Abstract
Genetic variations in DNA repair genes are thought to modulate DNA repair capacity and are suggested to be related to lung cancer risk. We conducted a meta-analysis of epidemiologic studies on the association between genetic polymorphisms in both base excision repair and nucleotide excision repair pathways, and lung cancer. We found xeroderma pigmentosum complementation group A (XPA) G23A (odds ratio (OR) = 0.76, 95% confidence interval (CI) = 0.61–0.94), 8-oxoguanine DNA glycosylase 1 (OGG1) Ser326Cys (OR = 1.22, 95% CI = 1.02–1.45), and excision repair cross-complementing group 2 (ERCC2) Lys751Gln (OR = 1.27, 95% CI = 1.10–1.46) polymorphisms were associated with lung cancer risk. Considering the data available, it can be conjectured that if there is any risk association between a single SNP and lung cancer, the risk fluctuation will probably be minimal. Advances in the identification of new polymorphisms and in high-throughput genotyping techniques will facilitate the analysis of multiple genes in multiple DNA repair pathways. Therefore, it is likely that the defining feature of future epidemiologic studies will be the simultaneous analysis of large samples of cases and controls.
1. Introduction
Sporadic cancer is a multifactorial disease that results from complex interactions between many genetic and environmental factors [1]. This means that there will not be a single gene or single environmental factor that has large effects on cancer susceptibility. Environmental factors (e.g., tobacco smoke, dietary factors, infectious agents, and radiation) add to the carcinogenic load to which humans are exposed, but exact numbers for added risk are generally less well established.
Cigarette smoke contains several thousand chemicals that are known to chemically modify DNA [2] and lead to the formation of mutations [3]. Most of these compounds are procarcinogens that must be activated by Phase I enzymes, such as cytochrome P450s. All activated carcinogens can bind to DNA and form DNA adducts that are capable of inducing mutations and initiating carcinogenesis. The capacity to repair DNA damage induced by activated carcinogens appears to be one of the host factors that may influence lung cancer risk. A critical cellular response that counteracts the carcinogenic effects of DNA damage is DNA repair.
Several studies have investigated whether reduced DNA repair capacity (DRC) is associated with an increased risk of cancer [4]. The reduced DRC of benzo(a)pyrene-7,8-diol-9,10-epoxide (an active form of benzo(a)pyrene)-DNA adducts is associated with an increased risk of lung cancer (2.1-fold, 95% confidence interval (CI) = 1.5–3.0) [5]. The reduced DRC has been shown to be associated with a 5.7-fold (95% CI = 2.1–15.7) increased risk of developing lung cancer [6]. Likewise, the reduced DRC of bleomycin-induced damage was found to be associated with an increased risk of lung cancer [7]. These studies suggested that a low DRC of various DNA repair mechanisms predisposes individuals to lung cancer, and this realization prompted us to search for defined DNA repair activities that may be risk factors for lung cancer. Polymorphisms in DNA repair genes may be associated with differences in the DRC of DNA damage and may influence an individual's risk of lung cancer, because the variant genotype in those polymorphisms might destroy or alter repair function.
At least four pathways of DNA repair operate on specific types of damaged DNA. Base excision repair (BER) operates on small lesions, while the nucleotide excision repair (NER) pathway repairs bulk lesions. Mismatch repair corrects replication errors. Double-strand DNA break repair (DSBR) actually consists of two pathways, homologous recombination (HR) and nonhomologous end-joining (NHEJ). The NHEJ repair pathway involves direct ligation of the two double strand break ends, while HR is a process by which double-strand DNA breaks are repaired through the alignment of homologous sequences of DNA. The following sections review the literature on DNA repair genes in more detail, specifically those involved in the NER and BER pathways.
It is believed that the predominant pathway used for removal of oxidized and many of the alkylated bases is BER. The process of BER is initiated by DNA glycosylases [e.g., 8-oxoguanine DNA glycosylase 1 (OGG1), endonuclease III homolog 1, thymine glycol-DNA-glycosylase], which are often promiscuous as far as their substrate specificity is concerned. The BER pathway can proceed through two different subpathways: short-patch and long-patch BER. These pathways are differentiated by the enzymes involved and the number of nucleotides removed. Short-patch BER replaces a single nucleotide by polymerase β and the newly synthesized DNA sealed by DNA ligase III/X-ray cross-complementing group 1 (XRCC1) heterodimer [8]. Long-patch BER inserts 2–13 nucleotides by concordant action of polymerase δ, proliferating cell nuclear antigen, flap endonuclease 1, and ligase I.
NER is a versatile DNA repair system that removes a wide range of DNA lesions including UV-induced lesions. There are two subpathways in NER. One is transcription-coupled DNA repair (TCR), which preferentially removes DNA damage that blocks ongoing transcription in the transcribed DNA strand of active genes. The other is global genome repair (GGR), which removes lesions throughout the genome, including those from the nontranscribed strand in the active gene [9]. Three rare, autosomal recessive inherited human disorders are associated with impaired NER activity: XP, CS, and trichothiodystrophy (TTD) [10]. XP has been studied most extensively. Seven different DNA NER genes, which correct seven distinct genetic XP complementation groups (XPA, XPB (excision repair cross-complementing group 3, ERCC3), XPC, XPD (ERCC2), XPE, XPF (ERCC4) and XPG (ERCC5, this gene causes CS)) and XPV have been identified [10]. XPA, ERCC3/XPB, ERCC2/XPD, ERCC4/XPF and ERCC5/XPG have a defect in TCR and GGR, while XPC and XPE have a defect in GGR only. ERCC6 and ERCC8 are also known as CS type B (CSB) and CSA, respectively.
