Abstract
Total ankle replacement (TAR) was first attempted in the 1970s, but poor results led to its being considered inferior to ankle fusion until the late 1980s and early 1990s. By that time, newer designs which more closely replicated the natural anatomy of the ankle, showed improved clinical outcomes.1 Currently, even though controversy still exists about the effectiveness of TAR compared to ankle fusion, TAR has shown promising mid-term results and should no longer be considered an experimental procedure. Factors related to improved TAR outcomes include: 1) better patient selection, 2) more precise knowledge and replication of ankle biomechanics, 3) the introduction of less-constrained designs with reduced bone resection and no need for cementation, and 4) greater awareness of soft-tissue balance and component alignment. When TAR is performed, a thorough knowledge of ankle anatomy, pathologic anatomy and biomechanics is needed along with a careful pre-operative plan. These are fundamental in obtaining durable and predictable outcomes. The aim of this paper is to outline these aspects through a literature review.
ANKLE BIOMECHANICS
Ankle biomechanics should be evaluated when planning TAR. Some important factors to consider include: 1) limb and ankle alignment; 2) bony and ligamentous anatomy of the ankle joint; 3) ankle motion which occurs in the sagittal, coronal, and transverse planes; and 4) both talocrural and subtalar joint contributions to motion in these three planes.
The ankle joint is composed of three articulations: the tibiotalar, fibulotalar, and tibiofibular joints. The talus has been described as the frustum of a cone with its apex oriented medially. When viewed from the top, the dome appears shaped like a wedge narrowed posteriorly/medially and wider anteriorly/laterally. The tibial plafond has a mirror shape compared to the talus, but with a longer radius of curvature. Thus, when the talus is plantarflexed, its narrowest portion sits in the ankle mortise and allows rotation between the talus and mortise. When the talus is maximally dorsiflexed, the tibiofibular syndesmosis accommodates the talus, and the wider portion of the talar articular surface locks into the ankle mortise, allowing little or no rotation between the talus and the mortise.2
The facets of both the medial and lateral malleoli are parallel to corresponding facets of the talus.3 There is articular contact at these facets from extreme plantarflex-ion through complete dorsiflexion.1 Different radii of curvature have been found between the talus and the mortise as well as between the talus and the mortise facets. The ankle joint was formerly thought to function as a simple hinge whose primary axis was transverse and perpendicular to the sagittal plane.1 It has been shown, however, that the primary axis is correlated with the transmalleolar plane and externally rotated an average of 23 degrees.34 Recent studies have demonstrated that the axis of rotation is not fixed but rather changes direction and position throughout ankle motion.5-7 The position and orientation of these axes account for coupled ankle motion. As the ankle is dorsiflexed, it rotates externally and everts. Conversely, as the ankle is plantar flexed, it rotates internally and inverts.
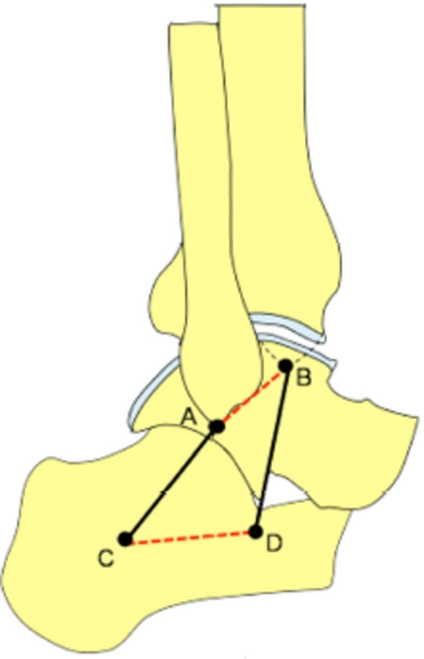
The role of the medial and lateral ankle ligaments is fundamental when describing ankle biomechanics. Leardini et al.6 formulated a two-dimensional four-bar linkage model of the ankle joint to describe dorsi/ plantar flexion in unloaded conditions (Figure 1). The experiments demonstrated that the human ankle joint complex behaves as a single-degree-of-freedom system during passive motion, with a moving axis of rotation. The four-bar-linkage model showed that the talus/cal-caneus complex and tibia/fibula complex rotate about each other on approximately inextensible line segments, represented by the calcaneofibular and tibiocalcaneal ligaments. In this model, the four bars described are: 1) the ligament; 3) the line connecting the proximal insertions of the calcaneofibular and tibiocalcaneal ligaments; and 4) the line connecting the distal insertions of the calcaneofibular and tibiocalcaneal ligaments (Figure 1). It was deduced that the ankle is a single-degree-of-freedom mechanism where mobility is allowed by the sliding of the articular surfaces upon each other and the isometric rotation of two ligaments about their origins and insertions, without tissue deformation.
Figure 1.
Two-dimensional four-bar linkage model of the ankle joint to describe dorsi/plantar flexion in unloaded conditions. The talus/ calcaneus complex and tibia/fibula complex rotate about each other on approximately inextensible line segments, represented by the calcaneofibular (AC) and tibiocalcaneal (BD) ligaments. The other two bars in this four-bar linkage model are formed by: 1) the bony segment connecting the proximal insertions of calcaneofibular and tibiocalcaneal ligaments (AB, red dashed line); and 2) the bony segment connecting the distal insertions of calcaneofibular and tibiocalcaneal ligaments (CD, red dashed line). Redrawn from Leardini etal. J Biomech. 1999 Jun;32(6):585-91.
The authors also described different radii of curvature of the posterior 75% of the talar articular surface and the anterior 25%. The posterior 75% showed a longer radius of curvature. This explains why, during dorsiflexion, the articular contact area is shifted anteriorly, with posterior joint distraction and, conversely, during plantarflexion the articular contact area is shifted posteriorly, with an anterior joint distraction. Furthermore, this model explains how, in a tibial reference frame, the instantaneous center of rotation moves from a posteroinferior to an anterosuperior position during a complete cycle from maximal plantarflexion to maximal dorsiflexion.6
The authors6 showed that the calcaneofibular and tibiocalcaneal ligaments, which are approximately isometric during the whole range of motion, control and guide passive motion and are therefore responsible for correct joint mobility. On the other hand, the tension and length of the other ankle ligaments changes according to joint position. The other ligaments are therefore responsible for joint stability.
