Abstract
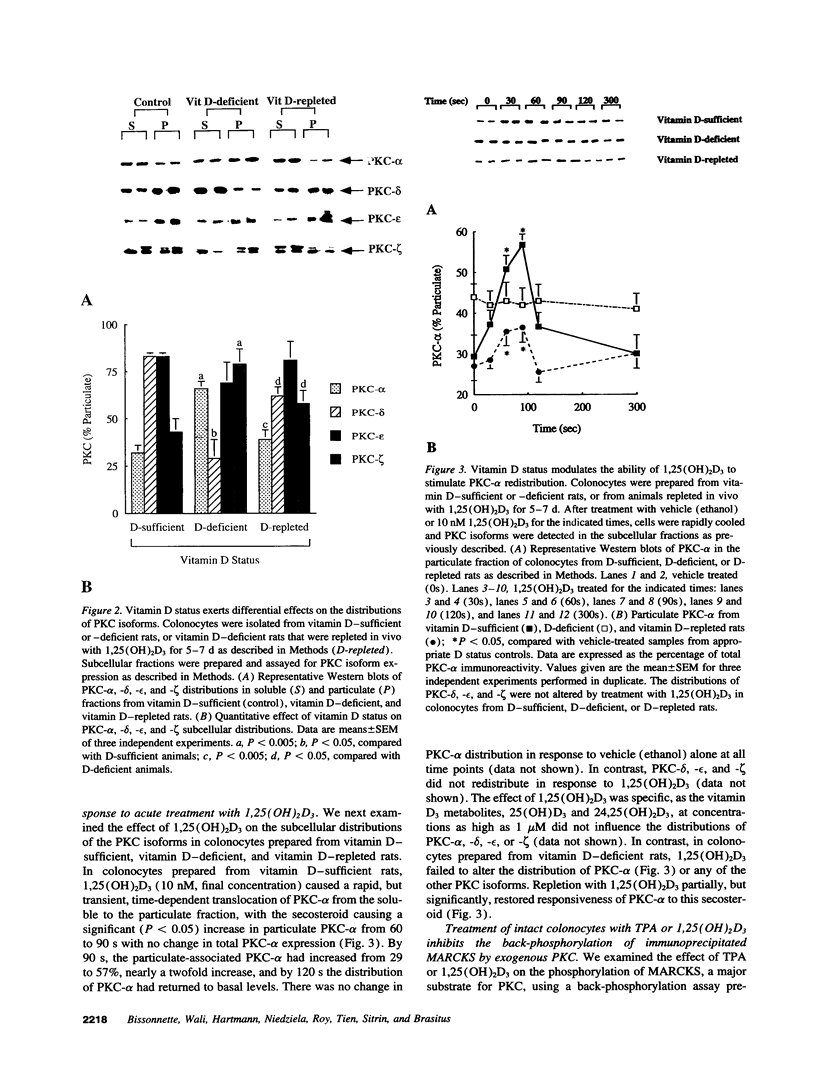
Considerable evidence that alterations in protein kinase C (PKC) are intimately involved in important physiologic and pathologic processes in many cells, including colonic epithelial cells, has accumulated. In this regard, phorbol esters, a class of potent PKC activators, have been found to induce a number of cellular events in normal or transformed colonocytes. In addition, our laboratory has demonstrated that the major active metabolite of vitamin D3, 1,25(OH)2D3, also rapidly (seconds-minutes) activated PKC and increased intracellular calcium in isolated rat colonocytes. These acute responses, however, were lost in vitamin D deficiency and partially restored with the in vivo repletion of 1,25(OH)2D3. The Ca(2+)-independent or novel isoforms of PKC expressed in the rat colon and the isoform-specific responses of PKC to acute treatment with phorbol esters or 1,25(OH)2D3 have not been previously characterized. Moreover, the effects of vitamin D status on PKC isoform expression, distribution, and response to agonists are also unknown. In the present experiments, in addition to PKC-alpha, rat colonocytes were found to express the novel isoforms delta, epsilon, and zeta by Western blotting using isoform-specific PKC antibodies. The tumor-promoting phorbol ester, 12-O-tetradecanoyl phorbol 13-acetate, caused time- and concentration-dependent translocations of all these isoforms except PKC-zeta. In vitamin D deficiency, there were no alterations in colonic PKC isoform expression but significant changes in the subcellular distribution of PKC-alpha, -delta, and -zeta. Acute treatment of colonocytes from D-sufficient, but not D-deficient, rats with 1,25(OH)2D3 caused a rapid transient redistribution of only PKC-alpha from the soluble to the particulate fraction. The alterations in PKC isoform distribution and PKC-alpha responsiveness to 1,25(OH)2D3 in vitamin D deficiency were partially, but significantly, restored with 5-7 d in vivo repletion of this secosteroid. Both 12-O-tetradecanoyl phorbol 13-acetate and 1,25(OH)2D3 activated endogenous PKC, as assessed by inhibition of myristoylated alanine-rich C kinase substrate back-phosphorylation by exogenous PKC. These studies indicate that PKC-alpha, -delta, and/or -epsilon likely mediate important phorbol ester-stimulated events described in the rat colon. In contrast, PKC-alpha is implicated in the rapid (s-min) PKC-dependent events initiated by 1,25(OH)2D3 in rat colonocytes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Albert K. A., Keum M. M., Wang J. K., Greengard P., Cohn Z. A. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988 Mar 24;332(6162):362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- Albert K. A., Nairn A. C., Greengard P. The 87-kDa protein, a major specific substrate for protein kinase C: purification from bovine brain and characterization. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7046–7050. doi: 10.1073/pnas.84.20.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa M. A., Firestein B. L., Godson C., Bell K. S., Insel P. A. Protein kinase C alpha mediates phospholipase D activation by nucleotides and phorbol ester in Madin-Darby canine kidney cells. Stimulation of phospholipase D is independent of activation of polyphosphoinositide-specific phospholipase C and phospholipase A2. J Biol Chem. 1994 Apr 8;269(14):10511–10516. [PubMed] [Google Scholar]
- Belleli A., Shany S., Levy J., Guberman R., Lamprecht S. A. A protective role of 1,25-dihydroxyvitamin D3 in chemically induced rat colon carcinogenesis. Carcinogenesis. 1992 Dec;13(12):2293–2298. doi: 10.1093/carcin/13.12.2293. [DOI] [PubMed] [Google Scholar]
- Bissonnette M., Tien X. Y., Niedziela S. M., Hartmann S. C., Frawley B. P., Jr, Roy H. K., Sitrin M. D., Perlman R. L., Brasitus T. A. 1,25(OH)2 vitamin D3 activates PKC-alpha in Caco-2 cells: a mechanism to limit secosteroid-induced rise in [Ca2+]i. Am J Physiol. 1994 Sep;267(3 Pt 1):G465–G475. doi: 10.1152/ajpgi.1994.267.3.G465. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Brandsch M., Miyamoto Y., Ganapathy V., Leibach F. H. Expression and protein kinase C-dependent regulation of peptide/H+ co-transport system in the Caco-2 human colon carcinoma cell line. Biochem J. 1994 Apr 1;299(Pt 1):253–260. doi: 10.1042/bj2990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. J., Bell R. M. Protein kinase C contains two phorbol ester binding domains. J Biol Chem. 1991 Sep 25;266(27):18330–18338. [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Alterations in protein kinase C in 1,2-dimethylhydrazine induced colonic carcinogenesis. Cancer Res. 1992 Apr 15;52(8):2216–2221. [PubMed] [Google Scholar]
- Craven P. A., Pfanstiel J., DeRubertis F. R. Role of activation of protein kinase C in the stimulation of colonic epithelial proliferation and reactive oxygen formation by bile acids. J Clin Invest. 1987 Feb;79(2):532–541. doi: 10.1172/JCI112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker L. V., Parker P. J. Protein kinase C--a question of specificity. Trends Biochem Sci. 1994 Feb;19(2):73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Dominguez I., Sanz L., Arenzana-Seisdedos F., Diaz-Meco M. T., Virelizier J. L., Moscat J. Inhibition of protein kinase C zeta subspecies blocks the activation of an NF-kappa B-like activity in Xenopus laevis oocytes. Mol Cell Biol. 1993 Feb;13(2):1290–1295. doi: 10.1128/mcb.13.2.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Cheng H. Y., Sharp G. W. Effects of phorbol esters on sodium and chloride transport in rat colon. Am J Physiol. 1986 Oct;251(4 Pt 1):G509–G517. doi: 10.1152/ajpgi.1986.251.4.G509. [DOI] [PubMed] [Google Scholar]
