Abstract
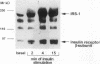
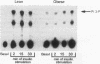
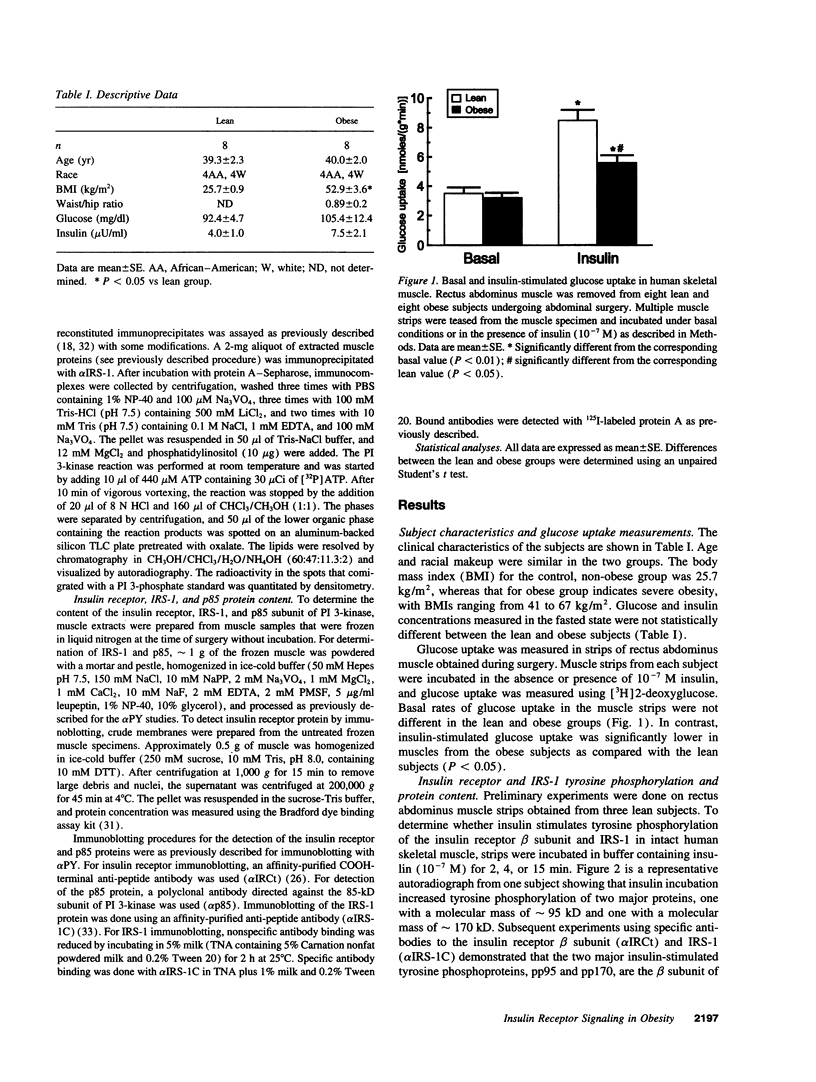
To determine whether the impaired insulin-stimulated glucose uptake in obese individuals is associated with altered insulin receptor signaling, we measured both glucose uptake and early steps in the insulin action pathway in intact strips of human skeletal muscle. Biopsies of rectus abdominus muscle were taken from eight obese and eight control subjects undergoing elective surgery (body mass index 52.9 +/- 3.6 vs 25.7 +/- 0.9). Insulin-stimulated 2-deoxyglucose uptake was 53% lower in muscle strips from obese subjects. Additional muscle strips were incubated in the basal state or with 10(-7) M insulin for 2, 15, or 30 min. In the lean subjects, tyrosine phosphorylation of the insulin receptor and insulin receptor substrate-1 (IRS-1), measured by immunoblotting with anti-phosphotyrosine antibodies, was significantly increased by insulin at all time points. In the skeletal muscle from the obese subjects, insulin was less effective in stimulating tyrosine phosphorylation (maximum receptor and IRS-1 phosphorylation decreased by 35 and 38%, respectively). Insulin stimulation of IRS-1 immunoprecipitable phosphatidylinositol 3-kinase (PI 3-kinase) activity also was markedly lower in obese subjects compared with controls (10- vs 35-fold above basal, respectively). In addition, the obese subjects had a lower abundance of the insulin receptor, IRS-1, and the p85 subunit of PI 3-kinase (55, 54, and 64% of nonobese, respectively). We conclude that impaired insulin-stimulated glucose uptake in skeletal muscle from severely obese subjects is accompanied by a deficiency in insulin receptor signaling, which may contribute to decreased insulin action.