The human ankle joint has unique articular characteristics. When loaded, the ankle joint has a smaller area of contact between the opposing articular surfaces compared to the knee and hip.8 At 500N of load, the contact area of the ankle joint averages 350mm,2 compared with 1120 mm2 for the knee and 1100 mm2 for the hip. Therefore, the smaller contact area makes the normal peak contact stress higher in the ankle than in the knee or hip. In addition, ankle joint articular cartilage differs from that of the knee and hip in thickness and tensile properties.9 The thickness of ankle articular cartilage ranges from <1 mm to slightly <2 mm.29
Ankle motion occurs in the sagittal, coronal, and transverse planes.10 Sagittal plane motion, which consists of plantarflexion and dorsiflexion, constitutes the greatest amount of motion in the healthy ankle. The range of sagittal plane motion has been reported to be 13° to 33° of dorsiflexion and 23° to 56° of plantarflexion.10 Most studies indicate that the range of motion required for normal walking is around 12° of dorsiflexion and 15° of plantarflexion.1 Transverse-plane motion is coupled with sagittal plane motion.10 Michelson and Helgemo reported that dorsiflexion resulted in an average of 7.2° ± 3.8° of external rotation of the foot relative to the leg and 1.9°± 4.12° degrees of internal rotation with plantarflexion.10,11 Coronal motion is described as varus or valgus rotation. Michelson et al. observed that both maximal dorsiflexion and plantarflexion of the ankle were associated with inversion of the ankle.10,12 They attributed coronal plane motion to the position of the deltoid ligament.10,12
It has been debated in the literature as to how much the talocrural and subtalar joints contribute to each motion.1 It has been shown that the talocrural joint has a greater contribution to dorsiflexion/plantarflexion and that the subtalar joint has a greater contribution to inversion/eversion.1 Axial rotation is thought to take place in approximately equal proportions at both joints.1 Furthermore, the key role of the talonavicular joint in dorsiflexion of the foot and motion of the other joints of the triple joint complex (subtalar, talonavicular, and calcaneocuboid joints) has been proven.13
BRIEF HISTORY
The first generation of TARs were formed by two components, a concave polyethylene tibial component and a convex metal (usually cobalt-chrome alloy) talar component. In some designs the materials were inverted for tibial and talar components. Constrained or unconstrained TAR designs were introduced, but poor results and high failure rates were recorded with both types. With constrained implants, the inability to dissipate the rotational forces produced by the continuous variation of rotational axis resulted in loosening as the main cause of failure.1 On the other hand, with unconstrained designs, instability occurred due to the excessive strain placed on surrounding soft tissues.1 First-generation implants all required large bone resection to allow cement fixation and component positioning. This has been shown to be another drawback and possible cause of loosening of these devices for two main reasons: 1) cementing techniques used in the 1970s would currently be considered inadequate, and 2) bony strength of the both tibia and talus rapidly decreases incremental to bone resection.14 It has been shown, as well, that bone strength was significantly higher in the talus (40% on average) than in the tibia, explaining the higher rate of loosening for the tibial component. In summary, first-generation implants were abandoned because of the high failure rates, most commonly due to cement fixation, over-constraint, lack of constraint, wound healing, component loosening and pain.
The second phase of implants began in the 1980s with the introduction of modern TARs, like the Buechel-Pappas Total Ankle Replacement (Endotec, South Orange, NJ) in the USA and the Scandinavian Total Ankle Replacement (STAR; Waldemar Link, Hamburg, Germany) in Europe. In 1992, the Agility Total Ankle System prosthesis (DePuy, Warsaw, IN), designed by Dr. Frank Alvine, was the first ankle implant to receive FDA approval.2 At the end of this phase, almost all TARs were semi-constrained, cementless (with minimal bone resection required), and using porous coatings to encourage bone ingrowth.1 Tibial metal-backed, polyethylene inserts and large contact areas on the tibia and talus became common features as well.
The last phase started in the late 1990s with the introduction of a few new implants, including the Salto (Torn-ier SA, Saint Ismier, France), HINTEGRA (Newdeal SA, Lyon), Mobility (DePuy, Warsaw, IN), TNK (Kyocera Corporation, Japan), and BOX (Finsbury Orthopaedics, Leatherhead, Surrey, UK). All these designs (with the exception of the TNK which is a ceramic implant) use the three-part mobile bearing system.1
INDICATIONS
Although patient selection seems to be critical to successful and predictable outcomes, indications for TAR are still being defined. The optimal patient is older (>50 years old) with end-stage ankle arthrosis, non-obese and with low physical demands.15,16 Patients with post-traumatic ankle arthrosis, especially younger patients, seem to have worse outcomes and are more likely to undergo revision than patients with other causes of arthrosis.17 However, some authors18 reported similar outcomes in patients less than and more than 50 years of age, and others19 have stated that TAR may have a role in less active, young (<50 years old) patients with post-traumatic arthrosis. Nevertheless, in general, weight and activity level should guide indications; less active and non-obese patients are less likely to place excessive demands on the TAR and undergo early failure and revision.
Another factor that needs to be taken into account is the presence of degenerative changes in other joints, such as the subtalar, midtarsal, knee, hip, and the contra-lateral ankle. These patients seem to benefit more from TAR than from arthrodesis because ankle arthrodesis shifts abnormal loads onto the neighboring joints and thus accelerates degenerative changes.15 In fact, it has been shown that at an average of eight years after ankle fusion, approximately 50% of the patients have clinically significant hindfoot arthritis20 and after an average of 22 years, virtually all patients develop hindfoot arthritis.21
In patients with combined subtalar and tibiotalar arthrosis, advocates of ankle fusion would perform a tibiocalcaneal fusion with retrograde nailing to take care of every cause of pain. Ankle arthroplasty has a role in these cases as well. If subtalar arthrosis is at an early stage and the patient's symptoms are mainly referable to the ankle joint, TAR-only can be performed. This allows better motion of the ankle and less stress across the subtalar joint. If the patient will become symptomatic because of subtalar degeneration, a subtalar fusion may be performed subsequently. If the patient has subtalar symptoms or severe arthrosis at first evaluation, subtalar fusion combined with TAR can be performed. The two procedures can be performed concurrently, or the TAR can be performed 45-60 days after the subtalar fusion.
