Abstract
Leukoaraiosis is a common finding in stroke patients, and has been strongly associated with risk of incident stroke and dementia. Leukoaraiosis may be an independent predictor of stroke outcomes, too. There is increasing evidence from neuroimaging to support the concept that some leukoaraiosis is caused by white matter infarcts, which may be particularly frequent in patients with aggressive small vessel diseases such as cerebral amyloid angiopathy. The relatively similar distribution of leukoaraiosis regardless of the distribution of vascular pathology suggests a conserved vulnerability to white matter injury across various vascular diseases, possibly related to resting patterns of blood flow. More insights into the pathophysiology of leukoaraiosis are sorely needed to reduce the burden of disability associated with this common condition.
Keywords: leukoaraiosis, intracerebral hemorrhage, ischemia, amyloid angiopathy, white matter disease, vascular cognitive impairment, outcomes
Leukoariaosis, or white matter lesions, are frequently seen on brain neuroimaging in older persons. Despite more than 15 years of extensive study, there remain significant unanswered questions regarding the pathogenesis of these very common lesions, which are strongly associated with cognitive dysfunction.
Clinical relevance of leukoaraiosis in stroke patients
There is without question an association between leukoaraiosis and stroke. Leukoaraiosis is more common and more severe in patients with ischemic stroke or intracerebral hemorrhage, compared to healthy persons. Leukoaraiosis is a feature of the small vessel cerebrovascular pathologies that lead to stroke, including hypertensive arteriopathy, cerebral amyloid angiopathy, and CADASIL. Most studies looking at the association between leukoaraiosis and stroke are cross-sectional, because few longitudinal studies that include brain imaging are of adequate size and duration to capture enough stroke events for analysis. One important exception is the Framingham Heart Study. Data from this study were recently used to show that the presence of severe leukoaraiosis at baseline more than doubles the future odds of stroke (Table 1).1 Additionally those with severe leukoaraiosis had more than double the odds of future all-cause mortality and almost four times the odds of subsequent dementia. These important confirmatory data are consistent with risk estimates from other large population-based studies including the Rotterdam Scan Study2 and the Cardiovascular Health Study,3 and demonstrate the clinical relevance of leukoaraiosis.
Table 1. Association of Extensive Leukoaraiosis with Incident Events in the Framingham Heart Study.
Data from 2,229 participants with baseline MRI, mean age 62±9 years, followed prospectively for 5-6 years on average. Extensive leukoaraiosis (n=313) was defined as white matter lesion volume, measured using quantitative methods and expressed as percent of the total intracranial volume, greater than one standard deviation above the age-adjusted mean.1
| Stroke | Dementia | Death | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Extensive Leukoaraiosis | 2.28 | 1.02-5.13 | 3.97 | 1.10-14.30 | 2.27 | 1.41-3.65 |
Leukoaraiosis may be more than a marker of increased stroke risk, however. There is accumulating evidence that leukoaraiosis is a surprisingly strong predictor of stroke outcomes, too. In the population-based Greater Cincinnati Stroke Study, ischemic stroke patients with severe leukoaraiosis had a 90-day modified Rankin scale (mRS) score that was on average 0.47 points higher than patients without leukoaraiosis (95% CI 0.07–0.87, p=0.02).4 Likewise, a single center study from Massachusetts General Hospital showed that mean mRS at six months after stroke was 0.8 higher in ischemic stroke patients in the top quartile of leukoaraiosis compared to the bottom quartile (p=0.01), and that leukoaraiosis was an independent predictor of the odds of a higher mRS after adjustment for potential confounders (p=0.05).5 These recent studies expand on our prior knowledge by showing that leukoaraiosis affects outcome from cortical ischemic stroke as well as small vessel stroke, in large samples with multivariable adjustment for other patient characteristics and ischemic stroke volume. One of the potential mechanisms for this association is that patients with leukoaraiosis may be at greater risk for ischemic stroke extension, possibly because leukoaraiosis may be a marker of poor collateral vessel health.6 Other possibilities include that leukoaraiosis may impair brain recovery mechanisms, may be associated with a lack of brain reserve capacity for injury, or may be a marker of risk of new vascular events. An increased frequency of tissue plasminogen activator-associated brain hemorrhage in patients with leukoaraiosis may account for some portion of the excess poor outcomes,7 but is unlikely to account for a large portion because few ischemic stroke patients receive thrombolysis. Further studies are needed to distinguish these mechanisms and to determine whether neurorehabilitation strategies need to take leukoaraiosis into account.
