Summary
Background
Many infants admitted to hospital undergo repeated invasive procedures. Oral sucrose is frequently given to relieve procedural pain in neonates on the basis of its effect on behavioural and physiological pain scores. We assessed whether sucrose administration reduces pain-specific brain and spinal cord activity after an acute noxious procedure in newborn infants.
Methods
In this double-blind, randomised controlled trial, 59 newborn infants at University College Hospital (London, UK) were randomly assigned to receive 0·5 mL 24% sucrose solution or 0·5 mL sterile water 2 min before undergoing a clinically required heel lance. Randomisation was by a computer-generated randomisation code, and researchers, clinicians, participants, and parents were masked to the identity of the solutions. The primary outcome was pain-specific brain activity evoked by one time-locked heel lance, recorded with electroencephalography and identified by principal component analysis. Secondary measures were baseline behavioural and physiological measures, observational pain scores (PIPP), and spinal nociceptive reflex withdrawal activity. Data were analysed per protocol. This study is registered, number ISRCTN78390996.
Findings
29 infants were assigned to receive sucrose and 30 to sterilised water; 20 and 24 infants, respectively, were included in the analysis of the primary outcome measure. Nociceptive brain activity after the noxious heel lance did not differ significantly between infants who received sucrose and those who received sterile water (sucrose: mean 0·10, 95% CI 0·04–0·16; sterile water: mean 0·08, 0·04–0·12; p=0·46). No significant difference was recorded between the sucrose and sterile water groups in the magnitude or latency of the spinal nociceptive reflex withdrawal recorded from the biceps femoris of the stimulated leg. The PIPP score was significantly lower in infants given sucrose than in those given sterile water (mean 5·8, 95% CI 3·7–7·8 vs 8·5, 7·3–9·8; p=0·02) and significantly more infants had no change in facial expression after sucrose administration (seven of 20 [35%] vs none of 24; p<0·0001).
Interpretation
Our data suggest that oral sucrose does not significantly affect activity in neonatal brain or spinal cord nociceptive circuits, and therefore might not be an effective analgesic drug. The ability of sucrose to reduce clinical observational scores after noxious events in newborn infants should not be interpreted as pain relief.
Funding
Medical Research Council.
Introduction
International clinical guidelines recommend that oral sucrose is given to relieve procedural pain in neonates.1 These recommendations are based on results from several randomised controlled clinical trials that conclude that sucrose is effective in reducing pain in preterm and term neonates.2 Because many infants admitted to hospital undergo repeated invasive procedures,3,4 and because there is increasing evidence of short-term and long-term adverse neurodevelopmental consequences,5–9 assessment of the effectiveness of sucrose analgesia in this patient group is essential. As a result, 44 randomised controlled trials investigating the effectiveness of sucrose analgesia in newborn infants are included in a recent Cochrane Review.2
A major challenge in analgesic trials in the infant population is definition of a reliable, quantitative outcome measurement of pain because verbal reports and visual analogue scales cannot be used.5,10 The most commonly used outcome measures are based on behavioural and physiological observations, and many validated composite pain measurement instruments, such as the premature infant pain profile (PIPP), are based on these observations.11–13 These methods might not, however, be an appropriate outcome measure for neonatal analgesic trials14–16 because they are largely based on human observation and judgment,17 and, whereas pain experience and pain behaviour are linked in adults,18 they might not be linked in neonates. For example, infants who do not display a change in facial expression after tissue damaging procedures might still display significant cortical responses,19 which suggests that infant behaviour is not a simple indication of pain activity in the brain. Many conditions, such as immaturity, neurological damage, and maternal drug misuse, can affect integrated sensorimotor function and consequent behaviour,20–22 but might not necessarily affect sensory pain processing.
Single heel lances evoke specific nociceptive brain activity recorded with neonatal electroencephalography (EEG)9,23 and spinal nociceptive reflexes recorded with electromyography (EMG).24 We undertook a randomised controlled trial of sucrose analgesia with use of this specific nociceptive brain activity as a direct measure of infant pain.
Methods
Study design and patients
We undertook a double-blind randomised study on the postnatal ward in the Elizabeth Garrett Anderson and Obstetric Wing, University College Hospital (UCH; London, UK), between Feb 25, 2009, and March 25, 2010. The participants were healthy newborn infants, born at 37–43 postmenstrual weeks, and less than 8 days old.