Patients amenable to triple arthrodesis or with previous triple arthrodesis, may benefit more from TAR than from a pantalar arthrodesis.15 As previously described, TAR and triple arthrodesis can be performed concurrently or with a two-stage procedure.22
Rheumatoid arthritis with multiple joint involvement is not a contraindication for TAR.23 Similar outcomes have been reported with TARs in osteoarthritis and rheumatoid arthritis in comparative studies.24 Furthermore, Cracchiolo et al. performed ankle fusion in 14 patients with rheumatoid arthritis and a history of previous hip and knee surgeries; they reported functional improvement in only one patient.25 On the other hand, some authors suggest that higher subsidence rates occur in patients with rheumatoid arthritis.19,26
It has to be mentioned here that, in very select patients, TAR can be indicated to treat painful ankle fusion. Few cases have been reported in the English literature and we refer readers to specific writings regarding this topic.27-28
Absolute contraindications to TAR include: 1) active infection, 2) peripheral vascular disease, 3) inadequate soft-tissue envelope, and 4)Charcot neuroarthropathy.15 Relative contraindications include: 1) young, active patients, 2) previous infection, 3) severe lower extremity malalignment, 4) marked ankle instability, 5) marked osteoporosis, and 6) osteonecrosis of the talus.15 Malalignment and instability are controversial factors and will be described in detail in the pre-operative planning section. In case of focal or superficial bone necrosis, designs that remove more talar bone can be indicated.15
WORK-UP AND PRE-OPERATIVE PLANNING
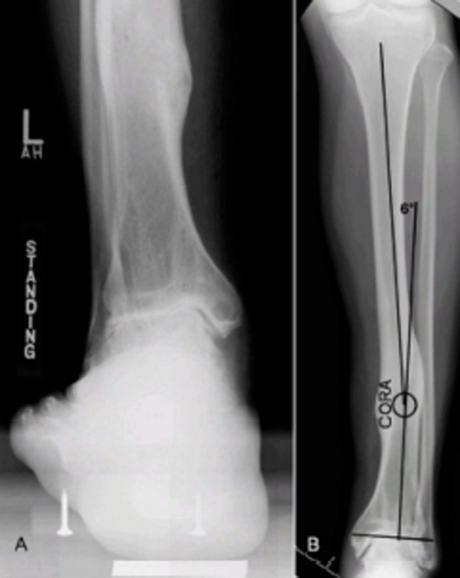
Weight bearing antero-posterior, lateral and mortise views of both ankles are required. The rearfoot alignment (Cobey/Saltzman) view (Figure 2A) is essential to evaluate the ankle joint and identify any calcaneal-to-tibial deformities. This view is obtained with the patient standing on an elevated platform, a cassette positioned 15° anteriorly inclined from vertical and the x ray beam oriented perpendicular to the film, aimed at the ankle. In case of diaphyseal deformities, antero-posterior and lateral views of the leg are required (Figure 2B).
Figure 2.
Left post-traumatic ankle arthritis. A) Cobey-Saltzman view. B) Antero-posterior radiograph of the left leg showing a varus deformity, with center of rotation of angulation (CORA) located at the distal third of the tibia.
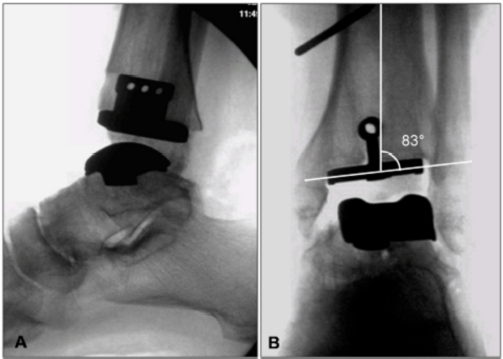
In the sagittal plane, the anterior distal tibial angle (ADTA) should be measured. The ADTA is formed by the mechanical axis of the tibia and the joint orientation line of the ankle in the sagittal plane and measures 80° ± 3° in the normal lower extremity (Figure 3B).29 An increased ADTA represents a recurvatum deformity.
Figure 3.
Pre-operative measurement. A) The lateral distal tibial angle (LDTA) is formed by the distal tibial articular surface and the anatomical axis of the tibia (normal values 89° ± 3°). B) The anterior distal tibial angle (ADTA) is formed by the mechanical axis of the tibia and the joint orientation line of the ankle in the sagittal plane (normal values 80° ± 3°). C) The tibial-talar angle (a) is defined by the tibial and talar articular surfaces in the ankle joint; if it measures > 10°, the joint is defined as incongruent.
