function mainly by attenuating sustained Smad3 activity. S1P1 function was dependent upon endogenous sphingosine kinase activity. Remarkably, two seemingly unrelated immunosuppressants FTY720 and rapamycin targeted the same S1P1 and mTOR pathway to regulate the dichotomy between TH1 and Treg cells. Our studies establish an S1P1-mTOR axis that controls T cell lineage specification.
Keywords: regulatory T cells, T cell differentiation, mTOR, immunosuppressant
INTRODUCTION
CD4+ helper T cells are central regulators of adaptive immune responses. In response to antigen stimulation, naïve CD4+ T cells proliferate and differentiate into T helper type 1 (TH1) cells, TH2 cells and TH17 cells to exert specific effector functions. These effector T cells are characterized by distinct patterns of cytokine secretion: interferon-γ (IFN-γ), interleukin 4 (IL-4) and IL-17 are the signature cytokines for TH1, TH2 and TH17 cells, respectively1. Naïve precursors can also develop into antigen-specific Foxp3+ Treg cells, known as induced Treg cells (iTreg), which act in synergy with naturally occurring Treg cells (nTreg) to establish immune tolerance and counter-balance effector T cell functions2,3. Induction of iTreg cells in the peripheral immune compartment is closely related to the generation of TH17 cells, as the differentiation of both lineages is dependent upon the pleiotropic cytokine transforming growth factor β (TGF-β; http://www.signaling-gateway.org/molecule/query?afcsid=A002271)4. Despite extensive progress in this area, it remains poorly understood how the differentiation of naïve precursors into opposing regulatory and effector lineages is regulated.
T cell differentiation is programmed by polarizing cytokines, which signal through cell surface cytokine receptors and intracellular pathways, ultimately leading to the expression of lineage-specific transcription factors and effector cytokines. IL-12, IL-4 and TGF-β drive the differentiation of TH1, TH2 and iTreg cells, respectively, while stimulation by TGF-β together with IL-6 or IL-21 promotes TH17 development1. Differentiation of T cells is further shaped by non-cytokine receptors, especially those in the nuclear receptor superfamily that recognize endogenous metabolites, environmental toxins and other immune modulators. Retinoic acid, a vitamin A metabolite, directs reciprocal TH17 and iTreg differentiation by activating the retinoic acid receptor5–7. Aryl hydrocarbon receptor, a transcription factor activated by environmental toxins, regulates Treg and TH17 cell differentiation in a ligand-specific fashion8,9. Aside from the cytokine and nuclear receptors, whether other classes of receptors directly control T cell lineage choices has not been well established.
Sphingosine 1-phosphate (S1P) is a bioactive lysophospholipid enriched in blood10. S1P signals through five known G protein-coupled receptors (GPCR): S1P1-S1P5. FTY720, a new immunosuppressant, induces T cell sequestration in lymphoid organs by acting on four of the five S1P receptors. FTY720 is effective in relapsing multiple sclerosis and has become the first oral therapy for the debilitating autoimmune disease11. However, mechanism of action for FTY270 is complex, and it remains controversial whether FTY720 acts as an agonist or a functional antagonist or both to regulate lymphocyte trafficking. Genetic approaches to alter the function of S1P1 [http://www.signaling-gateway.org/molecule/query?afcsid=A000813] indicate that S1P1 is the main S1P receptor that facilitates the egress of T cells from lymphoid organs12,13. Using gain- and loss-of-function genetic systems, we recently identified a novel role of S1P1 in the negative control of thymic generation and suppressive activity of nTreg cells in a process dependent upon the downstream Akt-mTOR [http://www.signaling-gateway.org/molecule/query?afcsid=A000094] pathway14.
To investigate the function of S1P1 in the lineage determination of peripheral T cells, we used a combination of genetic systems and pharmacological approaches. We found that S1P1 inhibited differentiation of Foxp3+ Treg cells while promoting the development of TH1 cells in a reciprocal manner. S1P1 antagonized TGF-β receptor function, through an inhibitory effect on Smad3 activity, to control the dichotomy between these two T cell lineages. Moreover, this regulatory function was dependent upon the effect of S1P1 to sustain mTOR activation in T cells. Our studies demonstrate that differentiation of pro-inflammatory TH1 and anti-inflammatory Treg cells is reciprocally regulated by S1P1-mTOR and the opposing TGF-β-Smad3 signaling.
RESULTS
S1P1 blocks differentiation of iTreg cells
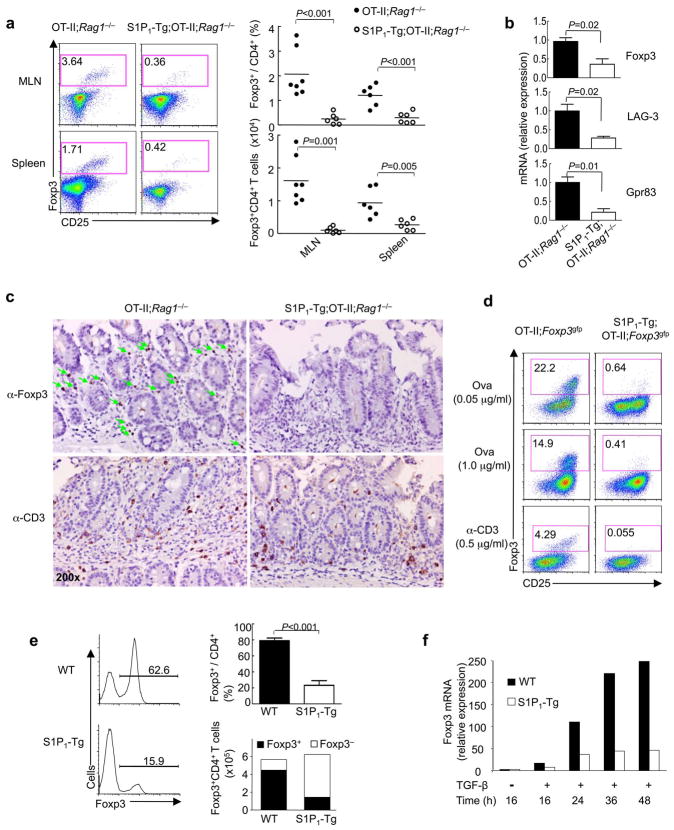
To gain insight into mechanisms of iTreg differentiation, we determined the role of S1P1 in this process. First, we crossed T cell-specific S1pr1-transgenic mice (hereafter called S1P1-Tg), which expressed 2–3 fold more S1P1 than wild-type mice (data not shown);13, with OT-II transgenic mice (CD4+ T cell receptors (TCR) specific for ovalbumin (Ova)) on a Rag1−/− background. Placing a TCR transgene on the Rag1−/− background results in all peripheral T cells having single antigen specificity without detectable Foxp3 expression, obviating the confounding effects of S1P1 function in the nTreg compartment14. In a model of oral antigen-induced iTreg generation6,7, Foxp3 induction in the mesenteric lymph nodes (MLN) and spleen of S1P1-Tg;OT-II;Rag1−/− mice was markedly reduced as compared with the control OT-II;Rag1−/− mice (Fig. 1a). Expression of Treg cell specific factors LAG-3 and Gpr83 was also decreased in S1P1-Tg;OT-II;Rag1−/− T cells (Fig. 1b). More pronounced effects were observed in the lamina propria, in which an extensive Foxp3+ population was induced in Ova-fed OT-II;Rag1−/− mice but not in S1P1-Tg;OT-II;Rag1−/− mice, despite similar numbers and distribution of total CD3+ cells in these mice (Fig. 1c). To formally exclude the contribution of the residual thymic derived nTreg cells, we crossed S1P1-Tg;OT-II mice with Foxp3gfp knockin mice that marked Foxp3 expression with GFP to distinguish Treg and conventional T cells14. We purified naive Foxp3− T cells from these mice and transferred them into wild-type immunocompetent mice. Administration of oral antigen to the recipients induced a sizable Foxp3+ population from wild-type donors, while the Foxp3+ population from S1P1-Tg donors was decreased by 70% (Supplementary Fig. 1). Taken together, S1P1 inhibits de novo induction of Foxp3-expressing iTreg cells from the peripheral T cell pool.