Medical charts were reviewed and, at the time of study, infants were assessed as clinically stable. All infants were awake; not receiving analgesic drugs, sedatives, or other psychotrophic agents; had not been fed for at least 30 min before start of the study; were supine; and were self-ventilating in air. Infants were not eligible for inclusion in the study if they showed signs of tissue damage on the lower limbs, had previous surgery, had intraventricular haemorrhage or periventricular leukomalacia, were born to diabetic mothers or opioid users, were asphyxiated at birth, or were born with congenital malformations or other genetic disorders. Infants were also excluded if they had contraindications to the administration of sucrose: high risk for necrotising enterocolitis, feeding intolerance, oesophageal atresia or tracheal oesophageal fistula, or active phase persistent pulmonary hypertension.
Ethics approval was obtained from the UCH ethics committee and informed written parental consent was obtained before each procedure. The study conformed to the standards set by the Declaration of Helsinki and Good Clinical Practice guidelines.
Randomisation and masking
Treatment randomisation and dispensing was done offsite at the UCH pharmacy. The pharmacy received the sterile sucrose solution (24% sucrose in purified water) and sterile water samples from Inspiration Healthcare (Respironics, Murrysville, PA, USA) in individual 15 mL vials, which could not be visually distinguished by the packaging. 60 samples were randomised by a block design with a 1:1 allocation for sucrose and sterile water. The samples were separated into six blocks of ten, in which each block contained five sucrose samples and five sterile water samples. The pharmacy labelled each sample with a randomisation code that corresponded to the identity of the solutions. Randomisation was achieved with a computer-generated randomisation code. Only the hospital pharmacy had access to the randomisation codes that could be used to identify the solution. A sealed copy of the randomisation chart was also stored in the neonatal unit in case an adverse event was reported. Throughout the study the researchers, clinicians, participants, and parents were masked to the identity of the solutions. Inspiration Healthcare was not involved in the study design, data collection, or data analysis. No interim analysis was done in this study.
Procedures
The noxious stimulus was a heel lance done to collect a clinically necessary blood sample. The foot was not squeezed for at least 30 s after the heel lance to ensure that the recorded responses could be timed from one discrete event. This procedure was done without impairing the clinical blood collection. No heel lances were done solely for the purpose of the study. Before the administration of the sucrose or sterile water a non-noxious control stimulus was applied to the infant's heel with a heel lancet. The lancet was rotated by 90° and placed against the heel, so that when the spring-loaded blade was released it did not contact the infants' heel. Infants experienced only the non-noxious tactile sensation and auditory click that occurs when the blade is released.
The sucrose solution or sterile water was administered directly onto the anterior surface of the tongue with a 1 mL syringe 2 min before a heel lance was done. In line with present clinical practice, the dose and expiry date of each solution was double-checked by two neonatal practitioners, and a neonatal nurse administered all solutions. A neonatal nurse was present during the studies and was responsible for the clinical care of the infants at all times. She was also responsible for reporting any adverse events to the consultant in charge.
Experimental recording techniques
A neonatal EEG cap (WaveGuard EEG cap, Advanced NeuroTechnology, Enschede, Netherlands) was used to record EEG activity in the infants. 32 recording electrodes were positioned according to the modified international 10/20 electrode placement system at Fz, Fp1, Fp2, F3, F4, F7, F8, FT9, FT10, FC5, FC6, Cz, CPz, C3, C4, CP3, CP4, CP5, CP6, Pz, POz, P3, P4, P9, P10, PP07, PP08, T7, T8, Oz, O1, and O2. Reference and ground electrodes were placed at FCz and the chest, respectively. Electrode to skin impedance was kept to a minimum by massaging the head with an EEG prepping gel before the cap was placed on the head. Conductive EEG gel was placed in the electrode cups, with a syringe, before the EEG cap was placed on the head. The EEG cap was sterilised after each study by the UCH Sterile Services Department.
EEG activity, from 0·05 to 70 Hz, was recorded with the Neuroscan (Scan 4.3) SynAmps2 EEG/EP recording system (Compumedics US, Charlotte, NC, USA). Signals were digitised with a sampling rate of 2 kHz and had a resolution of 24 bit. A 50 Hz notch filter was used. The heel lance was time-locked to the EEG recording with an accelerometer attached to the upper surface of the lancet.
Respiration was monitored with a movement transducer placed on the abdomen and heart rate measured with lead 1 electrocardiograph (ECG) electrodes placed on the chest. Oxygen saturation and heart rate were continuously measured with a Nellcor N-560 transcutaneous pulse oximeter (Covidien-Nellcor and Puritan Bennett Boulderm, CO, USA) that was placed on the foot contralateral to the site of stimulation. Pulse oximetry data were downloaded from the oximeter to a recording computer as they were acquired.
EMG activity, from 1 to 500 Hz, was recorded from the ipsilateral biceps femoris muscle with self-adhesive bipolar surface silver/silver chloride (Ag/AgCl) electrodes. Electrode to skin impedance was reduced by rubbing the skin with an EEG prepping gel. Electrodes were secured with self-adherent wrap and electrode leads were tied together to minimise electrical interference.