In the coronal plane, the lateral distal tibial angle (LDTA), the tibial-talar angle and the calcaneal tibial alignment should be measured. The LDTA (Figure 3A) is formed by the distal tibial articular surface and the anatomical axis of the tibia and measures 89° ± 3°.29 A decreased LDTA represents a varus deformity. The tibial-talar angle (Figure 3C) is defined by the tibial and talar articular surfaces in the ankle joint. When the tibial-talar angle is >10° the joint is defined as incongruent (unstable).30 The calcaneal-tibial alignment (measured on the Cobey/Saltzman view) is useful to confirm any varus or valgus deformities as well as to assess every talar compensation (inversion and eversion) to an abnormal LDTA. The subtalar joint can compensate 15° of eversion and 30° of inversion.29 Evaluation of subtalar joint compensation and range of motion is important because tibial realignment (with osteotomy or TAR) may unmask a subtalar deformity. If this distal (rearfoot) deformity is not addressed, further symptoms may develop.29
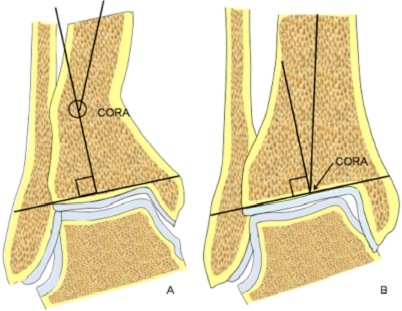
If an abnormal ADTA or LDTA is present (sagittal or coronal deformity), the center of rotation of angulation (CORA) is measured. The CORA is the intersection of the mid-diaphyseal line and the line starting from the middle of the joint and perpendicular to the abnormal ADTA or LDTA (Figure 4). The CORA can be located at the joint line level (usually due to anatomical joint line malalignment or to ankle degeneration) or proximally (usually due to tibial deformities/fractures).
Figure 4.
The center of rotation of angulation (CORA) is the intersection of the mid-diaphyseal line and the line starting from the middle of the joint and perpendicular to the abnormal ADTA or LDTA (LDTA in this figure). The CORA can be located proximally at the tibia (A) or at the joint line level (B).
Malalignment and instability should be thoroughly evaluated in pre-operative planning. Both can lead to edge-loading of the implant, polyethylene wear, progressive deformity and high early failure rates. Malalign-ment can be due to: 1) previous tibial fractures which result in diaphyseal or metaphyseal tibial malalignment (Figure 4A), either in the coronal or sagittal plane; 2) multi-articular degeneration of the hindfoot involving the subtalar and midtarsal joints (i.e., rheumatoid patients) which can result in varus or valgus malalignment; and 3) Ankle joint pathologies that include distal tibial articular surface malalignment, talar tilt due to ligamentous instability, or both (Table 1).
TABLE 1.
| Deformity type | Abnormal angles | Scheme |
|---|---|---|
| Varus tibial deformity-congruent joint | Decreased LDTA, CORA at the level of tibial articular surface, normal tibial-talar angle | 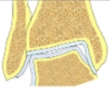 |
| Valgus tibial deformity-congruent joint | Increased LDTA, CORA at the level of tibial articular surface, normal tibial-talar angle | 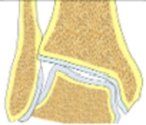 |
| Varus tibial deformity-incongruent joint | Decreased LDTA, CORA at the level of tibial articular surface, tibial-talar angle | 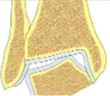 |
| Valgus tibial deform ity-incongruent joint | Increased LDTA, CORA at the level of tibial articular surface, tibial-talar angle | 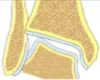 |
| Incongruent joint | Normal LDTA, tibial-talar angle > 10° | 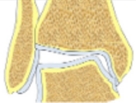 |
In diaphyseal or metaphyseal tibial malalignment, a varus/valgus or procurvatum/recurvatum deformity or both are present. In coronal malalignment, the LDTA is abnormal and the CORA is located in the tibial diaphysis or metaphysis (Figure 2B). In sagittal malalignment, the ADTA is abnormal and the CORA is located in the tibial diaphysis or metaphysis. Diaphyseal or metaphyseal tibial malalignment usually needs to be addressed with osteotomy before considering TAR.30 Only in cases of minimal ADTA or LDTA changes (<10°), can realignment be performed with the TAR tibial cut (Figure 5B). In this case, the choice of implant is very important. TAR instruments that allow distal tibial slope changes are required in cases of abnormal ADTA.
In varus or valgus hind-foot malalignment, ADTA and LDTA are normal, but calcaneal-to-tibial malalignment is present. Varus/valgus hindfoot malalignment is due to multi-articular degeneration, and subtalar or triple arthrodesis is usually sufficient to correct the deformity; otherwise calcaneal osteotomies can be combined.22 As previously mentioned, in these cases one-stage or two-stage procedures can be performed.
When the malalignment is in the ankle joint, it can be due to: a) tibial joint line deformity or progressive degeneration, b) an incongruent (unstable) joint, or c) both. These deformities are usually in the coronal plane and the surgeon can therefore encounter five different scenarios,30 summarized in Table 1.
Figure 5.
Post-traumatic left ankle arthritis in a varus congruent joint (same patient as Figure 2). A) Careful debridement of the osteophytes, synovial tissue and excessive capsule. B) Tibial guide positioning under fluoroscopy in order to obtain a neutralizing tibial cut (greater bone removal on the lateral side) to restore alignment. Even though the CORA is diaphyseal (see Figure 2), malalignment is minimal (6°) and can be addressed with the TAR, with no need for osteotomy
The most common scenario in ankle osteoarthritis is a varus hindfoot alignment due to talar tilt. Valgus alignment is rare and often associated with rheumatoid arthritis.31
Some authors report that pre-operative varus or valgus deformity did have a significant effect on TAR survivorship, with the likelihood of revision being directly proportional to the size of the angular deformity. They report that TAR should be undertaken with extreme caution in the presence of marked malalignment.32 Some authors have shown that patients with preoperative in-congruent joints were 10 times more likely to develop progressive edge-loading than patients with congruent joints.30 Conversely, Hobson et al.33 compared TAR results from a group of patients with coronal deformity up to 10° with a second group with 11° to 30° of deformity. They did not find significant differences between the two groups and stated that TAR can be safely performed in patients with a hindfoot deformity of up to 30°. They highlighted as well the importance of adequate correction of alignment and instability. Similarly, Kim et al.34 compared the results of TAR in neutral and in varus ankles (>10°) and concluded that results are similar in the two groups when appropriate additional procedures to correct deformity are carried out simultaneous with the TAR. The importance of ligamentous stability has also been stated by some biomechanical studies.35
For minimal distal tibial deformities (<10°), realignment can be performed with the tibial cut. For more severe deformities, a dome or wedge osteotomy is required prior to considering TAR. In pre-operative planning, a greater medial tibial resection must be planned in cases of valgus tibial deformity, while a greater lateral resection is needed for varus tibial deformity.