Figure 1. S1P1 inhibits de novo generation of Foxp3+ iTreg cells.
(a–c) Induction of Foxp3+ iTreg cells after feeding of OT-II;Rag1−/− and S1P1-Tg;OT-II;Rag1−/−mice with Ova in the drinking water for 5 days. (a) Foxp3 and CD25 expression in CD4 T cells and proportions and absolute numbers of Foxp3+ CD4 T cells. MLN, mesenteric lymph nodes. (b) mRNA expression of Foxp3, LAG-3 and Gpr83 in CD4 T cells isolated from MLN (n=3). (c) Distribution of Foxp3+ and CD3+ cells in the lamina propria. (d) Analysis of Foxp3 expression after naïve T cells from OT-II;Foxp3gfp and S1P1-Tg;OT-II;Foxp3gfp mice were stimulated with MLN-derived CD103+ DCs and the Ova peptide or anti-CD3 for 5 days. (e) Analysis of Foxp3 expression after wild-type (WT) and S1P1-Tg naïve T cells were activated with anti-CD3 and anti-CD28 in the presence of TGF-β (5 ng/ml). The right panels show proportions and absolute numbers of Foxp3+ and Foxp3− cells (n=3). (f) Kinetics of Foxp3 mRNA expression after wild-type and S1P1-Tg naïve cells were activated with anti-CD3 and anti-CD28 in the presence of TGF-β (5 ng/ml) for various times. Data represent three to four independent experiments.
A specialized subset of dendritic cells (DCs) in the mucosa expressing CD103 is tolerogenic by inducing Foxp3+ iTreg cells6,7. We purified CD103+ DCs from MLN and cultured them with naïve T cells from OT-II;Foxp3gfp mice (CD62LhiCD44loFoxp3−) in the presence of the cognate antigen or anti-CD3. Under these conditions, a substantial population of T cells from OT-II;Foxp3gfp mice was induced to express Foxp3, while S1P1-Tg;OT-II;Foxp3gfp T cells exhibited profound defects in Foxp3 induction (Fig. 1d). One key mechanism for CD103+ DC-mediated Treg cell differentiation involves the production of TGF-β6,7. Indeed, neutralizing TGF-β abrogated the function of these DCs to induce Foxp3 expression from wild-type and S1P1-Tg cells (data not shown). We conclude that S1P1 inhibits DC-induced iTreg generation.
Foxp3+ cells can be generated directly from naïve precursors by antigen stimulation in the presence of TGF-β15. When naïve cells were activated in the presence of TGF-β in an antigen-presenting cell (APC)-free condition, those expressing the S1P1 transgene were considerably impaired to differentiate into Foxp3+ iTreg cells (Fig. 1e). Wild-type and S1P1-Tg cells proliferated to a similar degree and comparably expressed apoptotic markers and Bcl2 (Fig. 1e and data not shown). Furthermore, crossing S1P1-Tg mice with Bcl2-transgenic animals failed to rectify the diminished iTreg differentiation (data not shown). Thus, the impaired iTreg generation was not due to altered T cell proliferation or survival, indicating that S1P1 inhibits TGF-β-mediated iTreg differentiation. We next explored the kinetics involved in S1P1-dependent inhibition of Foxp3 induction. In wild-type cells, Foxp3 mRNA was strongly induced at 24 h after TGF-β stimulation and continued to increase at 36–48 h. S1P1-Tg cells showed greatly reduced Foxp3 expression at 24–48 h (Fig. 1f). In addition, transduction of wild-type cells with S1P1-expressing retrovirus, followed by TGF-β treatment, resulted in diminished Foxp3 induction. Moreover, S1P1 exerted its inhibitory effect on Foxp3 induction even when transduction occurred 20–24 h after TGF-β stimulation (Supplementary Fig. 2), suggesting that S1P1 may interfere with sustained TGF-β signaling.
S1P1 inhibits iTreg generation and maintenance
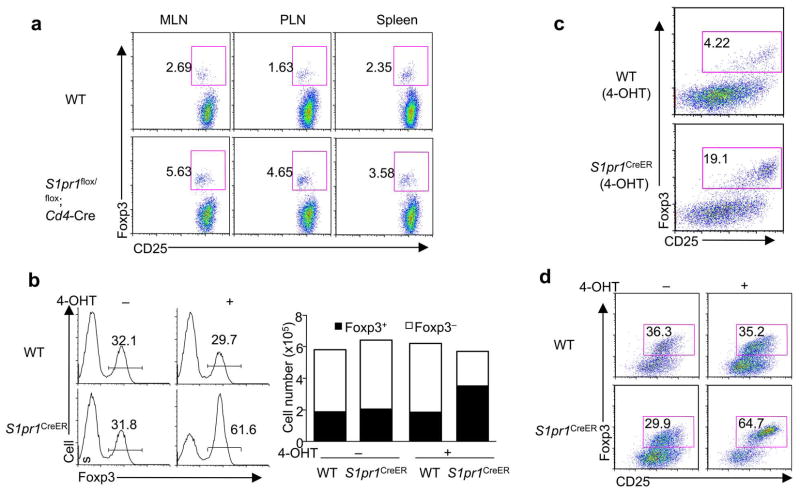
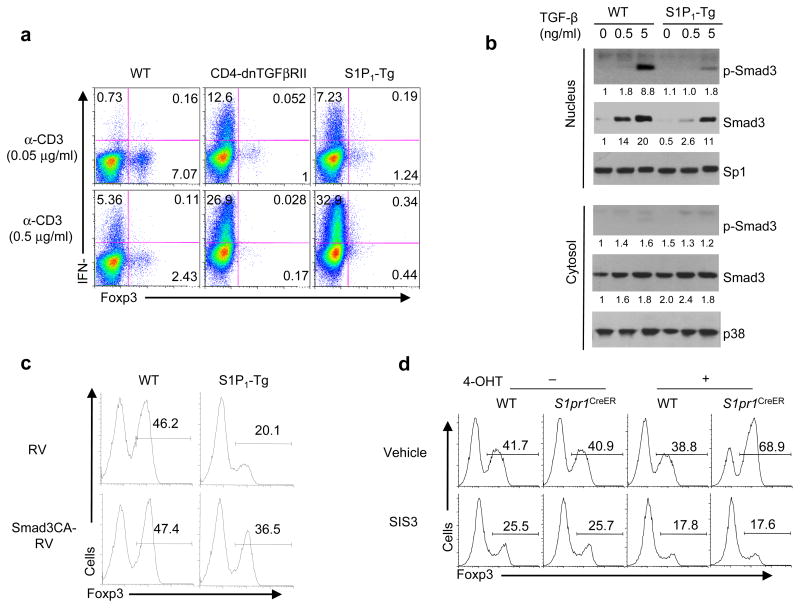
We then tested whether S1P1 is required to suppress iTreg differentiation. Given the marked reduction of peripheral T cells in S1pr1flox/flox mice crossed with Cd4-Cre transgenic mice14, we bred these mice onto the Foxp3gfp background and purified Foxp3− CD4 single-positive (CD4SP) thymocytes. When transferred into Rag1−/− mice, a subset of donor cells became Foxp3+. Deficiency of S1P1 resulted in an augmented Foxp3+ population, indicating increased iTreg generation in vivo (Fig. 2a). To more directly address the requirement of S1P1 in iTreg development from peripheral T cells, we crossed S1pr1flox/flox mice with Rosa26-Cre-ERT2 (called ‘S1pr1CreER mice’ here). After 4-hydroxytamoxifen (4-OHT) treatment, the floxed S1pr1 gene was deleted in naïve T cells (Supplementary Fig. 3a). When these cells were differentiated toward iTreg cells by exogenous TGF-β, they exhibited a higher frequency of the Foxp3+ population (Fig. 2b), associated with increased Foxp3 mRNA abundance (Supplementary Fig. 3b) and normal cell survival (data not shown). Moreover, CD103+ DC-induced Foxp3+ population was also enhanced in the absence of S1P1 (Fig. 2c). Therefore, S1P1 deficiency directly potentiates iTreg differentiation.