Full text
PDF

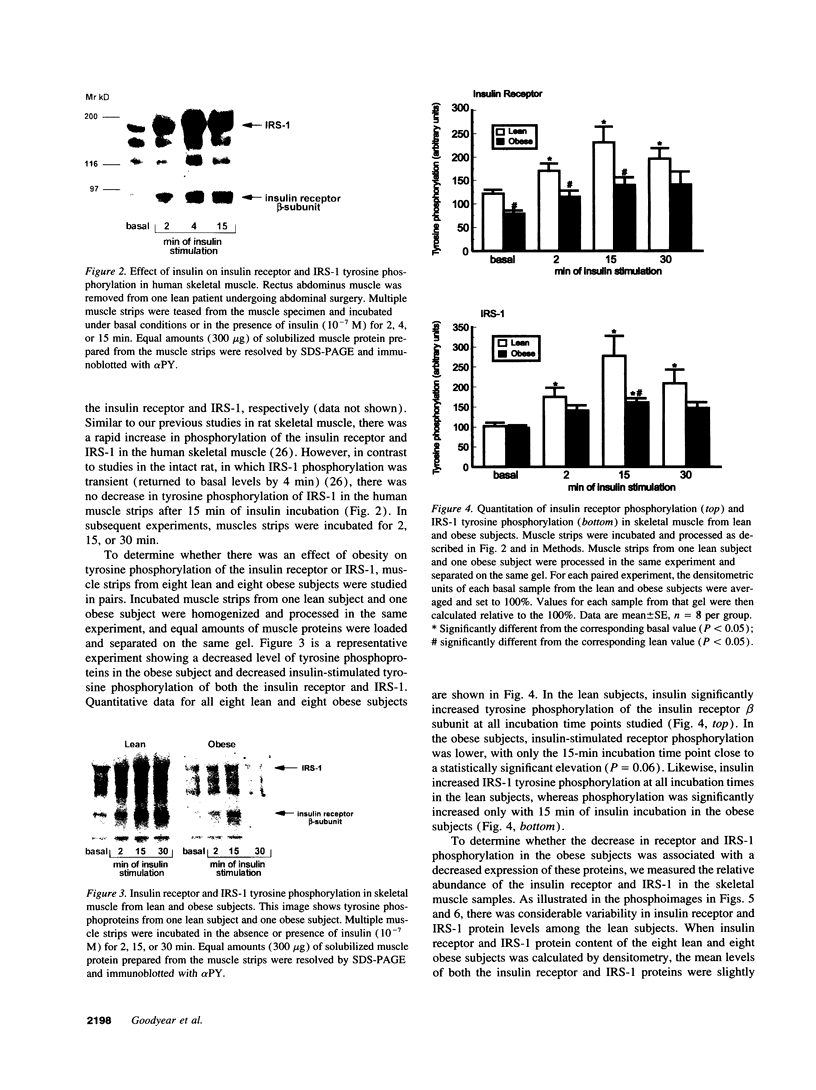
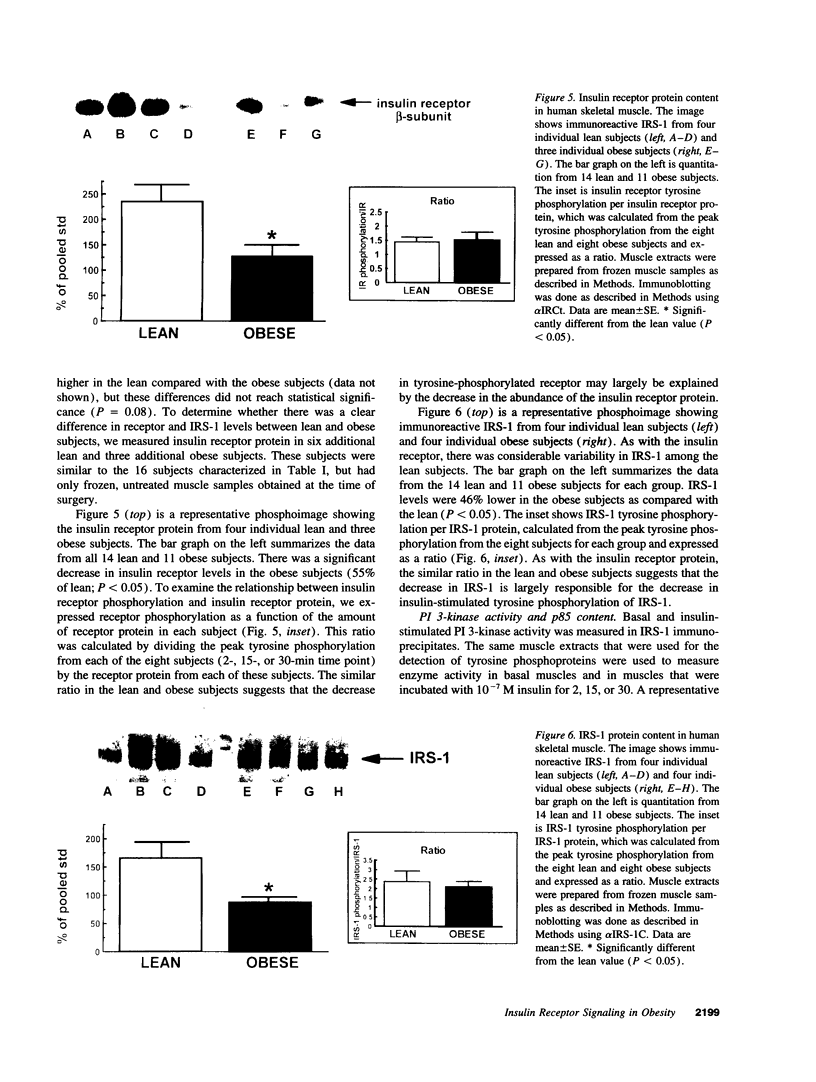
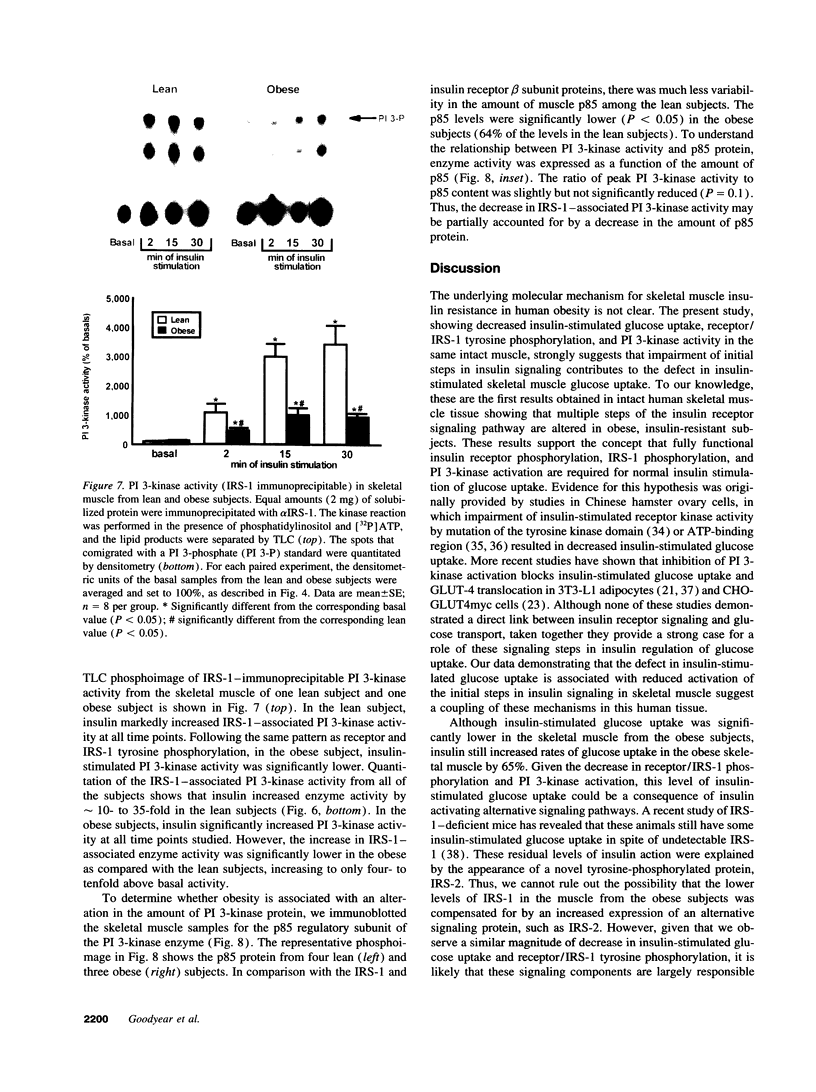
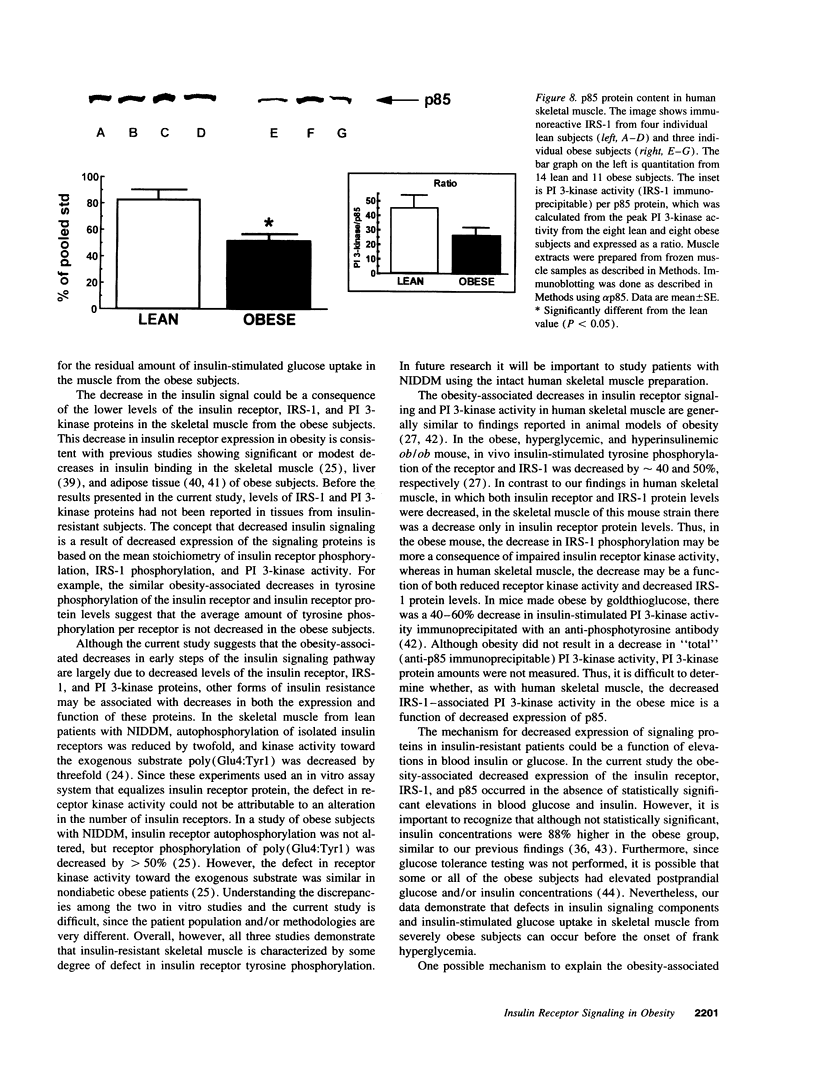



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki E., Lipes M. A., Patti M. E., Brüning J. C., Haag B., 3rd, Johnson R. S., Kahn C. R. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994 Nov 10;372(6502):186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Schroeder G. G., Kahn C. R., Myers M. G., Jr, Wilden P. A., Cahill D. A., White M. F. Insulin stimulation of phosphatidylinositol 3-kinase activity maps to insulin receptor regions required for endogenous substrate phosphorylation. J Biol Chem. 1992 Jan 15;267(2):1367–1374. [PubMed] [Google Scholar]
- Block N. E., Buse M. G. Effects of hypercortisolemia and diabetes on skeletal muscle insulin receptor function in vitro and in vivo. Am J Physiol. 1989 Jan;256(1 Pt 1):E39–E48. doi: 10.1152/ajpendo.1989.256.1.E39. [DOI] [PubMed] [Google Scholar]
- Bonen A., Tan M. H., Watson-Wright W. M. Insulin binding and glucose uptake differences in rodent skeletal muscles. Diabetes. 1981 Aug;30(8):702–704. doi: 10.2337/diab.30.8.702. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Ittoop O., Pories W. J., Meelheim D., Flickinger E. G., Thomas F., Jenquin M., Silverman J. F., Khazanie P. G., Sinha M. K. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J Clin Invest. 1986 Jul;78(1):249–258. doi: 10.1172/JCI112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Sinha M. K., Raju S. M., Ittoop O., Pories W. J., Flickinger E. G., Meelheim D., Dohm G. L. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest. 1987 May;79(5):1330–1337. doi: 10.1172/JCI112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. L., Auger K. R., Duckworth B. C., Hou W. M., Schaffhausen B., Cantley L. C. A tightly associated serine/threonine protein kinase regulates phosphoinositide 3-kinase activity. Mol Cell Biol. 1993 Mar;13(3):1657–1665. doi: 10.1128/mcb.13.