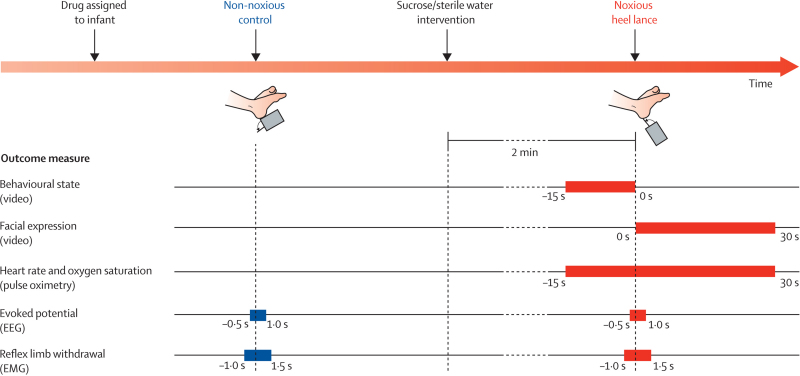
Facial expression was recorded with a portable tripod-mounted camcorder. A light emitting diode (LED), which flashed when either the non-noxious control event or noxious heel lance was done, was placed in the field of view of the camcorder. Figure 1 shows the experimental time line.
Figure 1.
Experimental time line
EEG=electroencephalography. EMG=electromyography.
Outcome measures
The primary outcome measure was pain-specific brain activity evoked by one time-locked heel lance, recorded with EEG and identified by principal component analysis (PCA). Secondary measures were baseline behavioural and physiological measures, observational pain scores (PIPP), and spinal nociceptive reflex withdrawal activity. The primary hypothesis was that administration of sucrose 2 min before a heel lance would reduce the evoked nociceptive-specific brain activity.
1500 ms EEG epochs corresponding to a noxious heel lance and non-noxious control event were considered for analysis (PCA). The EEG epochs included activity recorded 500 ms before and 1000 ms after each event. The segments were referenced to a common average, baseline corrected, high-pass filtered above 0·5 Hz, and aligned between 400 and 750 ms to correct for interindividual latency variability (maximum −20 to 50 ms). Infants were excluded from the analysis if technical failure occurred in the EEG recording or if movement artifact—defined as a voltage change greater than 50 μV over 50 ms in 15 or more electrodes—was identified in the alignment window.
Nociceptive-specific activity was characterised at electrode site Cz because we have previously reported nociceptive-specific activity at this electrode site,9,23 and because studies in adults show that similar potentials recorded at Cz are sensitive to pharmacological analgesic drugs.25
In the original research protocol we intended to use peak-to-peak amplitude detection. We deviated from the original protocol as instead we used PCA to analyse the data. This is an improved analytical technique that has been successfully used in EEG analysis and is more robust than peak-to-peak amplitude detection because it takes into account the overall signal rather than two single data points that can be easily affected by noise and are difficult to identify. We have successfully used this method in previous work to characterise evoked potentials generated after noxious and non-noxious stimulation in the human infant brain.9,23
PCA was used to decompose the EEG epochs recorded at Cz into basic waveforms, termed principal components.26 Epochs were considered as variables and timepoints as observations; the resultant covariance matrix was selected as the association matrix. The principal components represent systematic variation in the amplitude of the signal across timepoints in a cluster of epochs. The extent to which each component is represented in an individual EEG epoch is quantified by a unique weight. The weights of the first two principal components, which accounted for 84% of the total variance, were considered for analysis.
The PIPP score was calculated for each infant on the basis of behavioural and physiological observations.12 The facial expression component of the PIPP was analysed by trained observers (two neonatal research nurses) watching the videos for 30 s immediately after the heel lance. The videos were cut into 45 s epochs, which included 15 s before stimulus and 30 s after stimulus. The presence of each facial expression (nasolabial furrow, eye squeeze, and brow bulge) was assessed individually. The number of infants who had a facial expression score of zero was also calculated.22 The observers were fully masked to the treatment allocation and stimulus type when they analysed the videos. The observers did not know whether they were viewing video footage after the heel lance or the control stimulation. An additional level of masking was imposed by mixing the videos with other non-trial video footage. The facial expression component of the PIPP was rescored by two observers in 15 of 60 (25%) of the videos to assess intra-rater and inter-rater reliability, which was analysed by the Bland-Altman method.27 Bland-Altman plots showed good reliability with little bias (intra-rater bias 0·07; inter-rater bias 0·73). The limits of agreement for the intra-rater re-test were ±1·62. The limits of agreement for the inter-rater comparison were ±1·49.