Infarction: an Under-Recognized Cause of Leukoaraiosis?
The above studies suggest that preventing leukoaraiosis may reduce not only the population health burden from cognitive impairment but may also reduce neurological disability by improving outcomes from ischemic stroke. It is certainly possible that control of vascular risk factors will prevent progression of leukoaraiosis, although more randomized trials are needed to prove this. Much of the variation in leukoaraiosis severity is not accounted for by traditional risk factors, however, and there is a large heritable component to the risk. Therefore it is highly likely that there are not yet discovered disease mechanisms that play a role in the pathogenesis of leukoaraiosis. A large body of research has established validated reliable quantitative and semi-quantitative means of measuring leukoaraiosis and has established the clinical relevance of these lesions beyond any doubt; however fresh insights into pathogenesis have been relatively lacking. The fact that leukoaraiosis may have multiple causes including arterial diseases, such as hypertensive arteriopathy and cerebral amyloid angiopathy, and possibly venous diseases,8 further complicates investigation of pathogenic mechanisms. Studies to discriminate various mechanisms of leukoaraiosis should be a priority for future research.
Two recent studies have raised the possibility that white matter infarction may be a much more important contributor to leukoaraiosis than recognized previously. In one study that included 90 patients with acute lacunar stroke, only 25 of the strokes (28%) evolved into the typical cavitated lesion with CSF-like fluid in the center (Figure 1), with the remainder evolving into what would otherwise appear to be white matter lesions.9 This surprising finding suggests that the burden of small cerebral infarction is substantially underestimated by studies that define small infarcts on the basis of cavitated lacunar lesions. Furthermore, many studies have investigated the risk factors and consequences of cavitated lacunes and white matter lesions separately, however this distinction is possibly less relevant now that we recognize that many acute small infarcts do not cavitate. It seems probable that small infarcts, singly or in confluence, may in fact account for some portion of leukoaraiosis. Another study of 78 patients with cerebral amyloid angiopathy found that incidental small asymptomatic infarcts were seen on MRI done for other indications in 12 patients (15%, Figure 2).10 Patients with more cerebral microbleeds, indicating greater severity of cerebral amyloid angiopathy, were more likely to have small asymptomatic infarcts, suggesting that small infarcts may be a feature of aggressive small arterial diseases. These two studies are small and retrospective, and warrant confirmation in larger prospective cohorts. Improved sensitivity of neuroimaging for tiny infarcts may demonstrate that they are much more frequent than currently recognized. In fact, pathologic studies have shown that microinfarcts, too small to be seen by conventional neuroimaging, are relatively common in the brains of demented persons.
Figure 1. Acute Small Vessel Infarction May Evolve Into White Matter Lesions.