In incongruent joints, it is important to plan additional soft-tissue procedures based on the type of instability and to choose the correct implant. Indeed, it can be helpful in cases of severe deformity to use instruments that allow independent tibial and talar cuts (see surgical technique section).
As previously mentioned, talar avascular necrosis is a contraindication for TAR. However, in cases of focal or superficial bone necrosis, TAR can be performed. In these cases, designs that remove more talar bone are recommended.15
Once alignment planning has been performed, sizing of the tibial and talar components is evaluated with the specific phantom templates of the chosen implant.
SURGICAL TECHNIQUE
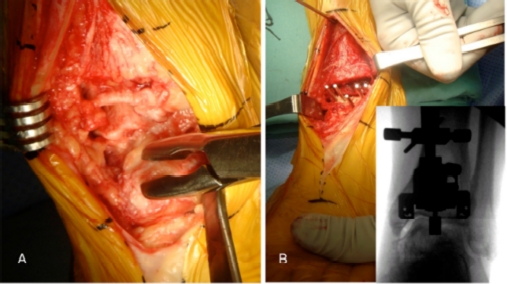
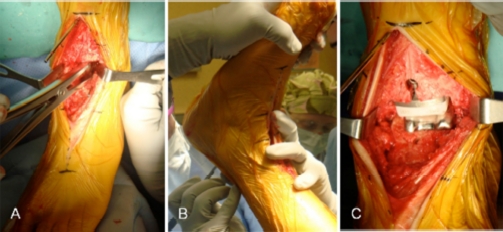
The patient is positioned supine, with a bump under the hip and a tourniquet on the proximal thigh. A midline 10 cm incision is made centered on the joint line. The superficial peroneal nerve is identified and protected throughout the procedure. The joint is approached through the bed of the tibialis anterior tendon (or the extensor hallucis longus). The goal is to achieve correct soft tissue balance and tibiotalar alignment, in order to position the tibial and talar components perpendicular to the plumb line of the body, i.e., parallel to the floor when the patient is standing. Therefore, careful debridement of osteophytes, synovial tissue and excessive capsule is carried out (Figure 5A). The medial and lateral gutters need to be debrided as well.
In congruent varus (or valgus) ankles, the tibial cut is then performed to neutralize the lateral distal tibial angle and to have a reference for talar tilt reduction (Figure 5B). In varus tibial deformity, more bone should be removed laterally compared to medially (Figure 5B), and vice versa in valgus tibial malalignment. Talar tilt is then reduced by sub-periosteal deltoid ligament release with a Cobb elevator (Figure 6A). If this is not sufficient, the tibialis posterior tendon is released as well.
Figure 6.
Post-traumatic left ankle arthritis in a varus congruent joint (same patient as Figure 2 and 5). A) After tibial and talar cuts, soft tissue balance is checked with a laminar spreader. B) After trial component positioning, the range of motion is checked. If there is insufficient dorsiflexion (not due to component malpositioning) percutaneous Achilles tendon lengthening is performed. C) Final components of Salto Talaris TAR implanted.
In incongruent varus (or valgus) ankles, the lateral distal tibial angle is normal and no neutralizing cuts are necessary. Talar tilt is reduced with intra-articular and extra-articular soft tissue procedures, as described above.
In cases of severe varus or valgus deformity (i.e. rheumatoid patients), tibial and talar bone cuts are necessary to re-align the joint, with a“sculpturing technique.“31 When performing tibial cuts with the talus still tilted, it is important to have TAR instruments that allow a talar cut independent of the tibial one. Then, soft tissue procedures can be performed to achieve a rectangular space between the tibial and talar cuts.
At this point, bone cuts are completed and trial components positioned, according to the surgical technique of the implant selected. With the trial components, range of motion and stability should be checked. If dorsiflexion is limited and this is not due to component malpositioning, a percutaneous Achilles’tendon lengthening is indicated (Figure 6B). If stability of the implant is not satisfactory due to varus tilt, reconstruction of the lateral ligaments may be necessary after definitive component positioning (Figures 6C, 7). Generally, if the implants are placed in anatomic alignment, with an appropriate polyethylene spacer, instability is uncommon. If ligamentous stabilization is required, this can be performed as for ankle instability, with anatomic repair (Brostrom36 or Brostrom-Gould37) with or without auto/allograft augmentation, or with non-anatomic reconstruction tenodeses (i.e., Watson-Jones,38 Evans,39 Chrisman-Snook40 procedures).
Figure 7.
Post-operative fluoroscopy. A) Lateral view, B) Antero-posterior view, showing a valgus correction of 7° on the coronal plane, in order to correct the 6° of diaphyseal varus deformity (see Figure 2).
Occasionally, despite these measures, hindfoot malalignment persists and requires correction with a separate hindfoot procedure (calcaneal osteotomy, sub-talar fusion), that can be performed concomitantly or as staged procedure, depending on the complexity of the index operation.
The post-operative regimen is dictated by the combined bony procedures (arthrodesis or osteotomy). If only TAR and soft tissue procedures were performed, the patient is immobilized in a walking plaster cast for 4-6 weeks. Weight bearing is usually allowed with crutches, unless markedly poor bone quality is identified. Then, the plaster is removed, free range of motion and weight bearing as tolerated are allowed.
RESULTS
Results with first-generation ankle replacements were found to be poor and it was recommended their use be discontinued for the Mayo,41“43 Conaxial (Beck-Stefee),44 Bath and Wessex,45 Newton,46 Waugh,1 Smith1 and Oregon1 implants.
With newer designs, encouraging results have been reported. Studies on long-term prosthetic survival rates (>10 years follow-up) of TAR have mainly been reported by inventors of the different prostheses.47 In fact, Buechel et al.48 reported a 12-year survival rate of 92% for 75 Beuchel-Pappas prostheses. Kofoed49 reported the results of 33 cemented STAR and of 25 uncemented STAR implants with a 70% survival rate (confidence limit, 60.3-78.5) for the cemented group and 95.4% survival rate (confidence limit, 91.0-99.9) for the uncemented group.49 Knecht et al.50 found a 10-year survival of 85% with 132 Agility prostheses.