The mean heart rate and oxygen saturation in the 15 s before the heel lance, and the maximum heart rate and the minimum oxygen saturation in the 30 s after the heel lance were used to calculate the physiological indices in the PIPP score. Custom-made data analysis software, written in MATLAB, was used to automatically calculate the mean baseline heart rate and oxygen saturation. The software also calculated change in heart rate and oxygen saturation from the heel lance. The data used these parameters to automatically calculate the physiological indices in the PIPP score.
The behavioural state score was calculated by observing the infant's sleep state and facial movements on the video footage in the 15 s before the heel lance. The latency to facial expression change was defined as the latency when the first PIPP facial feature (ie, nasolabial furrow, eye squeeze, and brow bulge) was observed after the heel lance in each infant.22
EMG analysis
2500 ms EMG epochs corresponding to a noxious heel lance were considered for analysis. The EMG epochs included activity recorded 1000 ms before and 1500 ms after each event. The segments were high-pass filtered above 10 Hz and rectified. Latency to flexion withdrawal reflex activity was defined as the time after stimulus when the EMG activity exceeded three standard deviations of the pre-stimulus baseline. Magnitude of flexion withdrawal reflex activity was measured in 250 ms periods after stimulus by calculation of the root mean square activity. The summary parameter for the magnitude of the spinal nociceptive reflex EMG activity was defined as the root mean square activity in 1000 ms after stimulus. Infants were not included in the EMG analysis when technical failure occurred in the EMG recording.
Statistical analysis
Data were analysed according to the trial protocol. A sample size of 40 infants had greater than 80% power to detect a 30% reduction in the amplitude of the nociceptive-specific brain activity as significant (p<0·05; two-tailed). We allowed for a total sample size of 60 infants (30 per group) to account for a high number of anticipated losses (ten per group) because of the possibility that technical failure could occur in any one of the physiological recordings (ie, video, EEG, or pulse oximetry; typically 20%; four per group) and that movement artifact could be recorded in the EEG (typically 20%; four per group).
Two-way nested analysis of variance was applied to the principal component weights to assess the effect of stimulation type (non-noxious control, noxious heel lance) and treatment (sterile water, sucrose). With this analysis we assessed whether noxious stimulation generated a specific component and whether this component was affected by sucrose or sterile water administration. Nociceptive-specific brain activity was identified by measurement of the principal components whose weights were significantly greater after the noxious lance compared with non-noxious control.
As with our previous publications, only one nociceptive-specific principal component was identified. To ensure that the activity defined in our participant sample could be generalised to other external samples, we compared the evoked activity with that reported in a previously published dataset9 and calculated the correlation coefficient between the observations. Two-way nested analysis of variance was applied in the same way to the root mean square values of the EMG reflex activity.
The effect of the treatment on all other outcome measures was summarised as mean values (with 95% CIs) and significance of the differences tested with unpaired Student's t tests. A significance level of 0·05 was used in all the tests. Data were analysed with MATLAB (version 7.8.0).
This study is registered, number ISRCTN78390996.
Role of the funding source
The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
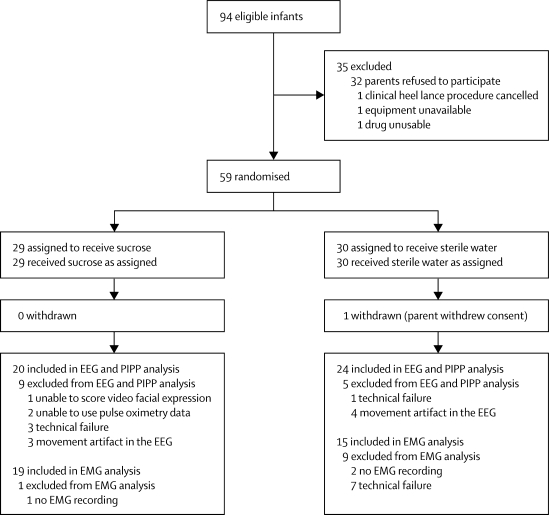
Figure 2 shows the trial profile. There were no deviations from the inclusion and exclusion criteria. 59 infants were included in our initial sample, of whom 44 were included in the final analysis. Table 1 shows the characteristics of the final population of infants. After sucrose or sterile water administration the mean heart rate, oxygen saturation, and behavioural scores in the 15 s before the heel lance did not differ significantly between infants who were given sucrose and those given sterile water (table 2).
Figure 2.
Trial profile
EEG=electroencephalography. PIPP=premature infant pain profile. EMG=electromyography.
Table 1.