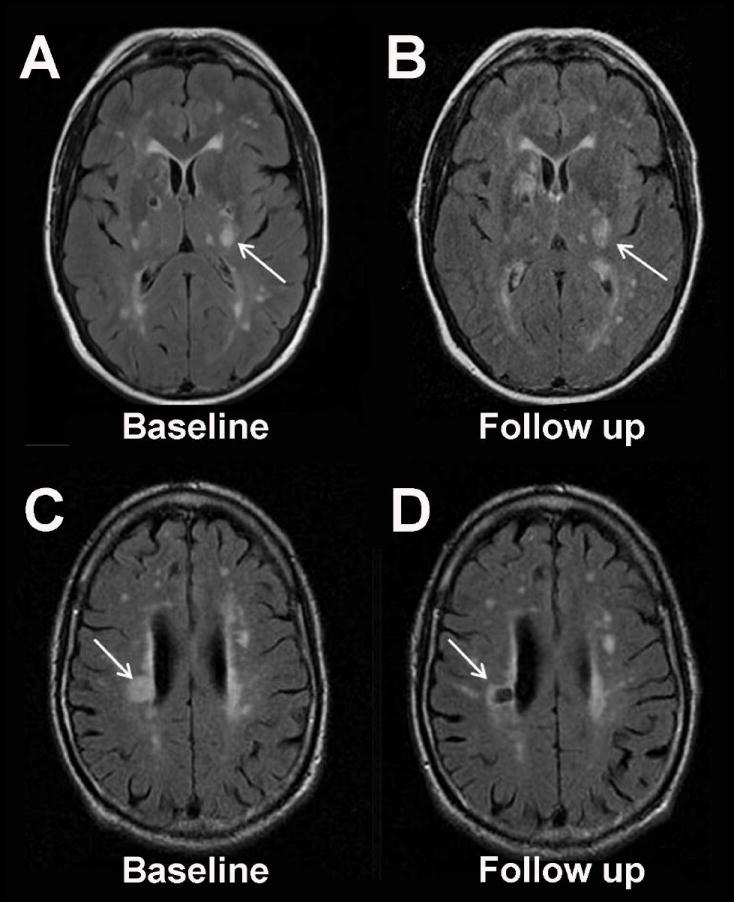
Recent data suggest that the majority of acute small artery infarctions on MRI evolve into T2-hyperintense white matter lesions (Panels A and B, fluid attenuated inversion recovery (FLAIR) MRI at 4 days and 65 days after acute infarction in the left internal capsule) rather than chronic cavitated lesions (Panels C and D, FLAIR MRI at 17 days and 7 months after acute infarction in the right corona radiata).9 This figure was graciously provided by Dr. Gillian M. Potter and Dr. Joanna M. Wardlaw.
Figure 2. Incidental Small Infarcts in Cerebral Amyloid Angiopathy.
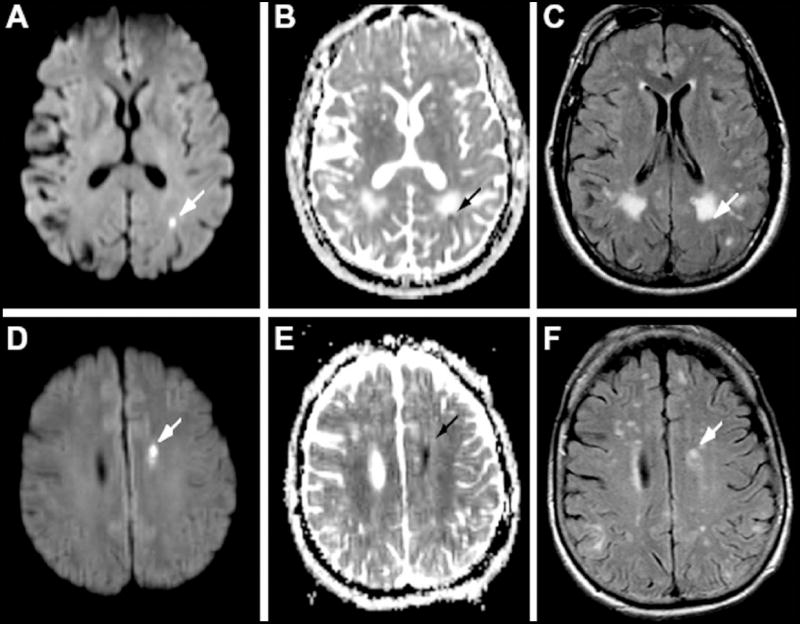
Incidental small acute cerebral infarcts can be detected with surprisingly high frequency in patients with cerebral amyloid angiopathy. Here, small lesions are seen in the periventricular white matter in 2 patients (panels A-C and D-F) with hyperintensity on diffusion-weighted sequence (A and D), hypointensity on apparent diffusion coefficient sequence (B and E) and hyperintensity on fluid attenuated inversion recovery (FLAIR, C and F). Reproduced with permission from Neurology.10
Distribution of Leukoaraiosis Does Not Match Distribution of Vascular Pathology
A puzzling feature of leukoaraiosis, with possible relevance to the pathogenesis of the lesions, is the relatively conserved anatomic distribution across individuals and different vascular diseases. As labeled in countless clinical radiology reports, leukoaraiosis is frequently considered “non-specific” with regard to the underlying cause, vascular or other. Exceptions are that in CADASIL there is relative preferential involvement of the external capsule and anterior temporal white matter, and in cerebral amyloid angiopathy there is relatively more posterior than anterior leukoaraiosis. Even so, in CADASIL and cerebral amyloid angiopathy patients as well as others, the greatest burden of lesions is in the periventricular white matter around the frontal and occipital horns, with varying amounts of involvement of areas of subcortical white matter. The probability of subcortical white matter involvement varies inversely with the distance from the ventricular margin.11