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham B., Vlahos C. J., Cheatham L., Wang L., Blenis J., Kahn C. R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994 Jul;14(7):4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. S., Friel J. C., Ruderman N. B. Regulation of phosphatidylinositol 3-kinase by insulin in rat skeletal muscle. Am J Physiol. 1993 Nov;265(5 Pt 1):E736–E742. doi: 10.1152/ajpendo.1993.265.5.E736. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Clarke J. F., Young P. W., Yonezawa K., Kasuga M., Holman G. D. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem J. 1994 Jun 15;300(Pt 3):631–635. doi: 10.1042/bj3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A. Insulin secretion, insulin resistance, and obesity. Int J Obes. 1982;6 (Suppl 1):73–82. [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Dhand R., Hiles I., Panayotou G., Roche S., Fry M. J., Gout I., Totty N. F., Truong O., Vicendo P., Yonezawa K. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994 Feb 1;13(3):522–533. doi: 10.1002/j.1460-2075.1994.tb06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm G. L., Elton C. W., Friedman J. E., Pilch P. F., Pories W. J., Atkinson S. M., Jr, Caro J. F. Decreased expression of glucose transporter in muscle from insulin-resistant patients. Am J Physiol. 1991 Mar;260(3 Pt 1):E459–E463. doi: 10.1152/ajpendo.1991.260.3.E459. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Endemann G., Yonezawa K., Roth R. A. Phosphatidylinositol kinase or an associated protein is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1990 Jan 5;265(1):396–400. [PubMed] [Google Scholar]