Characteristics of participating neonates
| Sucrose (N=20) | Sterile water (N=24) | |
|---|---|---|
| PMA at birth (weeks) | 39·8 (1·1) | 39·8 (1·3) |
| PMA at time of study (weeks) | 40·1 (1·1) | 40·3 (1·4) |
| Postnatal age at time of study (days) | 3 (2) | 3 (2) |
| Birthweight (g) | 3449 (453) | 3454 (443) |
| Boys | 11/20 (55%) | 15/24 (63%) |
| Apgar score at 1 min | 8·2 (1·5) | 8·0 (1·9) |
| Spontaneous vaginal delivery | 8/20 (40%) | 11/24 (46%) |
| Right heel lanced | 13/20 (65%) | 12/24 (50%) |
Data are mean (SD) or n/N (%). PMA=postmenstrual age.
Table 2.
Primary and secondary outcomes
| Sucrose (N=20) | Sterile water (N=24) | p value | |
|---|---|---|---|
| Primary outcome | |||
| Nociceptive-specific brain activity (mean weight) | 0·10 (0·04–0·16) | 0·08 (0·04–0·12) | 0·46 |
| Secondary outcomes | |||
| Mean baseline heart rate (bpm) | 132·6 (124·3–140·9) | 131·8 (122·2–141·5) | 0·90 |
| Mean baseline oxygen saturation (%) | 99·4% (98·8–100·1) | 97·4% (95·0–99·8) | 0·13 |
| Baseline behavioural score (from PIPP) | 1·3 (0·8–1·7) | 1·3 (0·8–1·8) | 0·91 |
| PIPP score | 5·8 (3·7–7·8) | 8·5 (7·3–9·8) | 0·02 |
| Latency to change in facial expression (s) | 3·8 (1·3–6·4) | 3·5 (1·0–6·1) | 0·86 |
| Facial non-responders | 7/20 (35%) | 0/24 (0%) | <0·0001 |
| Mean nociceptive reflex withdrawal activity (μV) | 36·11 (24·20–48·02) | 30·82 (18·51–43·13) | 0·49 |
| Mean latency to nociceptive reflex withdrawal activity (ms) | 363·3 (256·4–470·1) | 413·5 (262·0–564·9) | 0·56 |
Data are mean (95% CI) or n/N (%). bpm=beats per min. PIPP=premature infant pain profile.
After the heel lance, the PIPP score was significantly lower in infants who were given sucrose than in those given sterile water; however, we recorded no significant difference in the latency to change in facial expression between the two treatment groups (table 2). The number of infants who had a zero facial expression score after the noxious heel lance was significantly higher in the sucrose group than in the sterile water group (table 2).
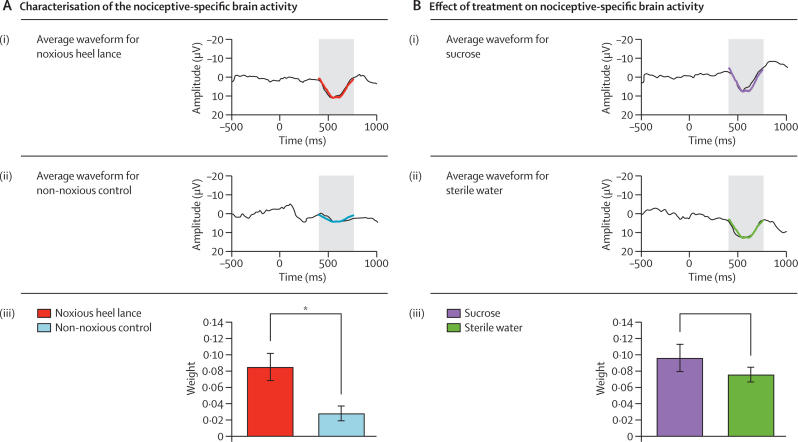
Nociceptive-specific brain activity was identified by one principal component that was significantly greater after the heel lance (mean principal component weight 0·09 [SE 0·02]) than after the non-noxious control event (0·03 [0·01]; p=0·006; figure 3A). The correlation coefficient between this component and a nociceptive-specific component calculated in an external sample of term infants in a previously published study9 was 92% (p<0·0001). The nociceptive-specific brain activity did not differ significantly between infants who received sucrose (mean principal component weight 0·10 [SE 0·03]) and those who received sterile water (0·08 [0·02]; p=0·46; figure 3B).
Figure 3.
Characterisation of the nociceptive-specific brain activity (A) and effect of sucrose or sterile water on the nociceptive-specific brain activity (B)
(A) Average waveform of the group data after (i) noxious heel lance and (ii) non-noxious control stimulus (alignment window 400–750 ms). (iii) Mean (SE) weight of the second principal component after the noxious heel lance and non-noxious control stimulus (*p=0·006). (B) Average waveform of the group data after the noxious heel lance, separated into two groups: (i) infants administered sucrose and (ii) infants administered sterile water (alignment window 400–750 ms). (iii) Mean (SE) weight of the nociceptive-specific component in the sucrose and sterile water groups (p=0·46).