Figure 2. S1P1 is required to restrain the generation and maintenance of Foxp3+ iTreg cells.
(a) Analysis of Foxp3 expression 4 weeks after Foxp3− CD4SP thymocytes from wild-type and S1pr1flox/flox;Cd4-Cre mice were transferred into Rag1−/− mice. PLN, peripheral lymph nodes. (b) Analysis of Foxp3 expression after naïve T cells from wild-type and S1pr1CreER mice were treated with 4-OHT and then activated in the presence of TGF-β for iTreg differentiation. The right panels show proportions and absolute numbers of Foxp3+ and Foxp3− T cells. (c) Analysis of Foxp3 expression after naïve T cells from wild-type and S1pr1CreER mice were treated with 4- OHT and then activated by CD103+ DCs and anti-CD3, without any exogenous cytokines. (d) Analysis of Foxp3 expression in mature iTreg cells upon acute deletion of S1P1. Naïve T cells from wild-type and S1pr1CreER mice were differentiated into iTreg cells. Foxp3+ (GFP+) cells were sorted, treated with 4-OHT and cultured with IL-2. Foxp3 expression was analyzed 4–5 days later. Data represent three independent experiments.
Continued cells, Foxp3 Foxp3 expression is necessary for Treg function. As compared with nTreg expression in iTreg cells is less stable and depends upon continuous TGF-β signaling16. To test the involvement of S1P1 in the maintenance of Foxp3 expression, we generated and sorted Foxp3+ (GFP+) iTreg cells from S1pr1CreER mice, and then treated them with 4-OHT to induce S1pr1 deletion in the mature iTreg cells. Upon TGF-β withdrawal, control iTreg cells that retained S1P1 expression readily lost Foxp3 expression, whereas iTreg cells deficient in S1P1 contained a much greater Foxp3+ population with increased Foxp3 and CD25 expression (Fig. 2d). Conversely, Foxp3+ iTreg cells generated from S1P1-Tg mice were unable to sustain Foxp3 expression in the absence of TGF-β (Supplementary Fig. 4). Therefore, S1P1 negatively regulates the maintenance of Foxp3 expression in iTreg cells.
S1P1 drives TH1 differentiation
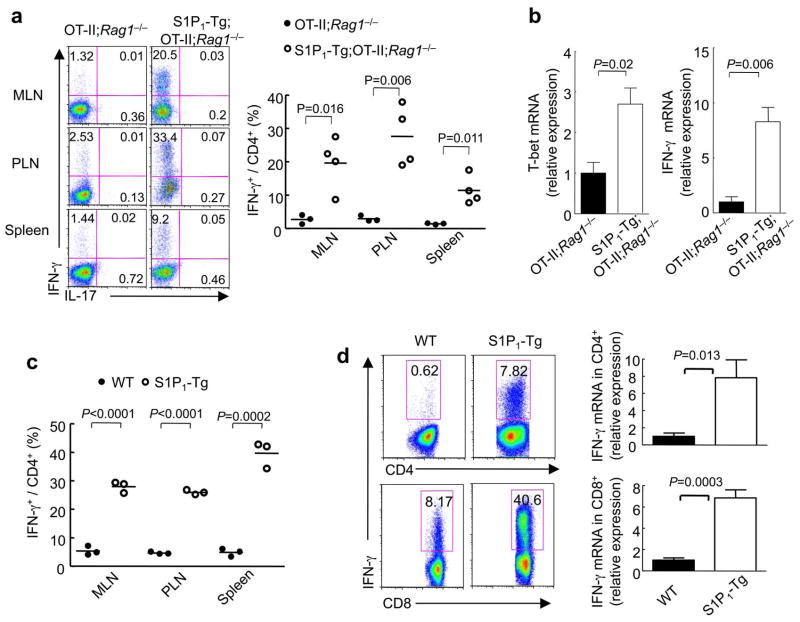
The inhibitory effects of S1P1 on iTreg induction and maintenance prompted us to investigate whether S1P1 diverges naïve T cells into alternative lineages using three in vivo systems. First, in the oral antigen model of OT-II;Rag1−/− mice, only a small fraction of IFN-γ+ cells was induced in OT-II;Rag1−/− mice, as would be expected for T cell responses under the tolerizing conditions. In contrast, S1P1-Tg;OT-II;Rag1−/− mice contained a markedly increased population (5–10 fold more abundant) of IFN-γ+ CD4 T cells in various lymphoid organs examined (Fig. 3a). Accordingly, quantitative RT-PCR identified elevated expression of IFN-γ and the TH1 transcriptional factor T-bet in S1P1-Tg cells following oral antigen exposure (Fig. 3b). IL-4 and IL-17 protein and RNA abundance were low and comparable between the two types of mice (Fig. 3a and data not shown), indicating selective differentiation of S1P1-Tg cells into the TH1 lineage.
Figure 3. S1P1 drives differentiation of TH1 cells.
(a,b) Analysis of TH1 differentiation after OT-II;Rag1−/− and S1P1-Tg;OT-II;Rag1−/− mice were fed with Ova in the drinking water for 5 days. (a) Expression of IFN-γ and IL-17 and proportions of IFN-γ+ CD4 T cells. (b) Quantitative RT-PCR analysis of T-bet and IFN-γ expression in CD4 T cells (n=3). (c) Proportions of IFN-γ+ CD4 T cells in gated donor cells from OT-II;Foxp3gfp and S1P1-Tg;OT-II;Foxp3gfp mice that were transferred to naïve wild-type mice and immunized with Ova intravenously. (d) IFN-γ expression in total CD4 and CD8 T cells from MLN of wild-type and S1P1-Tg mice, detected by intracellular staining 5 h post PMA/ionomycin stimulation (left), and quantitative RT-PCR after 24 h stimulation with anti-CD3/CD28 (right) (n=3–4). Data represent four independent experiments.
Second, we adoptively transferred T cells from OT-II or S1P1-Tg;OT-II mice (Thy1.1+) into naïve recipient and immunized recipients with Ova intravenously. Five days later, a substantially increased IFN-γ+ population was detected among S1P1-Tg donor-derived cells, as compared with wild-type donors (Fig. 3c). Also, secretion of IFN-γ, but not of IL-4 or IL-17, was enhanced in the S1P1-Tg cell transfer group after ex vivo peptide stimulation (data not shown).
Third, we determined the proportions of T cells capable of producing IFN-γ in S1P1-Tg mice by intracellular cytokine staining. Compared with MLN cells from wild-type mice, which had few IFN-γ producing CD4+ and CD8+ T cells, a higher percentage of S1P1-Tg cells produced IFN-γ (Fig. 3d). Quantitative RT-PCR revealed 7–8 fold more IFN-γ in S1P1-Tg CD4+ and CD8+ T cells as compared with the wild-type counterparts (Fig. 3d). Taken together, S1P1 promotes IFN-γ production and TH1 differentiation in vivo.