- Ferrannini E., Bjorkman O., Reichard G. A., Jr, Pilo A., Olsson M., Wahren J., DeFronzo R. A. The disposal of an oral glucose load in healthy subjects. A quantitative study. Diabetes. 1985 Jun;34(6):580–588. doi: 10.2337/diab.34.6.580. [DOI] [PubMed] [Google Scholar]
- Folli F., Saad M. J., Backer J. M., Kahn C. R. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J Biol Chem. 1992 Nov 5;267(31):22171–22177. [PubMed] [Google Scholar]
- Freidenberg G. R., Henry R. R., Klein H. H., Reichart D. R., Olefsky J. M. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J Clin Invest. 1987 Jan;79(1):240–250. doi: 10.1172/JCI112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. E., Sherman W. M., Reed M. J., Elton C. W., Dohm G. L. Exercise training increases glucose transporter protein GLUT-4 in skeletal muscle of obese Zucker (fa/fa) rats. FEBS Lett. 1990 Jul 30;268(1):13–16. doi: 10.1016/0014-5793(90)80960-q. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Maianu L., Hancock J. A., Golichowski A. M., Baron A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes. 1992 Apr;41(4):465–475. doi: 10.2337/diab.41.4.465. [DOI] [PubMed] [Google Scholar]
- Giorgetti S., Ballotti R., Kowalski-Chauvel A., Tartare S., Van Obberghen E. The insulin and insulin-like growth factor-I receptor substrate IRS-1 associates with and activates phosphatidylinositol 3-kinase in vitro. J Biol Chem. 1993 Apr 5;268(10):7358–7364. [PubMed] [Google Scholar]
- Giorgino F., Almahfouz A., Goodyear L. J., Smith R. J. Glucocorticoid regulation of insulin receptor and substrate IRS-1 tyrosine phosphorylation in rat skeletal muscle in vivo. J Clin Invest. 