- Frawley B. P., Jr, Tien X. Y., Hartmann S. C., Wali R. K., Niedziela S. M., Davidson N. O., Sitrin M. D., Brasitus T. A., Bissonnette M. TPA causes divergent responses of Ca(2+)-dependent and Ca(2+)-independent isoforms of PKC in the nuclei of Caco-2 cells. Biochim Biophys Acta. 1994 Jun 30;1222(2):301–305. doi: 10.1016/0167-4889(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Grabarek J., Ware J. A. Protein kinase C activation without membrane contact in platelets stimulated by bryostatin. J Biol Chem. 1993 Mar 15;268(8):5543–5549. [PubMed] [Google Scholar]
- Guillem J. G., O'Brian C. A., Fitzer C. J., Johnson M. D., Forde K. A., LoGerfo P., Weinstein I. B. Studies on protein kinase C and colon carcinogenesis. Arch Surg. 1987 Dec;122(12):1475–1478. doi: 10.1001/archsurg.1987.01400240123023. [DOI] [PubMed] [Google Scholar]
- Halline A. G., Davidson N. O., Skarosi S. F., Sitrin M. D., Tietze C., Alpers D. H., Brasitus T. A. Effects of 1,25-dihydroxyvitamin D3 on proliferation and differentiation of Caco-2 cells. Endocrinology. 1994 Apr;134(4):1710–1717. doi: 10.1210/endo.134.4.8137734. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Hoffman F. J., Jr, Janis R. A. Effects of calcium channel antagonists on the phosphorylation of major protein kinase C substrates in the rat hippocampus. Biochem Pharmacol. 1993 Aug 17;46(4):677–681. doi: 10.1016/0006-2952(93)90554-a. [DOI] [PubMed] [Google Scholar]
- Khare S., Tien X. Y., Wilson D., Wali R. K., Bissonnette B. M., Scaglione-Sewell B., Sitrin M. D., Brasitus T. A. The role of protein kinase-C alpha in the activation of particulate guanylate cyclase by 1 alpha,25-dihydroxyvitamin D3 in CaCo-2 cells. Endocrinology. 1994 Jul;135(1):277–283. doi: 10.1210/endo.135.1.7912183. [DOI] [PubMed] [Google Scholar]
- Khare S., Wilson D. M., Tien X. Y., Dudeja P. K., Wali R. K., Sitrin M. D., Brasitus T. A. 1,25-Dihydroxycholecalciferol rapidly activates rat colonic particulate guanylate cyclase via a protein kinase C-dependent mechanism. Endocrinology. 1993 Nov;133(5):2213–2219. doi: 10.1210/endo.133.5.8104780. [DOI] [PubMed] [Google Scholar]
- Khare S., Wilson D. M., Tien X. Y., Wali R. K., Bissonnette M., Brasitus T. A. Protein kinase C mediates the calcium-induced activation of rat colonic particulate guanylate cyclase. Arch Biochem Biophys. 1994 Oct;314(1):200–204. doi: 10.1006/abbi.1994.1430. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mukhtar H., Athar M. Dietary anticarcinogens and cancer prevention. Cleve Clin J Med. 1988 Nov-Dec;55(6):507–508. doi: 10.3949/ccjm.55.6.507. [DOI] [PubMed] [Google Scholar]
- Nemere I., Dormanen M. C., Hammond M. W., Okamura W. H., Norman A. W. Identification of a specific binding protein for 1 alpha,25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J Biol Chem. 1994 Sep 23;269(38):23750–23756. [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pence B. C., Buddingh F. Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis. 1988 Jan;9(1):187–190. doi: 10.1093/carcin/9.1.187. [DOI] [PubMed] [Google Scholar]