To establish whether S1P1 regulates intrinsic T cell differentiation, we sorted naïve T cells from wild-type and S1P1-Tg mice and cultured them under nonpolarizing conditions (TH0). A larger proportion of S1P1-Tg cells spontaneously differentiated into IFN-γ+ cells associated with increased T-bet expression (Supplementary Fig. 5a and data not shown). Higher IFN-γ expression was also observed in S1P1-Tg;OT-II cells (Supplementary Fig. 5a) and wild-type cells transduced with S1P1-expressing retrovirus (data not shown). Conversely, deletion of S1P1 diminished IFN-γ expression (Supplementary Fig. 5b). Therefore, S1P1 promotes IFN-γ expression and TH1 differentiation.
S1P1 directs reciprocal TH1 and iTreg differentiation
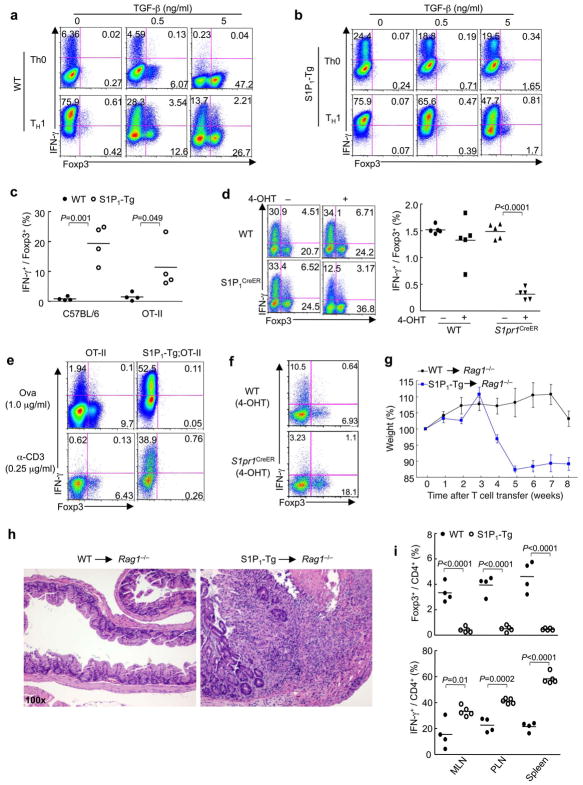
We next determined the mechanisms for the altered iTreg and TH1 differentiation in S1P1-Tg and deficient cells. TGF-β is a pleiotropic cytokine with pronounced effects on cell fate determination of multiple T cell lineages 17, but whether TGF-β directly coordinates development of TH1 and iTreg cells from naïve precursors is not well understood. We therefore differentiated wild-type naïve cells under TH0 and TH1 conditions in the presence of TGF-β and measured Foxp3 and IFN-γ expression simultaneously. As expected, a subset of cells expressed IFN-γ but few Foxp3+ cells were detected under nonpolarizing conditions. Addition of TGF-β induced Foxp3 and terminated IFN-γ expression. Under TH1 conditions, most T cells became IFN-γ positive, yet addition of TGF-β decreased IFN-γ+ population and simultaneously induced Foxp3+ population, resulting in the co-existence of TH1 and iTreg cells (Fig. 4a). The effects of TGF-β to promote Foxp3 over IFN-γ expression were profoundly lost in S1P1-Tg cells (Fig. 4b). In particular, when TGF-β was added to differentiating TH1 cells to foster development of both TH1 and iTreg cells, the ratio of IFN-γ+: Foxp3+ cells was reversed in S1P1-Tg cells (Fig. 4c). This reciprocal change was also observed in antigen-specific S1P1-Tg;OT-II T cells (Fig. 4c). Conversely, deletion of S1P1 resulted in the expansion of iTreg cells at the expense of TH1 cells (Fig. 4d). Therefore, S1P1 mediates reciprocal TH1 and iTreg differentiation in vitro.
Figure 4. S1P1 regulates reciprocal TH1 and iTreg differentiation and immune homeostasis in vivo.
(a,b) Analysis of IFN-γ and Foxp3 expression in wild-type (a) and S1P1-Tg (b) cells differentiated under the specified conditions. (c,d) Ratios of IFN-γ+ and Foxp3+ populations in wild-type and S1P1-Tg cells (c) and 4-OHT treated wild-type and S1pr1CreER cells (d) that were differentiated under TH1 conditions in the presence of TGF-β. (e,f) Analysis of IFN-γ+ and Foxp3+ populations in wild-type and S1P1-Tg cells (e) and 4-OHT treated wild-type and S1pr1CreER cells (f) that were activated by CD103+ DCs and Ova or anti-CD3, without any exogenous cytokines. (g-i) Analysis of T cell-dependent colitis and in vivo differentiation. Wild-type or S1P1-Tg naive T cells were transferred in combination with wild-type Treg cells (CD45.1+) into Rag1−/− mice. (g) Changes in body weight. (h) Representative intestine histology. (i) Proportions of Foxp3+ and IFN-γ+ CD4 T cells derived from naïve T cell donors. Data represent three independent experiments.
As an independent system of T cell differentiation, we primed T cells with tolerogenic CD103+ DCs, without any exogenous cytokines6,7. Under these conditions, distinct populations of Foxp3+ and IFN-γ+ cells were generated from wild-type cells. T cells with increased S1P1 signaling produced IFN-γ instead of Foxp3 (Fig. 4e), whereas those deficient in S1P1 preferentially developed into Foxp3+ Treg cells instead of IFN-γ+ TH1 cells (Fig. 4f). These results further indicate an intrinsic role of S1P1 in directing TH1 and iTreg lineage commitment.
Previous studies showed that TH1 cells and IFN-γ are pathogenic in colitis18, whereas iTreg cells act in synergy with nTreg cells to establish mucosal tolerance19. To determine the in vivo relevance of S1P1-mediated T cell differentiation, we transferred naïve T cells from wild-type or S1P1-Tg (CD45.2+) mice in conjunction with wild-type congenic (CD45.1+) Treg cells into Rag1−/− mice. In this system, in situ developed iTreg cells from naïve donors, together with nTreg donor cells, are required to control colitis19; wild-type nTreg cells were used here to circumvent the confounding effects in S1P1-Tg nTreg cells14. While there was minimal weight loss and inflammation in the wild-type co-transfer group, transfer of S1P1-Tg naïve cells and wild-type Treg cells resulted in significant weight loss associated with severe colitis and leukocyte infiltration (Fig. 4g,h). To dissect the underlying mechanisms, we measured expression of Foxp3 and IFN-γ in cells derived from naïve cell donors (CD45.2+). As compared with wild-type donor cells, we observed fewer Foxp3+ iTreg cells from S1P1-Tg mice and a significant increase of IFN-γ-expressing T cells (Fig. 4i). Therefore, S1P1 controls the reciprocal relationship between iTreg and TH1 cells in vivo.
Discrete mechanisms in iTreg and TH1 differentiation
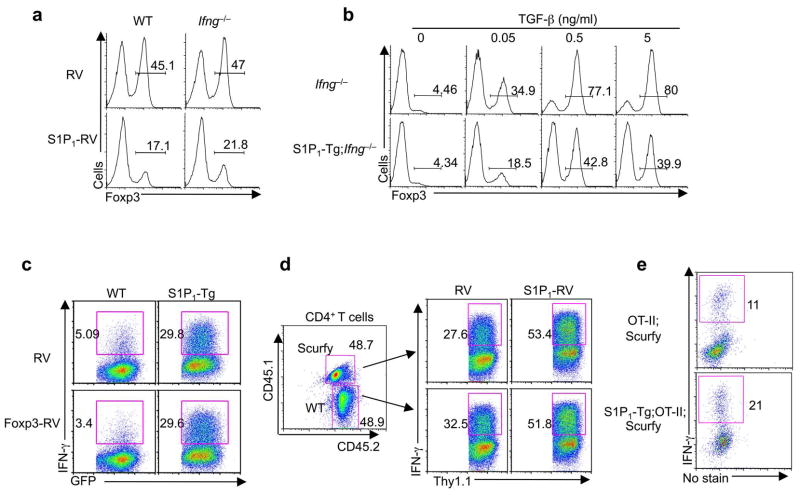
We next determined whether control of TH1 and iTreg differentiation by S1P1 is interdependent. IFN-γ can inhibit iTreg generation20, although conflicting conclusions also exist21. We tested whether inhibition of iTreg differentiation by S1P1 is a secondary consequence of increased IFN-γ production. Retroviral transduction of S1P1 into either Ifng−/− or wild-type cells resulted in diminished Foxp3+ induction under iTreg conditions (Fig. 5a). Also, the S1P1 transgene downregulated iTreg generation in both Ifng+/+ and Ifng−/− backgrounds (Fig. 5b). Thus, S1P1 regulates iTreg differentiation independent of its role in facilitating IFN-γ expression.