1993 May;91(5):2020–2030. doi: 10.1172/JCI116424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgino F., Chen J. H., Smith R. J. Changes in tyrosine phosphorylation of insulin receptors and a 170,000 molecular weight nonreceptor protein in vivo in skeletal muscle of streptozotocin-induced diabetic rats: effects of insulin and glucose. Endocrinology. 1992 Mar;130(3):1433–1444. doi: 10.1210/endo.130.3.1531627. [DOI] [PubMed] [Google Scholar]
- Handberg A., Vaag A., Damsbo P., Beck-Nielsen H., Vinten J. Expression of insulin regulatable glucose transporters in skeletal muscle from type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1990 Oct;33(10):625–627. doi: 10.1007/BF00400207. [DOI] [PubMed] [Google Scholar]
- Heydrick S. J., Jullien D., Gautier N., Tanti J. F., Giorgetti S., Van Obberghen E., Le Marchand-Brustel Y. Defect in skeletal muscle phosphatidylinositol-3-kinase in obese insulin-resistant mice. J Clin Invest. 1993 Apr;91(4):1358–1366. doi: 10.1172/JCI116337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Zorzano A., Böni-Schnetzler M., Nemenoff R. A., Powers A., Pilch P. F., Ruderman N. B. Intrinsic differences of insulin receptor kinase activity in red and white muscle. J Biol Chem. 1986 Nov 15;261(32):14939–14944. [PubMed] [Google Scholar]
- Kanai F., Ito K., Todaka M., Hayashi H., Kamohara S., Ishii K., Okada T., Hazeki O., Ui M., Ebina Y. Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI3-kinase. Biochem Biophys Res Commun. 1993 Sep 15;195(2):762–768. doi: 10.1006/bbrc.1993.2111. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kelly K. L., Ruderman N. B., Chen K. S. Phosphatidylinositol-3-kinase in isolated rat adipocytes. Activation by insulin and subcellular distribution. J Biol Chem. 1992 Feb 15;267(5):3423–3428. [PubMed] [Google Scholar]