Henricson et al.47 reported on survivorship of 531 different TARs implanted in Sweden from 1993 to 2005 and recorded in the Swedish Ankle Arthroplasty Register. The estimated overall five-year survival rate was 0.78 (95%CI: 0.74-0.82) and the 10-year survival rate was 0.62 (0.52-0.72). Gender and rheumatoid arthritis were not correlated with higher failure rates. Conversely, lower age at the index surgery implied an increased risk of revision (p = 0.002, RR 0.98, CI: 0.96-0.99). For the three surgeons who had implanted the majority of the STAR ankles, survival rates became significantly higher after the first 30 cases, increasing at five years from 0.70 (0.57-0.77) to 0.86 (0.80-0.93) 5
Fevang et al.51 reported on survivorship of 257 TARs recorded in the Norwegian Arthroplasty Register and implanted between 1994 and 2005. The overall five-year and 10-year survival were 89% and 76%, respectively. The authors found no significant influence from age, sex, type of implant, diagnosis, or cementation on the risk of revision.51
A recent systematic review was conducted to compare the outcomes of different TARs available on the market.52 Thirteen Level IV studies reporting on 1105 total ankle arthroplasties (234 Agility™, 344 STAR, 153 Buechel-Pappas™, 152 HINTEGRA®, 98 Salto™, 70 TNK, 54 Mobility™) were included. Residual pain was common (range, 27%-60%), superficial wound complications occurred in 0% to 14.7%, deep infections occurred in 0% to 4.6% of ankles, and ankle function improved after total ankle arthroplasty. The overall failure rate was approximately 10% at five years with a wide range (range, 0%-32%) between different centers. The authors concluded that superiority of an implant design over another could not be supported by the available data.52
SooHoo et al.53 compared the reoperation rates following ankle arthrodesis and TAR. Patients with TAR had an increased risk of device-related infection and of having a major revision procedure. The rates of major revision surgery after ankle replacement were 9% at one year and 23% at five years compared with 5% and 11% following ankle arthrodesis. Patients treated with ankle arthrodesis had a higher rate of subtalar fusion at five years postoperatively (2.8%) than did those treated with ankle replacement (0.7%).53 Conversely, similar rates of revision for TAR and ankle fusion have been reported by Haddad et al. in their systematic review.54
Saltzman et al.55 wrote a preliminary report (24 months follow-up) of a prospective controlled trial of STAR versus ankle fusion. The authors found that major complications and need for secondary surgical intervention were more common in the TAR group than in the ankle fusion group. However, ankles treated with STAR ankle replacement had better function and equivalent pain relief compared to ankles treated with fusion.
CONCLUSIONS
Even though many aspects are still being defined (indications, long-term outcomes of the newer designs, etc.), TAR should no longer be considered inferior to ankle fusion or as an experimental procedure. However, surgeons should remember that TAR is not for every patient and that the appropriate indication, based on the evidence available, is fundamental to obtaining durable and predictable outcomes. Ankle fusion is still a valid alternative for patients who are not amenable to TAR. A thorough knowledge of ankle anatomy, pathologic anatomy and biomechanics together with a careful pre-operative planning are mandatory to successful technical performance of total ankle replacement surgery.
REFERENCES
- 1.Vickerstaff JA, Miles AW, Cunningham JL. A brief history of total ankle replacement and a review of the current status. Med Eng Phys. 2007;29(10):1056–64. doi: 10.1016/j.medengphy.2006.11.009. Dec. [DOI] [PubMed] [Google Scholar]
- 2.Chou LB, Coughlin MT, Hansen S, Jr, Haskell A, Lundeen G, Saltzman CL, Mann RA. Osteoarthritis of the ankle: the role of arthroplasty. J Am Acad Orthop Surg. 2008;16(5):249–59. doi: 10.5435/00124635-200805000-00003. May. [DOI] [PubMed] [Google Scholar]
- 3.Inman VT. The joints of the ankle. Baltimore: Williams&Wilkins; 1979. [Google Scholar]
- 4.Isman RE, Inman VT. Anthropometric studies of the human foot and ankle. Bull Pros Res. 1969;10:97–129. [Google Scholar]
- 5.Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32:63–70. doi: 10.1016/s0021-9290(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 6.Leardini A, O'Connor JJ, Catani F. A geometric model of the human ankle joint. J Biomech. 1999;32:585–91. doi: 10.1016/s0021-9290(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 7.Lundberg A, Goldie I, Kalin B. Kinematics of the ankle/foot complex: plantarflexion and dorsiflexion. Foot Ankle. 1989;9:194–200. doi: 10.1177/107110078900900409. [DOI] [PubMed] [Google Scholar]
- 8.Kimizuka M, Kurosawa H, Fukubayashi T. Load-bearing pattern of the ankle joint. Arch Orthop Trauma Surg. 1980;96:45–49. doi: 10.1007/BF01246141. [DOI] [PubMed] [Google Scholar]