- Sanz L., Berra E., Municio M. M., Dominguez I., Lozano J., Johansen T., Moscat J., Diaz-Meco M. T. Zeta PKC plays a critical role during stromelysin promoter activation by platelet-derived growth factor through a novel palindromic element. J Biol Chem. 1994 Apr 1;269(13):10044–10049. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Sitrin M. D., Halline A. G., Abrahams C., Brasitus T. A. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazine-induced colonic carcinogenesis in the rat. Cancer Res. 1991 Oct 15;51(20):5608–5613. [PubMed] [Google Scholar]
- Taniguchi H., Manenti S., Suzuki M., Titani K. Myristoylated alanine-rich C kinase substrate (MARCKS), a major protein kinase C substrate, is an in vivo substrate of proline-directed protein kinase(s). A mass spectroscopic analysis of the post-translational modifications. J Biol Chem. 1994 Jul 15;269(28):18299–18302. [PubMed] [Google Scholar]
- Thelen M., Rosen A., Nairn A. C., Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991 May 23;351(6324):320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Trilivas I., McDonough P. M., Brown J. H. Dissociation of protein kinase C redistribution from the phosphorylation of its substrates. J Biol Chem. 1991 May 5;266(13):8431–8438. [PubMed] [Google Scholar]
- Wali R. K., Baum C. L., Bolt M. J., Brasitus T. A., Sitrin M. D. 1,25-dihydroxyvitamin D3 inhibits Na(+)-H+ exchange by stimulating membrane phosphoinositide turnover and increasing cytosolic calcium in CaCo-2 cells. Endocrinology. 1992 Sep;131(3):1125–1133. doi: 10.1210/endo.131.3.1324151. [DOI] [PubMed] [Google Scholar]
- Wali R. K., Baum C. L., Sitrin M. D., Bolt M. J., Dudeja P. K., Brasitus T. A. Effect of vitamin D status on the rapid actions of 1,25-dihydroxycholecalciferol in rat colonic membranes. Am J Physiol. 1992 Jun;262(6 Pt 1):G945–G953. doi: 10.1152/ajpgi.1992.262.6.G945. [DOI] [PubMed] [Google Scholar]
- Wali R. K., Baum C. L., Sitrin M. D., Brasitus T. A. 1,25(OH)2 vitamin D3 stimulates membrane phosphoinositide turnover, activates protein kinase C, and increases cytosolic calcium in rat colonic epithelium. J Clin Invest. 1990 Apr;85(4):1296–1303. doi: 10.1172/JCI114567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikel C. S., Spann C. L., Chambers C. P., Crane J. K., Linden J., Hewlett E. L. Phorbol esters enhance the cyclic GMP response of T84 cells to the heat-stable enterotoxin of Escherichia coli (STa). Infect Immun. 1990 May;58(5):1402–1407. doi: 10.1128/iai.58.5.1402-1407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetsel W. C., Khan W. A., Merchenthaler I., Rivera H., Halpern A. E., Phung H. M., Negro-Vilar A., Hannun Y. A. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992 Apr;117(1):121–133. doi: 10.1083/jcb.117.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]