To investigate the relationship between vascular pathology and regional leukoaraiosis we compared the leukoaraiosis distribution between patients with vascular pathologies that have a near inverse distribution, cerebral amyloid angiopathy and hypertensive arteriopathy.12 Cerebral amyloid angiopathy affects the arteries of the leptomeninges and cortex, with little involvement of the penetrating arteries in the white matter and essentially no involvement of the perforating arteries at the base of the brain and brainstem. By contrast, hypertensive arteriopathy most severely affects the penetrating arteries at the base of the brain with relatively less involvement of the arteries of the cortex. Thus, one might expect striking differences in the distribution of leukoaraiosis between these vastly different vascular pathologies.
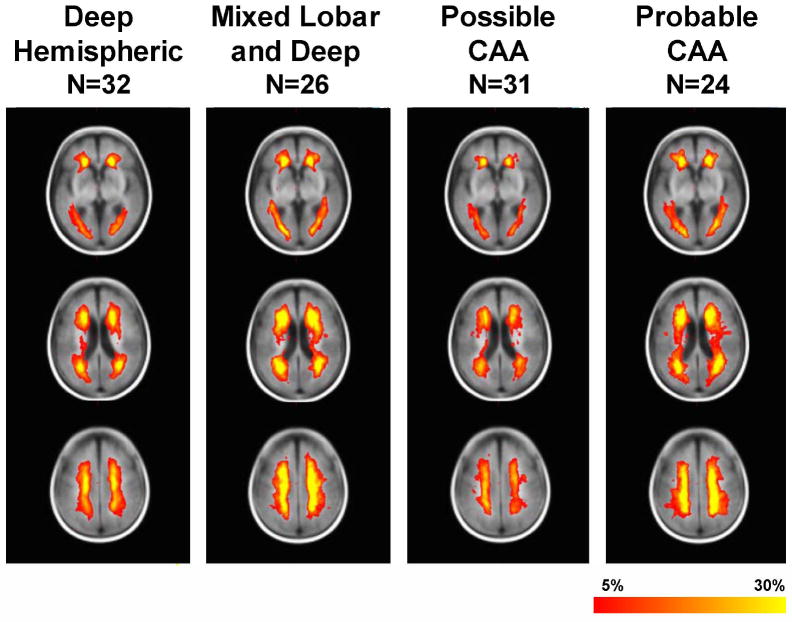
To test this hypothesis we compared probability maps of leukoaraiosis distribution in 32 patients presenting with deep hemispheric intracerebral hemorrhage caused by hypertension, 55 patients with probable or possible cerebral amyloid angiopathy by validated research criteria, and 26 patients with mixed lobar and deep hemorrhages that may have either hypertensive arteriopathy or a combination of hypertensive arteriopathy plus cerebral amyloid angiopathy (not unexpected because both are relatively common vascular pathologies of aging).12 The overall distribution of leukoaraiosis was much more similar than different across the groups (Figure 3), notwithstanding a greater frequency of brainstem hyperintensity in the group with presumed hypertensive hemorrhage, expected because vascular amyloid is not found to any significant extent pathologically in the brainstem arteries.
Figure 3. Distribution of Leukoaraiosis in Patients with Hypertensive Deep Hemispheric Intracerebral Hemorrhage Compared to Cerebral Amyloid Angiopathy-Associated Hemorrhage.
The distribution of leukoaraiosis is substantially similar despite the near inverse distribution of vascular pathology in hypertensive arteriopathy (maximally affecting the penetrating arteries to the deep hemispheric brain structures) compared to cerebral amyloid angiopathy (affecting the cortex and meninges). The color code (red to yellow) is proportional to the frequency of leukoaraiosis at that voxel.