- 9.El-Khoury GY, Alliman KJ, Lundberg HJ, Rud-ert MJ, Brown TD, Saltzman CL. Cartilage thickness in cadaveric ankles: Measurement with double contrast multi-detector row CT arthrography versus MR imaging. Radiology. 2004;233:768–773. doi: 10.1148/radiol.2333031921. [DOI] [PubMed] [Google Scholar]
- 10.Castro MD. Ankle biomechanics. Foot Ankle Clin. 2002;7(4):679–93. doi: 10.1016/s1083-7515(02)00049-9. Dec. [DOI] [PubMed] [Google Scholar]
- 11.Michelson JD, Helgemo SL., Jr. Kinematics of the axially loaded ankle. Foot Ankle Int. 1995;16(9):577–82. doi: 10.1177/107110079501600912. Sep. [DOI] [PubMed] [Google Scholar]
- 12.Michelsen JD, Ahn UM, Helgemo SL. Motion of the ankle in a simulated supination-external rotation fracture model. J Bone Joint Surg Am. 1996;78(7):1024–31. doi: 10.2106/00004623-199607000-00006. Jul. [DOI] [PubMed] [Google Scholar]
- 13.Astion DJ, Deland JT, Otis JC, Kenneally S. Motion of the hindfoot after simulated arthrodesis. J Bone Joint Surg Am. 1997;79(2):241–6. doi: 10.2106/00004623-199702000-00012. Feb. [DOI] [PubMed] [Google Scholar]
- 14.Hvid I, Rasmussen O, Jensen NC, Nielsen S. Trabecular bone strength profiles at the ankle joint. Clin Orthop Relat Res. 1985;(199):306–12. Oct. [PubMed] [Google Scholar]
- 15.Easley ME, Vertullo CJ, Urban WC, Nunley JA. Total ankle arthroplasty. J Am Acad Orthop Surg. 2002;10(3):157–67. doi: 10.5435/00124635-200205000-00002. May-Jun. [DOI] [PubMed] [Google Scholar]
- 16.Saltzman CL. Total ankle arthroplasty. State of the art. Instr Course Lect. 1999;48:263–268. [PubMed] [Google Scholar]
- 17.Hintermann B. Short- and mid-term results with the STAR total ankle prosthesis. Orthopade. 1999;28:792–803. doi: 10.1007/PL00003669. [DOI] [PubMed] [Google Scholar]
- 18.Kofoed H, Lundberg-Jensen A. Ankle arthroplasty in patients younger and older than 50 years: A prospective series with long-term follow-up. Foot Ankle Int. 1999;20:501–506. doi: 10.1177/107110079902000807. [DOI] [PubMed] [Google Scholar]
- 19.Gould JS, Alvine FG, Mann RA, Sanders RW, Walling AK. Total ankle replacement: A surgical discussion. Part I: Replacement systems, indications, and contraindications. Am J Orthop. 2000;29:604–609. [PubMed] [Google Scholar]
- 20.Muir DC, Amendola A, Saltzman CL. Long-term outcome of ankle arthrodesis. Foot Ankle Clin. 2002;7(4):703–8. doi: 10.1016/s1083-7515(02)00048-7. Dec. [DOI] [PubMed] [Google Scholar]
- 21.Coester LM, Saltzman CL, Leupold J, Pontarelli W. Long-term results following ankle arthrodesis for post-traumatic arthritis. J Bone Joint Surg Am. 2001;83:219–228. doi: 10.2106/00004623-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Bonnin M, Judet T, Colombier JA, Buscayret F, Graveleau N, Piriou P. Midterm results of the Salto Total Ankle Prosthesis. Clin Orthop Relat Res. 2004;(424):6–18. doi: 10.1097/01.blo.0000132407.75881.a0. Jul. [DOI] [PubMed] [Google Scholar]
- 23.van der Heide HJ, Schutte B, Louwerens JW, van den Hoogen FH, Malefijt MC. Total ankle prostheses in rheumatoid arthropathy: Outcome in 52 patients followed for 1-9 years. Acta Orthop. 2009;80(4):440–4. doi: 10.3109/17453670903153568. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kofoed H, Sorensen TS. Ankle arthroplasty for rheumatoid arthritis and osteoarthritis: Prospective long-term study of cemented replacements. J Bone Joint Surg Br. 1998;80:328–332. doi: 10.1302/0301-620x.80b2.8243. [DOI] [PubMed] [Google Scholar]
- 25.Cracchiolo A, III, Cimino WR, Lian G. Arthrodesis of the ankle in patients who have rheumatoid arthritis. J Bone Joint Surg Am. 1992;74(6):903–9. Jul. [PubMed] [Google Scholar]
- 26.Doets HC, Brand R, Nelissen RG. Total ankle arthroplasty in inflammatory joint disease with use of two mobile-bearing designs. J Bone Joint Surg Am. 2006;88(6):1272–84. doi: 10.2106/JBJS.E.00414. Jun. [DOI] [PubMed] [Google Scholar]
- 27.Greisberg J, Assal M, Flueckiger G, Hansen ST., Jr Takedown of ankle fusion and conversion to total ankle replacement. Clin Orthop Relat Res. 2004;(424):80–8. doi: 10.1097/01.blo.0000132460.27102.d6. Jul. [DOI] [PubMed] [Google Scholar]
- 28.Hintermann B, Barg A, Knupp M, Valderrabano V. Conversion of painful ankle arthrodesis to total ankle arthroplasty. J Bone Joint Surg Am. 2009;91(4):850–8. doi: 10.2106/JBJS.H.00229. Apr. [DOI] [PubMed] [Google Scholar]
- 29.Mendicino RW, Catanzariti AR, Reeves CL. Percutaneous supramalleolar osteotomy for distal tibial (near articular) ankle deformities. J Am Podiatr Med Assoc. 2005;95(1):72–84. doi: 10.7547/0950072. Jan-Feb. [DOI] [PubMed] [Google Scholar]
- 30.Haskell A, Mann RA. Ankle arthroplasty with preoperative coronal plane deformity: short-term results. Clin Orthop Relat Res. 2004;(424):98–103. doi: 10.1097/01.blo.0000132248.64290.52. Jul. [DOI] [PubMed] [Google Scholar]
- 31.Kofoed H. Concept and use of the Scandinavian total ankle replacement. Foot Ankle Spec. 2009;2(2):89–94. doi: 10.1177/1938640009331488. Apr. [DOI] [PubMed] [Google Scholar]
- 32.Wood PL, Prem H, Sutton C. Total ankle replacement: medium-term results in 200 Scandinavian total ankle replacements. J Bone Joint Surg Br. 2008;90(5):605–9. doi: 10.1302/0301-620X.90B5.19677. May. [DOI] [PubMed] [Google Scholar]