- King P. A., Horton E. D., Hirshman M. F., Horton E. S. Insulin resistance in obese Zucker rat (fa/fa) skeletal muscle is associated with a failure of glucose transporter translocation. J Clin Invest. 1992 Oct;90(4):1568–1575. doi: 10.1172/JCI116025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusari J., Kenner K. A., Suh K. I., Hill D. E., Henry R. R. Skeletal muscle protein tyrosine phosphatase activity and tyrosine phosphatase 1B protein content are associated with insulin action and resistance. J Clin Invest. 1994 Mar;93(3):1156–1162. doi: 10.1172/JCI117068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamphere L., Lienhard G. E. Components of signaling pathways for insulin and insulin-like growth factor-I in muscle myoblasts and myotubes. Endocrinology. 1992 Nov;131(5):2196–2202. doi: 10.1210/endo.131.5.1385098. [DOI] [PubMed] [Google Scholar]
- McGuire M. C., Fields R. M., Nyomba B. L., Raz I., Bogardus C., Tonks N. K., Sommercorn J. Abnormal regulation of protein tyrosine phosphatase activities in skeletal muscle of insulin-resistant humans. Diabetes. 1991 Jul;40(7):939–942. doi: 10.2337/diab.40.7.939. [DOI] [PubMed] [Google Scholar]
- Obermaier-Kusser B., White M. F., Pongratz D. E., Su Z., Ermel B., Muhlbacher C., Haring H. U. A defective intramolecular autoactivation cascade may cause the reduced kinase activity of the skeletal muscle insulin receptor from patients with non-insulin-dependent diabetes mellitus. J Biol Chem. 1989 Jun 5;264(16):9497–9504. [PubMed] [Google Scholar]
- Parker P. J., Waterfield M. D. Phosphatidylinositol 3-kinase: a novel effector. Cell Growth Differ. 1992 Oct;3(10):747–752. [PubMed] [Google Scholar]
- Pedersen O., Bak J. F., Andersen P. H., Lund S., Moller D. E., Flier J. S., Kahn B. B. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990 Jul;39(7):865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L. M., Ganguly S., Smith C. J., Cobb M. H., Rubin C. S., Rosen O. M. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6792–6796. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pories W. J., MacDonald K. G., Jr, Morgan E. J., Sinha M. K., Dohm G. L., Swanson M. S., Barakat H. A., Khazanie P. G., Leggett-Frazier N., Long S. D. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992 Feb;55(2 Suppl):582S–585S. doi: 10.1093/ajcn/55.2.582s. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Kapeller R., White M. F., Cantley L. C. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M. J., Araki E., Miralpeix M., Rothenberg P. L., White M. F., Kahn C. R. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest. 1992 Nov;90(5):1839–1849. doi: 10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu P. V., Takegawa K., Fry M. J., Stack J. H., Waterfield M. D., Emr S. D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993 Apr 2;260(5104):88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Shoelson S. E., Chatterjee S., Chaudhuri M., White M. F. YMXM motifs of IRS-1 define substrate specificity of the insulin receptor kinase. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2027–2031. doi: 10.1073/pnas.89.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M. K., Pories W. J., Flickinger E. G., Meelheim D., Caro J. F. Insulin-receptor kinase activity of adipose tissue from morbidly obese humans with and without NIDDM. Diabetes. 1987 May;36(5):620–625. doi: 10.2337/diab.36.5.620. [DOI] [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Kahn C. R. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988 Mar 5;263(7):3440–3447. [PubMed] [Google Scholar]
- Walmsley A. R. The dynamics of the glucose transporter. Trends Biochem Sci. 1988 Jun;13(6):226–231. doi: 10.1016/0968-0004(88)90089-8. [DOI] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr 14;332(6165):644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]