Thus, our quantitative study provides evidence to support the clinical impression that leukoaraiosis distribution is more similar than different across widely different vascular diseases, and is thus relatively non-specific. An interesting question, heretofore little considered, is the reason why the leukoaraiosis distribution is so relatively constant. Our data suggest that there may be a relatively conserved regional vulnerability to ischemia, related either to white matter vulnerability to ischemic injury, or resting blood flow patterns. The periventricular region is the end-zone of white matter perfusion and SPECT atlas data confirm that blood flow is lowest there; it is possible that flow-limiting vascular pathology at diverse locations might therefore result in the lowest perfusion of this vulnerable region.13 Furthermore, our data in patients with cerebral amyloid angiopathy show that leukoaraiosis occurs at a distant site (the periventricular white matter) from the site of vascular pathology (predominantly in the cortex or leptomeninges). This implicates a flow-related mechanism for the pathogenesis of leukoaraiosis, rather than blood-brain barrier dysfunction with increased permeability, at least in cerebral amyloid angiopathy.
Conclusions
Leukoaraiosis is a common neuroimaging finding that is associated with stroke outcome as well as stroke incidence. Although the pathogenesis of leukoaraiosis is probably multifactorial, new evidence suggest that some portion of leukoaraiosis is the consequence of small white matter infarcts in the vulnerable periventricular region in patients with aggressive cerebral small arterial diseases. A challenge to the field is to move beyond measurement of leukoaraiosis and associations with typical vascular risk factors and outcomes, to identification of novel risk factors that shed light on pathogenesis and offer potential therapeutic targets. While this review has highlighted recent neuroimaging findings with implications for pathogenesis, further insights will likely also come from studies of serum biomarkers, genetics and vascular and brain pathology.
Acknowledgments
The author gratefully acknowledges the support of the Canadian Institutes of Health Research, Alberta Heritage Fund for Medical Research, Canadian Stroke Network, Heart and Stroke Foundation of Canada and the National Institute for Neurological Disorders and Stroke (R01 NS062028 and AG26484).
Footnotes
There are no commercial conflicts of interest.
References
- 1.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O’Leary DH, Furberg CD. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 4.Kissela B, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Flaherty ML, Air E, Broderick J, Tsevat J. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke. 2009;40:530–536. doi: 10.1161/STROKEAHA.108.521906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arsava EM, Rahman R, Rosand J, Lu J, Smith EE, Rost NS, Singhal AB, Lev MH, Furie KL, Koroshetz WJ, Sorensen AG, Ay H. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology. 2009;72:1403–1410. doi: 10.1212/WNL.0b013e3181a18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, Wu O, Gonzalez RG, Koroshetz WJ, Sorensen AG. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 7.Demchuk AM, Khan F, Hill MD, Barber PA, Silver B, Patel S, Levine SR for the NINDS rt-PA Studty Group. Importance of leukoaraiosis on ct for tissue plasminogen activator decision making: Evaluation of the NINDS rt-pa stroke study. Cerebrovascular Diseases. 2008;26:120–125. doi: 10.1159/000139658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40:S48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 9.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, Wardlaw JM. Counting cavitating lacunes underestimates the burden of lacunar infarction. Stroke. 2010;41:267–272. doi: 10.1161/STROKEAHA.109.566307. [DOI] [PubMed] [Google Scholar]
- 10.Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE, Greenberg SM. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–1235. doi: 10.1212/01.wnl.0000345666.83318.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (wmh): Exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith EE, Nandigam KRN, Chen Y-W, Jeng J, Salat DH, Halpin A, Frosch M, Wendell L, Fazen LE, Rosand J, Viswanathan A, Greenberg SM. MRI markers of small vessel disease in lobar and deep hemispheric intracerebral hemorrhage. Stroke. 2010 doi: 10.1161/STROKEAHA.110.579078. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, Blacker D, Albert MS, Guttmann CR, Greenberg SM. Spatial distribution of white-matter hyperintensities in alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]