Figure 5. S1P1 mediates iTreg and TH1 differentiation through discrete mechanisms.
(a) Analysis of Foxp3 expression after wild-type and Ifng−/− naïve T cells were transduced with control (RV) and S1P1-expressing retrovirus (S1P1-RV) and activated in the presence of TGF-β differentiation. (b) Analysis of Foxp3 expression after naïve T cells from Ifng −/− and for iTregS1P1-Tg;Ifng−/− mice were differentiated in the presence of different doses of TGF-β. (c) Analysis of IFN-γ expression in wild-type and S1P1-Tg cells activated under TH0 conditions and transduced with control (RV) and Foxp3-expressing retrovirus (Foxp3-RV) linked with a GFP reporter; gated GFP+ cells are shown in the FACS plots. (d) Analysis of IFN-γ expression in Foxp3-deficient cells. Wild-type (CD45.2+) and Scurfy (CD45.1+.2+) bone marrow cells were mixed at 1:1, and transferred into sublethally irradiated Rag1−/− mice. At 6 weeks after reconstitution, naïve T cells from the two donor populations were purified, activated under TH0 conditions, and transduced with control (RV) and S1P1-expressing retrovirus (S1P1-RV) linked with a Thy1.1 reporter; gated Thy1.1+ cells are shown in the FACS plots. (e) Analysis of IFN-γ expression in Foxp3-deficient cells after naïve T cells from OT-II and S1P1-Tg;OT-II mice bred onto the Scurfy background were activated by Ova and irradiated splenic APC. Data represent three independent experiments.
Conversely, we tested whether the reduced Foxp3 expression in S1P1-Tg cells is responsible for their enhanced IFN-γ expression. To this end, we transduced the Foxp3 gene into wild-type and S1P1-Tg cells, and found that forced Foxp3 expression had no effects at reducing the expression of IFN-γ in S1P1-Tg cells (Fig. 5c). To further assess the function of Foxp3 in S1P1-mediated TH1 differentiation, we examined whether Foxp3 deficiency affects the ability of S1P1 to drive TH1 differentiation. To prevent the lymphoproliferative autoimmune phenotype due to Foxp3 deficiency, we constructed mixed bone marrow chimeras by co-transferring bone marrow cells from Foxp3-deficient Scurfy mice (CD45.1+.2+) and wild-type mice (CD45.2+) into Rag1−/− recipients; the presence of functional Treg cells derived from the wild-type donors prevented autoimmune activation of Scurfy T cells14. Naïve T cells from these two donor populations were purified and transduced with S1P1-expressing retrovirus. S1P1 expression was equally effective to promote IFN-γ expression in wild-type and Foxp3-deficient cells (Fig. 5d). As an independent approach, we bred OT-II and S1P1-Tg;OT-II mice with Scurfy mice, in which the TCR transgene ameliorated the confounding effects of autoimmune inflammation22. Naïve T cells from these mice were stimulated with Ova peptide, and increased TH1 differentiation was observed in those expressing the S1P1 transgene (Fig. 5e). Therefore, either ectopic expression or deficiency of Foxp3 did not prevent S1P1 from driving TH1 differentiation, suggesting that S1P1 mediates TH1 differentiation independent of its effects at inhibiting Foxp3 expression.
Aged S1P1-Tg mice developed high titers of autoantibodies, concomitant with spontaneous T cell activation14. Since IFN-γ production is associated with the pathogenesis of systemic autoimmune disease, we tested whether increased IFN-γ in S1P1-Tg cells contributes to the breakdown of immune tolerance. As compared with S1P1-Tg mice, S1P1-Tg;Ifng−/− mice contained significantly reduced titers of anti-double stranded (ds) DNA antibody (data not shown). In contrast, enhanced expression of CD44 on S1P1-Tg cells, indicative of their spontaneous activation in vivo14, was observed irrespective of the IFN-γ background (data not shown), consistent with our previous observation that spontaneous T cell activation was mainly due to defective Treg cells in S1P1-Tg mice14. Therefore, S1P1 regulates immune homeostasis through both Treg-dependent and independent mechanisms.
S1P1 antagonizes TGF-β-Smad3 signaling
We noticed that the exacerbated colitis following S1P1-Tg cell transfer phenocopied the abnormalities in T cells with transgenic expression of a dominant negative TGF-β receptor (CD4-dnTGFβRII) in a similar model23,24. Moreover, mice with impaired TGF-β receptor signaling in T cells spontaneously differentiate into TH1 cells in vivo25,26. We hypothesize that S1P1 mediates reciprocal TH1 and iTreg differentiation by antagonizing TGF-β receptor signaling. To test this hypothesis, we first compared the response of S1P1-Tg cells with that of CD4-dnTGFβRII cells. In response to CD103+ DCs, both S1P1-Tg and CD4-dnTGFβRII T cells were skewed into IFN-γ+ TH1 cells rather than Foxp3+ Treg cells (Fig. 6a). Similarly, in response to exogenous TGF-β, CD4-dnTGFβRII and S1P1-Tg cells had similar defects in upregulating Foxp3; instead these cells expressed IFN-γ (Supplementary Fig. 6). Therefore, CD4-dnTGFβRII mice phenocopy the abnormalities seen in S1P1-Tg mice, suggesting that S1P1 likely affects TGF-β receptor signaling.
Figure 6. S1P1 attenuates TGF-β-Smad3 signaling.
(a) Analysis of Foxp3 expression in wild-type, CD4-dnTGFβRII, and S1P1-Tg cells activated by CD103+ DCs and anti-CD3. (b) Analysis of Smad3 nuclear translocation after 2 d stimulation of wild-type and S1P1-Tg cells. The numbers below each panel show the quantification of band intensity relative to the loading controls. (c) Analysis of Foxp3 expression in wild-type and S1P1-Tg cells transduced with control (RV) and constitutively active Smad3 retrovirus (Smad3CA-RV) and activated in the presence of TGF-β for iTreg differentiation. (d) Analysis of Foxp3 expression in 4-OHT treated wild-type and S1pr1CreER cells that were activated in the presence of TGF-β and a Smad3 inhibitor SIS3 (5 μM).