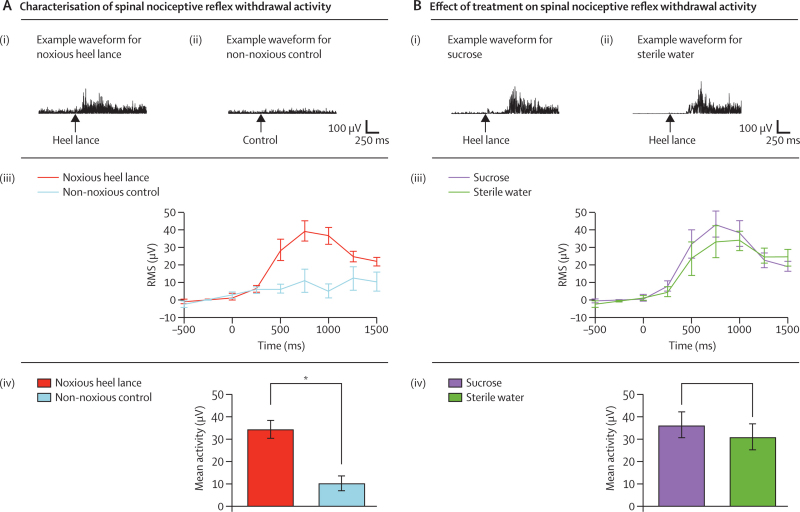
Spinal reflex withdrawal activity, recorded by EMG of the biceps femoris of the stimulated leg, was significantly greater after the heel lance (mean activity 33·78 μV [SE 4·02] than after the non-noxious control event (8·84 μV [3·67]; p<0·0001; figure 4A). The magnitude of the spinal reflex withdrawal activity did not differ significantly between infants who received sucrose (mean activity 36·11 μV [SE 5·67]) and those who received sterile water (30·82 μV [5·74]; p=0·49; figure 4B). Furthermore, mean latency to nociceptive reflex withdrawal activity did not differ significantly between the two groups (table 2). No adverse events were recorded during this study.
Figure 4.
Characterisation of the spinal nociceptive reflex withdrawal activity (A) and effect of sucrose or sterile water on spinal nociceptive reflex withdrawal activity (B)
(A) Example spinal reflex withdrawal activity in one infant after: (i) noxious heel lance and (ii) non-noxious control stimulus. (iii) Magnitude (mean [SE]) of the spinal reflex withdrawal activity after noxious heel lance and non-noxious control stimulus represented as the root mean square (RMS) activity in 250 ms time periods in infants. (iv) Mean (SE) spinal reflex withdrawal activity in infants after the noxious heel lance and non-noxious control stimulus (*p<0·0001). (B) Example spinal nociceptive reflex withdrawal activity after a noxious heel lance in two infants who received: (i) sucrose and (ii) sterile water. (iii) Magnitude (mean [SE]) of the spinal nociceptive reflex withdrawal activity after noxious heel lance in infants given sucrose or sterile water represented as the RMS activity in 250 ms time periods. (iv) Mean (SE) spinal nociceptive reflex withdrawal activity in infants given sucrose or sterile water (p=0·49).
Discussion
This randomised controlled trial measured the effect of oral sucrose on procedural pain in infants, with direct measures of brain and spinal cord activity as an outcome measure for pain. The results show that although, as previously reported, sucrose significantly reduces the PIPP score—a composite observational behavioural and physiological measure2—it has no effect on the neural activity in sensory pain circuits in the brain or the spinal cord. Although true pain perception cannot be measured in non-verbal populations, neural activity in nociceptive pathways is a more direct measure than behavioural and physiological assessment. The finding that sucrose does not change neural activity strongly suggests that pain perception is not affected by this intervention.