- 33.Hobson SA, Karantana A, Dhar S. Total ankle replacement in patients with significant pre-operative deformity of the hindfoot. J Bone Joint Surg Br. 2009;91(4):481–6. doi: 10.1302/0301-620X.91B4.20855. Apr. [DOI] [PubMed] [Google Scholar]
- 34.Kim BS, Choi WJ, Kim YS, Lee JW. Total ankle replacement in moderate to severe varus deformity of the ankle. J Bone Joint Surg Br. 2009;91(9):1183–90. doi: 10.1302/0301-620X.91B9.22411. Sep. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K, Crevoisier XM, Kitaoka HB, Zhao KD, Berglund LJ, Kaufman KR, An KN. Analysis of joint laxity after total ankle arthroplasty: cadaver study. Clin Biomech (Bristol, Avon) 2009;24(8):655–60. doi: 10.1016/j.clinbiomech.2009.06.007. Oct. [DOI] [PubMed] [Google Scholar]
- 36.Broström L. Sprained ankles: VI. Surgical treatment of “chronic” ligament ruptures. Acta Chir Scand. 1966;132:551–565. [PubMed] [Google Scholar]
- 37.Gould N, Seligson D, Gassman J. Early and late repair of lateral ligament of the ankle. Foot Ankle. 1980;1:84–89. doi: 10.1177/107110078000100206. [DOI] [PubMed] [Google Scholar]
- 38.Watson-Jones R. Recurrent forward dislocation of the ankle joint. J Bone Joint Surg Br. 1952;134:519. [Google Scholar]
- 39.Evans DL. Recurrent instability of the ankle: A method of surgical treatment. Proc R Soc Med. 1953;46:343–344. [PMC free article] [PubMed] [Google Scholar]
- 40.Chrisman OD, Snook GA. Reconstruction of lateral ligament tears of the ankle: An experimental study and clinical evaluation of seven patients treated by a new modification of the Elmslie procedure. J Bone Joint Surg Am. 1969;51:904–912. [PubMed] [Google Scholar]
- 41.Stauffer RN, Segal NM. Total ankle ar throplasty: 4 years' experience. Clin Orthop Relat Res. 1981;160:217–21. [PubMed] [Google Scholar]
- 42.Kitaoka HB, Patzer GL, Ilstrup DM, Wallrichs SL. Survivorship analysis of the Mayo total ankle arthroplasty. J Bone Joint Surg Am. 1994;76:974–9. doi: 10.2106/00004623-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Kitaoka HB, Patzer GL. Clinical results of the Mayo total ankle arthroplasty. J Bone Joint Surg Am. 1996;78:1658–64. doi: 10.2106/00004623-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Wynn AH, Wilde AH. Long-term follow-up of the Conaxial (Beck-Steffee) total ankle arthroplasty. Foot Ankle. 1992;13:303–6. doi: 10.1177/107110079201300601. [DOI] [PubMed] [Google Scholar]
- 45.Carlsson A, Henricson A, Linder L, Nilsson J, Redlund-Johnell I. A 10-year survival analysis of 69 Bath and Wessex ankle replacements. Foot Ankle Surg. 2001;7:39–44. [Google Scholar]
- 46.Newton III SE. Total ankle arthroplasty. Clinical study of fifty cases. J Bone Joint Surg Am. 1982;64:104–11. [PubMed] [Google Scholar]
- 47.Henricson A, Skoog A, Carlsson A. The Swedish Ankle Arthroplasty Register: An analysis of 531 arthroplasties between 1993 and 2005. Acta Orthop. 2007;78(5):569–74. doi: 10.1080/17453670710014248. Oct. [DOI] [PubMed] [Google Scholar]
- 48.Buechel F, Sr, Buechel F, Jr, Pappas M. Twenty-year evaluation of cementless mobile-bearing total ankle replacements. Clin Orthop Relat Res. 2004;(424):19–26. doi: 10.1097/01.blo.0000132243.41419.59. [DOI] [PubMed] [Google Scholar]
- 49.Kofoed H. Scandinavian Total Ankle Replacement (STAR) Clin Orthop Relat Res. 2004;(424):73–9. doi: 10.1097/01.blo.0000132414.41124.06. [DOI] [PubMed] [Google Scholar]
- 50.Knecht S, Estin M, Callaghan J, Zimmerman M, Alliman K, Alvine F, Saltzman C. The agility total ankle arthroplasty: seven to sixteen-year follow-up. J Bone Joint Surg (Am) 2004;86(6):1161–71. [PubMed] [Google Scholar]
- 51.Fevang BT, Lie SA, Havelin LI, Brun JG, Skredderstuen A, Furnes O. 257 ankle arthroplasties performed in Norway between 1994 and 2005. Acta Orthop. 2007;78(5):575–83. doi: 10.1080/17453670710014257. Oct. [DOI] [PubMed] [Google Scholar]
- 52.Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199–208. doi: 10.1007/s11999-009-0987-3. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.SooHoo NF, Zingmond DS, Ko CY. Comparison of reoperation rates following ankle arthrodesis and total ankle arthroplasty. J Bone Joint Surg Am. 2007;89(10):2143–9. doi: 10.2106/JBJS.F.01611. Oct. [DOI] [PubMed] [Google Scholar]
- 54.Haddad SL, Coetzee JC, Estok R, Fahrbach K, Banel D, Nalysnyk L. Intermediate and long-term outcomes of total ankle arthroplasty and ankle arthrodesis. A systematic review of the literature. J Bone Joint Surg Am. 2007;89(9):1899–905. doi: 10.2106/JBJS.F.01149. Sep. [DOI] [PubMed] [Google Scholar]
- 55.Saltzman CL, Mann RA, Ahrens JE, Amendola A, Anderson RB, Berlet GC, Brodsky JW, Chou LB, Clanton TO, Deland JT, Deorio JK, Horton GA, Lee TH, Mann JA, Nunley JA, Thordarson DB, Walling AK, Wapner KL, Coughlin MJ. Prospective controlled trial of STAR total ankle replacement versus ankle fusion: initial results. Foot Ankle Int. 2009;30(7):579–96. doi: 10.3113/FAI.2009.0579. Jul. [DOI] [PubMed] [Google Scholar]