We next investigated the molecular mechanisms by which S1P1 and TGF-β receptor interact. Smad3 is an important transcription factor to mediate TGF-β effects in iTreg generation27. TGF-β stimulation resulted in prolonged activation of Smad3 in wild-type T cells (Supplementary Fig. 7a). Immediately after TGF-β stimulation, Smad3 phosphorylation was comparable between wild-type and S1P1-Tg cells (data not shown). However, S1P1-Tg cells were unable to maintain Smad3 phosphorylation after 16 h of TGF-β treatment, and more pronounced reduction was observed at 24–48 h of stimulation (Supplementary Fig. 7a). Accordingly, nuclear translocation of total and phosphorylated Smad3 was profoundly reduced in S1P1-Tg cells after 2 days of stimulation (Fig. 6b). To understand the importance of sustained Smad3 activation, we transduced a dominant negative Smad3 molecule into wild-type cells at 24 h after initiation of iTreg differentiation, which was effective at inhibiting Foxp3 induction (Supplementary Fig. 7b). Moreover, increased p-Smad3 was observed in S1P1-deficient cells at and after 24 h of stimulation (Supplementary Fig. 8). To directly determine whether Smad3 mediates S1P1 function, we introduced constitutively active Smad3 into S1P1-Tg cells, which restored Foxp3 expression in S1P1-Tg cells by up to 60% (Fig. 6c). Conversely, treatment of S1P1-deficient T cells with the Smad3 inhibitor reduced iTreg differentiation (Fig. 6d). Therefore, S1P1 antagonizes TGF-β-dependent effects on T cell differentiation mainly by attenuating Smad3 signaling.
The S1P1-mTOR axis is targeted by immunosuppressants
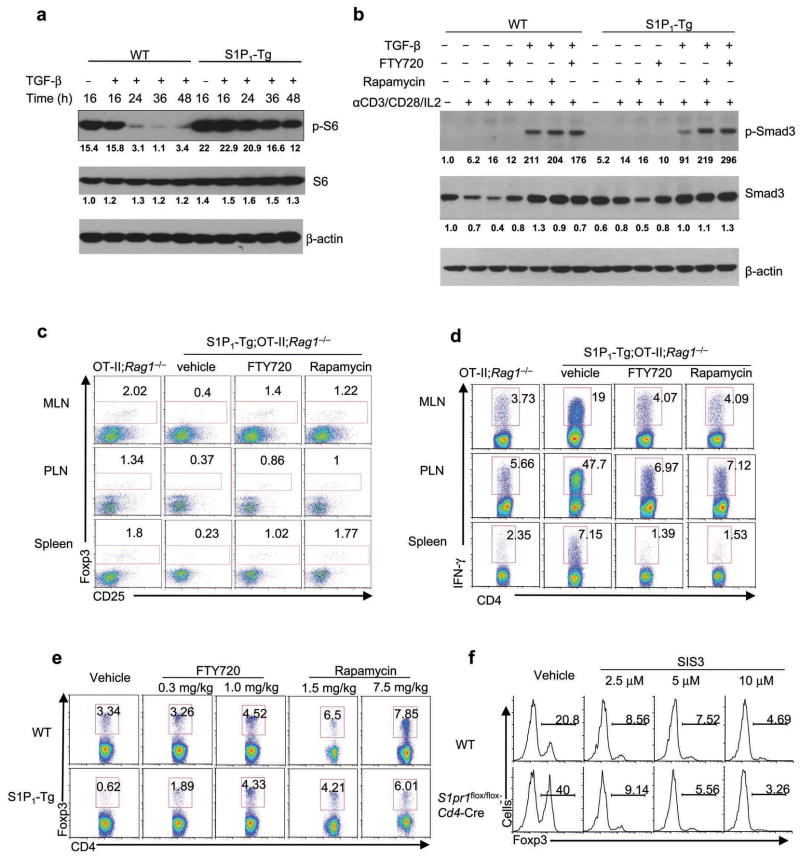
We further explored the signaling pathway utilized by S1P1 to antagonize TGF-β-Smad3 signaling. The Akt-mTOR pathway has recently been shown to restrain iTreg generation28–31. However, S1P1 activates mTOR in nTreg cells but not naïve T cells immediately after TCR stimulation14. Given the effects of S1P1 on sustained but not early Smad3 activation, we examined mTOR activity at later time points by assessing the phosphorylation of the ribosomal protein S6, a well-established target of mTOR. S1P1-Tg cells exhibited increased amounts of p-S6 after 16 h of TCR stimulation, and more prominent increase was observed at later time points, indicating an important role for S1P1 to sustain mTOR activation (Fig. 7a).
Figure 7. S1P1-mTOR axis is targeted by FTY720 and rapamycin.
(a) Kinetics of mTOR activation in wild-type and S1P1-Tg cells stimulated for various times. (b) Analysis of Smad3 and S6 activation in wild-type and S1P1-Tg cells treated with FTY720 and rapamycin and activated for 2 days. (c,d) Analysis of Foxp3 (c) and IFN-γ expression (d) in OT-II;Rag1−/− and S1P1-Tg;OT-II;Rag1−/− mice fed with Ova in the drinking water for 5 days, accompanied by daily FTY720 (1 mg/kg) or rapamycin (3 mg/kg) treatment. (e) Analysis of Foxp3 expression in thymic CD4SP cells from wild-type and S1P1-Tg mice treated daily with FTY720 and rapamycin for a total of 5 days. Data represent three independent experiments. (f) Induction of Foxp3 in thymic Treg precursors from wild-type and S1pr1flox/flox;Cd4-Cre mice stimulated with IL-2 (50 U/ml) in the presence of SIS3.
This newly revealed role of S1P1-mediated reciprocal differentiation of iTreg and TH1 cells led us to examine possible therapeutic implications. Both rapamycin (Sirolimus), an inhibitor of mTOR, and FTY720 have been shown to modulate iTreg cells28–30,32. To address whether FTY720 and rapamycin share an immunosuppressive mechanism, we first examined mTOR activation in S1P1-Tg cells treated with FTY720 or rapamycin. FTY720 and rapamycin both decreased p-S6 (Supplementary Fig. 9). Moreover, both treatments restored p-Smad3 activity in S1P1-Tg cells to that observed in wild-type cells (Fig. 7b). These results identified an S1P1-mTOR axis that acts to interfere with Smad3 signaling and is targeted by immunosuppressive drugs.
To further investigate the effects of the immunosuppressants on immune responses, we administered oral Ova antigen to OT-II;Rag1−/− mice and treated them daily with FTY720 or rapamycin. Such treatments increased iTreg cells and reduced IFN-γ+ TH1 cells (Supplementary Fig. 10). Treatment of S1P1-Tg;OT-II;Rag1−/− mice with FTY720 or rapamycin reversed the altered differentiation of iTreg and TH1 cells, to similar to untreated wild-type control mice (Fig. 7c,d). Thus, FTY720 and rapamycin modulate reciprocal TH1 and iTreg differentiation in vivo. To ascertain whether this reflects intrinsic effects, we treated T cells with these drugs in vitro. Treatment of S1P1-Tg cells with FTY720 or rapamycin increased iTreg and reduced TH1 differentiation (Supplementary Fig. 11). Taken together, FTY720 and rapamycin directly modulate reciprocal differentiation of iTreg and TH1 cells by targeting the S1P1-mTOR axis.
We next asked whether FTY720 and rapamycin also target thymic nTreg cells. We treated wild-type and S1P1-Tg mice with FTY720 or rapamycin for 5 days, and assessed the frequency and number of Foxp3+ CD4SP thymocytes. As previously reported, S1P1-Tg mice contained decreased thymic nTreg cells14. After FTY720 or rapamycin treatment, nTreg cells in these mice were similar to wild-type mice (Fig. 7e). Accordingly, the diminished in vitro differentiation of S1P1-Tg nTreg precursors (CD25+Foxp3–) into mature Foxp3+ cells was rescued by FTY720 and rapamycin (Supplementary Fig. 12). Therefore, FTY720 and rapamycin affect thymic development of nTreg cells by targeting the S1P1-mTOR axis. To further explore whether Smad3 mediates S1P1 signaling in thymic nTreg cell differentiation, we treated nTreg precursors from wild-type and S1pr1flox/flox;Cd4-Cre mice with the Smad3 inhibitor SIS3. Blocking Smad3 activity ablated the increased nTreg differentiation observed in precursors lacking S1P1 (Fig. 7f). Although TGF-β is required for Treg survival in the thymus33, SIS3 treatment reduced Foxp3 induction even in Bcl2-Tg mice (data not shown), indicating that the effect of Smad3 in nTreg differentiation is independent of cell survival. Taken together, the drug-sensitive S1P1-mTOR axis interferes with Smad3 signaling to control development of both nTreg and iTreg subsets.