Any measure of pain in this group is necessarily indirect, and whether the electrophysiological measures reported in this study are indicative of the conscious pain experience of the newborn infant cannot be shown. Nevertheless, behavioural and physiological output measures need integration and control of several somatic motor and autonomic circuits, which are affected by several developmental homoeostatic and external factors.10,19–21 By comparison, the nociceptive-evoked activity in the brain and spinal sensory circuits recorded in this study are a more direct measure of pain activity in the infant CNS. In the adult brain, the magnitude of nociceptive-evoked potentials directly correlates with perceived pain intensity.28,29 The nociceptive-evoked activity in flexor muscles, which cause reflex withdrawal of the limb, is also a proven functional measure of spinal and supraspinal pain processing.30 Pharmacological analgesic drugs, such as tramadol and morphine, depress nociceptive-evoked brain activity and flexion reflexes, and result in concurrent reduction in perceived pain intensity in adults.25,31,32
Our results accord with previous trials showing that sucrose decreases infant PIPP scores,2 but they show that such scores do not reliably reflect nociceptive activity in the brain of newborn infants, possibly because of the site of action of sucrose in the brain. The reduction in nociceptive behaviour recorded after oral sucrose in young rats33 persists after midbrain transection, suggesting that forebrain neural circuits are not needed for this effect.34 Sucrose reduction of nociceptive withdrawal reflexes in adult rats—a conditioned effect of all hedonistic foods thought to prevent eating from ending—also involves brainstem endogenous inhibitory mechanisms.35 Thus in infants exposed to noxious procedures, sucrose could mediate a brainstem inhibition of behaviour, and inhibit facial motor activity, while strong pain activation still occurs in the forebrain. This notion is especially important in view of the increasing evidence for short-term and long-term adverse effects of infant pain experience on neurodevelopment.5–9 The absence of evidence for an analgesic action of sucrose in this study, together with uncertainty over the long-term benefits of repeated sucrose administration,36 suggest that sucrose should not be used routinely for procedural pain in infants without further investigation.
This double-blind randomised controlled trial used new electrophysiological methods to assess the effectiveness of analgesic drugs in newborn infants. The conclusions that we can draw from this study are limited by the small sample size (n=44), which could mean that this study was not powered to observe subtle effects that sucrose might have on CNS processing. Significant group differences in infant nociceptive brain activity have, however, been recorded in sample sizes of only 15 infants.9 This single-centre trial should be repeated in a larger sample of infants, and this new method used to test the effect of other known pharmacological analgesic drugs, such as morphine.
In conclusion, our results show that although oral sucrose does reduce observed pain behaviour, it has no significant effect on the magnitude of spinal nociceptive reflexes or on the acute activation of pain networks in the brain. Sucrose seems to blunt facial expression activity after painful procedures, but our data suggest that it does not reduce direct nociceptive activity in central sensory circuits, and therefore might not be an effective analgesic drug.
Acknowledgments
Acknowledgments
This work was supported by the Medical Research Council. We thank Siân Roberts (UCL, London, UK) for help with data acquisition, the UCL/UCH Comprehensive Biomedical Research Centre, the staff at UCH, and all the parents and infants who took part in this study.
Contributors
The work presented here was undertaken in collaboration between all authors. RS, AW, JM, SB, and MF defined the research idea and designed the study's method. RS and MF wrote the report. RS, LC, DP, and JY contributed to research data collection and to the study design. DP, JY, and JM were responsible for the clinical care of the participants. RS, LC, LF, DP, JY, AW, SB, JM, and MF reiewed and edited the report. LF, RS, and LC did the statistical analysis. All authors have seen and approved the final version of this report.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Anand KJ. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173–180. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 2.Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2010;1 doi: 10.1002/14651858.CD001069.pub3. CD001069. [DOI] [PubMed] [Google Scholar]
- 3.Carbajal R, Rousset A, Danan C. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 4.Simons SH, van DM, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med. 2003;157:1058–1064. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5:35–50. doi: 10.1038/ncpneuro0984. [DOI] [PubMed] [Google Scholar]
- 6.Hermann C, Hohmeister J, Demirakca S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125:278–285. doi: 10.1016/j.pain.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Hohmeister J, Demirakca S, Zohsel K, Flor H, Hermann C. Responses to pain in school-aged children with experience in a neonatal intensive care unit: cognitive aspects and maternal influences. Eur J Pain. 2009;13:94–101. doi: 10.1016/j.ejpain.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Hohmeister J, Kroll A, Wollgarten-Hadamek I. Cerebral processing of pain in school-aged children with neonatal nociceptive input: an exploratory fMRI study. Pain. 2010;150:220–221. doi: 10.1016/j.pain.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Slater R, Fabrizi L, Worley A, Meek J, Boyd S, Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. Neuroimage. 2010;52:583–589. doi: 10.1016/j.neuroimage.2010.04.253. [DOI] [PubMed] [Google Scholar]