Sphingosine kinases control T cell differentiation
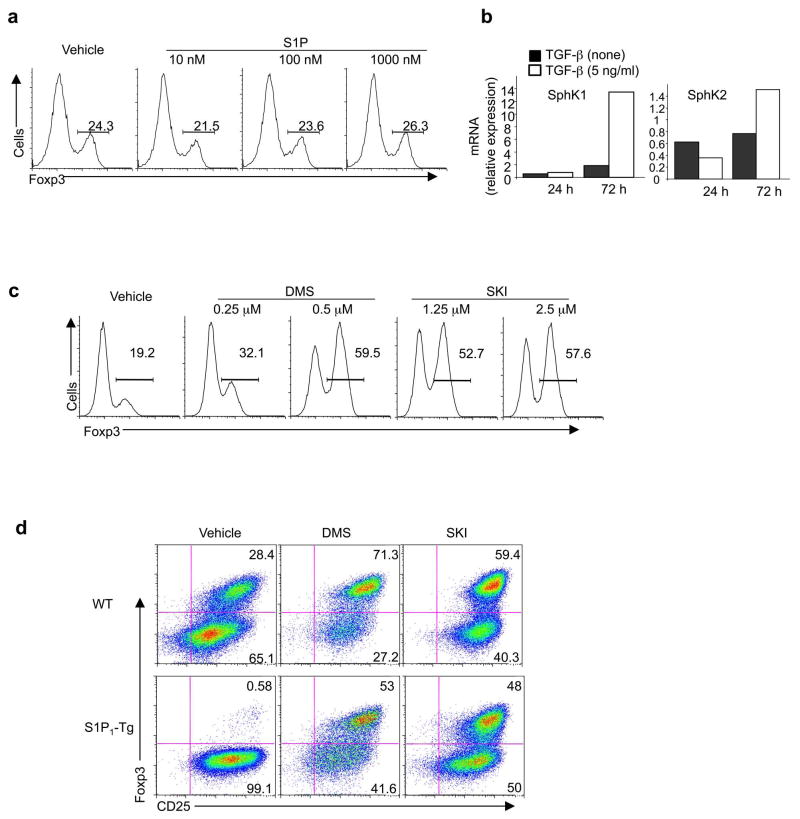
Given an essential role of S1P1 in Treg cell differentiation, we explored mechanisms of S1P1 activation. Supplement of exogenous S1P to naïve T cells did not alter their differentiation into iTreg cells (Fig. 8a). We thus tested whether the endogenously produced S1P is involved. S1P is synthesized by one of the two sphingosine kinases (SphKs) on the substrate sphingosine34. In differentiating T cells, SphK1 was strongly upregulated by the treatment of TGF-β (Fig. 8b), suggesting the likely involvement of intrinsic SphK activity in iTreg cell generation. To test this hypothesis, we treated naïve T cells with N,N,-dimethylsphingosine (DMS) or sphingosine kinase inhibitor (SKI), two widely used inhibitors of SphK activity. Blocking SphK activity strongly upregulated Foxp3 induction (Fig. 8c). Treatment of S1P1-Tg cells with DMS or SKI restored their abilities to differentiate into iTreg cells (Fig. 8d). Similarly, blocking SphK activity considerably rectified the defects of S1P1-Tg cells in the reciprocal TH1 and Treg cell differentiation (Supplementary Fig. 13a). Thus, T cell differentiation mediated by S1P1 is dependent upon intrinsic SphKs, whose expression is upregulated by TGF-β as a feedback mechanism to limit TGF-β responses (Supplementary Fig. 13b).
Figure 8. SphK activity regulates T cell differentiation.
(a) Analysis fo Foxp3 expression in wild-type cells treated with various concentrations of S1P in a serum-free medium. (b) Analysis of SphK1 and SphK2 mRNA expression in cells activated in the absence or presence of TGF-β. Levels in naïve T cells were set to 1. (c) Analysis of Foxp3 expression after naïve T cells were pre-treated with DMS or SKI, and activated under iTreg conditions for 5 days. (d) Analysis of Foxp3 and CD25 expression in wild-type and S1P1-Tg naïve T cells were pre-treated with DMS (0.25 μM) or SKI (2.5 μM), and activated under iTreg conditions for 5 days. Data represent three independent experiments.
DISCUSSION
Our results demonstrate that differentiation of TH1 and Treg cells is reciprocally regulated. S1P1 is a switch factor that drives the development of TH1 cells at the expense of Treg generation. S1P1 function is dependent upon endogenous SphK activity, and it signals through mTOR and intersects with TGF-β signaling mainly by attenuating the sustained Smad3 activity. Although TGF-β and mTOR have pleiotropic functions in regulating multiple CD4 T cell lineages17,30, the interplay between S1P1-mTOR and TGF-β-Smad3 signaling with dynamic kinetics selectively controls the reciprocal differentiation of TH1 and Treg cells. We propose that this novel lineage commitment process controls the balance between immunity and tolerance and contributes to mechanisms of action for immunosuppressive therapy.
The dichotomy of TH1 and Treg cell fate determination is reminiscent of the reciprocal differentiation of iTreg and TH17 cells 1,4. Moreover, the effect on T cell differentiation by S1P1, a G protein-coupled receptor for the endogenous bioactive lipid, is analogous to the control of reciprocal Treg and TH17 differentiation mediated by non-cytokine receptors such as retinoid acid receptor and aryl hydrocarbon receptor that recognize certain endogenous metabolites and environmental toxins5–9. These results collectively establish a new paradigm of T cell lineage specification controlled by non-cytokine immune modulators. Unlike inhibition of TH17 cell differentiation by Foxp322,35,36, Foxp3 ectopic expression or complete ablation did not prevent S1P1 from driving TH1 differentiation. Moreover, S1P1 blocked iTreg generation independent of its effects to drive TH1 differentiation. Thus, S1P1 regulates TH1 and iTreg differentiation through discrete mechanisms, further supporting the reciprocal nature of these two lineages.
Elevated S1P1 expression is associated with various autoimmune diseases37. A direct role for S1P1 in immune homeostasis is highlighted by the development of autoimmunity in aged S1P1-Tg mice14, which we attributed to defects in both Treg and naïve T cells. Also, transfer of either regulatory or naïve S1P1-Tg cells into Rag1−/− mice exacerbated the development of colitis14. These observations are reminiscent of TGF-β-dependent immune homeostasis via Treg-dependent and independent mechanisms25. Interestingly, premature egress of CD4+CD8+ thymocytes into peripheral lymphoid organs and tissues has been observed in another strain of S1P1-transgenic mice that utilized an artificial promoter to drive the transgene expression in both T and B cells38. We did not observe these alterations in multiple lines of founder mice expressing the S1P1 transgene throuhg the T cell-selective CD2 promoter/enhancer13 (data not shown). The simplest interpretation for the discordance is the use of different promoters for the transgene expression. Importantly, diminished thymic nTreg cells were apparent in both types of transgenic mice14,38, highlighting the key function of S1P1 in Treg cell differentiation.