- 10.Ranger M, Johnston CC, Anand KJ. Current controversies regarding pain assessment in neonates. Semin Perinatol. 2007;31:283–288. doi: 10.1053/j.semperi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Simons SH, van DM, van Lingen RA. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003;290:2419–2427. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- 12.Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Taddio A, Shah V, Katz J. Reduced infant response to a routine care procedure after sucrose analgesia. Pediatrics. 2009;123:e425–e429. doi: 10.1542/peds.2008-3028. [DOI] [PubMed] [Google Scholar]
- 14.Anand KJ, Aranda JV, Berde CB. Analgesia and anesthesia for neonates: study design and ethical issues. Clin Ther. 2005;27:814–843. doi: 10.1016/j.clinthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald M. When is an analgesic not an analgesic? Pain. 2009;144:9. doi: 10.1016/j.pain.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Walker SM. Pain in children: recent advances and ongoing challenges. Br J Anaesth. 2008;101:101–110. doi: 10.1093/bja/aen097. [DOI] [PubMed] [Google Scholar]
- 17.Prkachin KM. Assessing pain by facial expression: facial expression as nexus. Pain Res Manag. 2009;14:53–58. doi: 10.1155/2009/542964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puntillo KA, Morris AB, Thompson CL, Stanik-Hutt J, White CA, Wild LR. Pain behaviors observed during six common procedures: results from Thunder Project II. Crit Care Med. 2004;32:421–427. doi: 10.1097/01.CCM.0000108875.35298.D2. [DOI] [PubMed] [Google Scholar]
- 19.Slater R, Cantarella A, Franck L, Meek J, Fitzgerald M. How well do clinical pain assessment tools reflect pain in infants? PLoS Med. 2008;5:e129. doi: 10.1371/journal.pmed.0050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberlander TF, Grunau RE, Fitzgerald C, Whitfield MF. Does parenchymal brain injury affect biobehavioral pain responses in very low birth weight infants at 32 weeks' postconceptional age? Pediatrics. 2002;110:570–576. doi: 10.1542/peds.110.3.570. [DOI] [PubMed] [Google Scholar]
- 21.Oberlander TF, Jacobson SW, Weinberg J, Grunau RE, Molteno CD, Jacobson JL. Prenatal alcohol exposure alters biobehavioral reactivity to pain in newborns. Alcohol Clin Exp Res. 2010;34:681–692. doi: 10.1111/j.1530-0277.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slater R, Cantarella A, Yoxen J. Latency to facial expression change following noxious stimulation in infants is dependent on postmenstrual age. Pain. 2009;146:177–182. doi: 10.1016/j.pain.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Slater R, Worley A, Fabrizi L. Evoked potentials generated by noxious stimulation in the human infant brain. Eur J Pain. 2010;14:321–326. doi: 10.1016/j.ejpain.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Andrews K, Fitzgerald M. Cutaneous flexion reflex in human neonates: a quantitative study of threshold and stimulus-response characteristics after single and repeated stimuli. Dev Med Child Neurol. 1999;41:696–703. doi: 10.1017/s0012162299001425. [DOI] [PubMed] [Google Scholar]
- 25.Truini A, Panuccio G, Galeotti F. Laser-evoked potentials as a tool for assessing the efficacy of antinociceptive drugs. Eur J Pain. 2010;14:222–225. doi: 10.1016/j.ejpain.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Boxtel GJM. Computational and statistical methods for analyzing event-related potential data. Behav Res Methods Instrum Comput. 1998;30:87–102. [Google Scholar]
- 27.Altman DG, Bland JM. Measurement in medicine—the analysis of method comparison studies. Statistician. 1983;32:307–317. [Google Scholar]
- 28.Lee MC, Mouraux A, Iannetti GD. Characterizing the cortical activity through which pain emerges from nociception. J Neurosci. 2009;29:7909–7916. doi: 10.1523/JNEUROSCI.0014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts K, Papadaki A, Goncalves C. Contact heat evoked potentials using simultaneous EEG and fMRI and their correlation with evoked pain. BMC Anesthesiol. 2008;8:8. doi: 10.1186/1471-2253-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. doi: 10.1016/j.pneurobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz J, Beck H, Bromm B. Differential changes of laser evoked potentials, late auditory evoked potentials and P300 under morphine in chronic pain patients. Electroencephalogr Clin Neurophysiol. 1997;104:514–521. doi: 10.1016/s0168-5597(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 32.Willer JC. Studies on pain. Effects of morphine on a spinal nociceptive flexion reflex and related pain sensation in man. Brain Res. 1985;331:105–114. doi: 10.1016/0006-8993(85)90719-x. [DOI] [PubMed] [Google Scholar]
- 33.Anseloni V, Ren K, Dubner R, Ennis M. Ontogeny of analgesia elicited by non-nutritive suckling in acute and persistent neonatal rat pain models. Pain. 2004;109:507–513. doi: 10.1016/j.pain.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Anseloni VC, Ren K, Dubner R, Ennis M. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience. 2005;133:231–243. doi: 10.1016/j.neuroscience.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 35.Foo H, Mason P. Analgesia accompanying food consumption requires ingestion of hedonic foods. J Neurosci. 2009;29:13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holsti L, Grunau RE. Considerations for using sucrose to reduce procedural pain in preterm infants. Pediatrics. 2010;125:1042–1047. doi: 10.1542/peds.2009-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]