S1P1 attenuates TGF-β-Smad3 function via signaling through mTOR. Even though Smad3 is activated within minutes after TGF-β stimulation, the sustained Smad3 activity, which is antagonized by the S1P1-mTOR axis, is essential for iTreg differentiation. This is in agreement with the observed 48 h delay in Foxp3 expression following TGF-β treatment16. The effect of S1P1 on mTOR signaling in naive T cells is distinct from its function in nTreg cells, in which it facilitates immediate mTOR activation14, suggesting cell context-specific regulation of mTOR signaling. Despite this, the S1P1-mTOR axis negatively regulates the differentiation of both thymic nTreg cells and extrathymic iTreg cells, via antagonizing TGF-β-Smad3 signaling. Although the roles of TGF-β in nTreg development remain controversial33,39, a requirement for Smad3 signaling in this process is established by the significant reduction of thymic nTreg cells in Smad3−/− mice40. This probably reflects the engagement of multiple and potentially opposing signaling pathways by TGF-β, as well as the pleiotropic effects of TGF-β on T cell development and homeostasis17. Taken together, while diverse mechanisms promote the generation of nTreg and iTreg cells3, a common pathway involving S1P1-mTOR and the antagonistic interactions with TGF-β-Smad3 signaling negatively controls the differentiation of both Treg subsets.
mTOR signaling is necessary for the differentiation of multiple effector T cells including TH1, TH2 and TH17 cells while at the same time, inhibiting iTreg generation30. Here we found that the S1P1-mTOR axis selectively controls TH1 and Treg differentiation, suggesting that other receptors may mediate mTOR activation for TH2 and TH17 differentiation. Although previous reports showed that a human S1P1 transgene is capable of promoting TH2 and TH17 cell generation in vitro41,42, we found no evidence of S1P1 involvement in these processes upon antigen stimulation in vivo. Whether S1P1 regulates these processes under more defined conditions requires further testing. Additional molecules involved in Akt-mTOR signaling, including Rictor (mTORC2), Cbl-b, Foxo1 and Foxo3, has recently been shown to modulate T cell responses30,43,44. Given the potent effects of S1P1 on Treg cell induction and maintenance and TH1 cell generation, S1P1 may engage multiple pathways associated with Akt-mTOR. Furthermore, direct interactions between Smad3 and the Akt, mTOR and Foxo pathways have been observed outside the immune system45,46, suggesting that Smad3 may integrate these diverse pathways to direct T cell differentiation.
S1P is maintained at high levels in the blood and lymph by the actions of SphKs but kept at low levels in lymphoid organs by S1P lyase. Separate sources appear to provide S1P to plasma and lymph47, and a recent study indicates that neural crest-derived pericytes produce S1P to induce thymocyte egress38. Combining pharmacological and genetic approaches, we found that S1P1-mediated regulation of T cell differentiation requires endogenous SphK activity. Although exogenously added S1P has no apparent effects on Treg cell generation, we cannot fully exclude the contribution of the exogenous source of S1P in this process, because partioning of S1P distribution might not be recapitulated in the isolated cell cultures. Nonetheless, our results clearly demonstrate a key role for the endogenously produced S1P in T cell differentiation in autocrine and/or paracrine manners. Moreover, SphK1 expression is strongly upregulated by TGF-β stimulation, which likely serves as a feedback mechanism to restrain prolonged TGF-β effects on Foxp3 expression. In growth factor-activated cells, S1P has been found to be produced and secreted out of the cell to activate S1P receptors in its vicinity in a model known as “inside-out” signaling of S1P34. To our knowledge, our work represents the first time to identify the similar modes of action for S1P in T cell responses.
We further demonstrated that S1P1 links fundamental processes of T cell lineage commitment to immunosuppressive therapy. FTY720 and rapamycin are thought to affect distinct molecular and cellular pathways in T cells by acting on S1P1 to induce lymph node sequestration and on mTOR to block cell cycle entry, respectively10,31. Here we found that these two potent immunosuppressants target the same S1P1-mTOR axis to modulate the balance between pro-inflammatory TH1 and anti-inflammatory Treg cells in vivo. These effects are likely to be T cell intrinsic, as we were able to recapitulate their functions using in vitro culture systems. Therefore, regulation of T cell lineage choices by the S1P1-mTOR axis can be further explored to develop novel therapeutics for autoimmunity and transplant rejection.
Supplementary Material
Acknowledgments
The authors acknowledge R. Proia (National Institutes of Health) for S1pr1flox/flox mice, A. Rudensky (Memorial Sloan-Kettering Institute) for Foxp3gfp knockin mice, T. Ludwig (Columbia University) for Rosa26-Cre-ERT2 mice, D. Littman (New York University) for Foxp3-expressing retroviral constructs, D. Green for Bcl2-Tg mice and stimulating discussions, and R. Cross, G. Lennon and S. Morgan for cell sorting. This work is supported by US National Institutes of Health (K01 AR053573 and administrative supplement, R01 NS064599, and Cancer Center Support Grant CA021765), the Arthritis Foundation, the Lupus Research Institute, and the American Lebanese Syrian Associated Charities (to H.C.).
Footnotes
AUTHOR CONTRIBUTIONS
G.L. designed and performed in vivo and cellular experiments and contributed to writing the manuscript; K.Y. designed and performed biochemical analyses and cellular and molecular experiments; S.B. contributed to in vivo and cellular experiments and gene expression analysis; S.S. contributed to cell isolation and gene expression analysis and managed the mouse colony; H.C designed experiments, wrote the manuscript, and provided overall direction.
References
- 1.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 5.Mucida D, et al. Reciprocal Th-17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 6.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 9.Quintana FJ, et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 10.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kappos L, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 12.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 13.Chi H, Flavell RA. Cutting edge: regulation of T cell trafficking and primary immune responses by sphingosine 1-phosphate receptor 1. J Immunol. 2005;174:2485–2488. doi: 10.4049/jimmunol.174.5.2485. [DOI] [PubMed] [Google Scholar]
- 14.Liu G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-β. J Immunol. 2007;178:7667–7677. doi: 10.4049/jimmunol.178.12.7667. [DOI] [PubMed] [Google Scholar]
- 17.Li MO, Flavell RA. TGF-β: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 19.Haribhai D, et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei J, et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, et al. Role of IFN-γ in induction of Foxp3 and conversion of CD4+CD25−s T cells to CD4+ Tregs. J Clin Invest. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 24.Fahlen L, et al. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 28.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sehrawat S, Rouse BT. Anti-Inflammatory Effects of FTY720 against Viral-Induced Immunopathology: Role of Drug-Induced Conversion of T Cells to Become Foxp3+ Regulators. J Immunol. 2008;180:7636–7647. doi: 10.4049/jimmunol.180.11.7636. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–653. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou L, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitano M, et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- 38.Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328:1129–1135. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, et al. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 40.Martinez GJ, et al. Smad3 differentially regulates the induction of regulatory and inflammatory T cell differentiation. J Biol Chem. 2009;284:35283–35286. doi: 10.1074/jbc.C109.078238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Huang MC, Goetzl EJ. Type 1 sphingosine 1-phosphate G protein-coupled receptor (S1P1) mediation of enhanced IL-4 generation by CD4 T cells from S1P1 transgenic mice. J Immunol. 2007;178:4885–4890. doi: 10.4049/jimmunol.178.8.4885. [DOI] [PubMed] [Google Scholar]
- 42.Huang MC, Watson SR, Liao JJ, Goetzl EJ. Th17 augmentation in OTII TCR plus T cell-selective type 1 sphingosine 1-phos–ate receptor double transgenic mice. J Immunol. 2007;178:6806–6813. doi: 10.4049/jimmunol.178.11.6806. [DOI] [PubMed] [Google Scholar]
- 43.Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merkenschlager M, von Boehmer H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J Exp Med. 2010;207:1347–1350. doi: 10.1084/jem.20101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated Smad3 activation. EMBO J. 2006;25:58–69. doi: 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 47.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, et al. Transforming growth factor beta-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci USA. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang K, et al. Hsp90 regulates activation of interferon regulatory factor 3 and TBK-1 stabilization in Sendai virus-infected cells. Mol Biol Cell. 2006;17:1461–1471. doi: 10.1091/mbc.E05-09-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.