The aim of this article is to review and evaluate associations between genes in the BER and NER pathways, focusing on genetic polymorphisms in OGG1, XRCC1, XPA, and ERCC2 genes, which have been reported a sufficient number of studies to conduct a meta-analysis. The details of the OGG1, XRCC1, XPA, and ERCC2 genes are given in Table 1
Table 1.
The details of the OGG1, XRCC1, XPA, and ERCC/2XPD genes.
| Gene symbol | Gene name | Gene location | Polymorphism | DNA repair capacity |
|---|---|---|---|---|
| OGG1 | 8-oxoguanine DNA glycosylase | 3p26.2 | Ser326Cys (rs1052133) | The Cys/Cys genotype may be associated with a lower DNA repair capacity |
|
| ||||
| XRCC1 | X-ray repair complementing defective repair in Chinese hamster cells 1 | 19q13.2 | Arg194Trp (rs1799782), Arg280His (rs25489), Arg399Gln (rs25487) | Although the Arg399Gln, Arg194Trp, and Arg280His polymorphisms have been suggested to be functional, there is no direct evidence on its functional consequences |
|
| ||||
| XPA | Xeroderma pigmentosum, complementation group A | 9q22.3 | G23A (rs 1800975) | The G allele may be associated with a higher DNA repair capacity |
|
| ||||
| ERCC2/XPD | Excision repair cross-complementing group 2/ Xeroderma pigmentosum, complementation group D | 19q13.3 | Asp312Asn (rs1799793), Lys751Gln (rs13181) | The 312Asn and 751Gln alleles are reported to be associated with lower DNA repair capacity |
2. Materials and Methods
2.1. Identification and Eligibility of Relevant Studies
We conducted MEDLINE, Current Contents, and Web of Science searches using “OGG1", “XRCC1", “XPA", “ERCC2/XPD", “lung cancer", and “polymorphism" as keywords to search for papers published (from January 1, 1966 through December 31, 2009). Additional articles were identified through the references cited in the first series of articles selected. Articles included in the meta-analysis were in English language, with human subjects. Case-control studies were eligible if they had determined the distribution of the relevant genotypes in lung cancer cases and in concurrent controls using a molecular method for genotyping. For overlapping studies, only the first published one was selected. Using the MEDLINE database, we identified 18 genetic epidemiological studies that provided information on lung cancer occurrence associated with the OGG1 Ser326Cys polymorphism. Also, we identified 22 studies of the XRCC1 Arg399Gln polymorphism, 12 studies of XRCC1 Arg194Trp polymorphism, and 10 studies of the XRCC1 Arg280 His polymorphism. As for NER polymorphisms, we identified 6 studies for the XPA G23A polymorphism, 16 studies for the Asp312Asn polymorphism, and 19 studies for the Lys751Gln polymorphism. No additional articles through Current Contents or Web of Science have been identified.
2.2. Data Extraction and Assessment of Study Quality
For each study, characteristics such as authors, year of publication, ethnic group of the study population, source of control population, number of genotyped cases and controls, crude odds ratio (OR), and the method for quality control of genotyping were noted. For studies including subjects of different ethnic groups, data were extracted separately for each ethnic group whenever possible.
Methods for defining study quality in genetic studies are more clearly delineated than those for observational studies. We combined only studies with allelic frequencies being in Hardy-Weinberg equilibrium (HWE) (Pearson χ 2 test, P ≥ .05) because departure from HWE can imply the presence of genotyping error, possible ethnic admixture in the population, or selection bias (lack of representativeness of the general population). We assessed the homogeneity of the study population (Caucasian or Asian).
2.3. Meta-Analysis
Data were combined using both a fixed effects (the inverse variance-weighted method) and a random effects (DerSimonian and Laird method) models [11]. The Cochran Q statistics test is used for the assessment of heterogeneity. The fixed effects model is used when the effects are assumed to be homogenous, while the random effects model is used when they are heterogenous. In the absence of between-study heterogeneity, the two methods provide identical results. The presence of heterogeneity can result from differences in the selection of controls, age distribution, prevalence of lifestyle factors, histological type of lung cancer, stage of lung cancer, and so on. The random effects model incorporates an estimate of the between-study variance and tends to provide wider CIs when the results of the constituent studies differ among themselves. As the random effects model is more appropriate when heterogeneity is present [11], the summary OR and prevalence were essentially based on the random effects model. The meta-analyses were performed on crude ORs, since the adjusted ORs were not comparable because of the inclusion of different covariates in the multivariate regression models. Using individuals with the homozygous common genotype as the reference group, we calculated ORs for individuals with the heterozygous genotype and homozygous rare genotype separately whenever possible (information available in at least two studies). In some cases, we combined the heterozygous genotype with the homozygous rare genotype due to a low prevalence of the rare allele in several polymorphisms. The Q statistic was considered significant for P < .10 [12, 13]. Publication bias is always a concern in meta-analysis. The presence of publication bias indicates that nonsignificant or negative findings remain unpublished. To test for publication bias, both Begg's [14] and Egger's [15] tests are commonly used to assess whether smaller studies reported greater associations than larger studies. Publication bias is considered significant for P < .10. For each genetic comparison, subgroup analysis was stratified by the ethnicity and, if possible, histological type of lung cancer. All of the calculations were performed using STATA Version 10.1 (Stata Corporation, College Station, TX) software.
3. Results
3.1. OGG1 Ser326Cys Polymorphism
Table 2 shows the individual ORs from each study and summary ORs of the OGG1 Ser326Cys polymorphism [16–33]. Two studies [24, 26] were excluded from the meta-analysis because genotype distribution in control population significantly deviates from HWE. Combining data from all 17 populations on the basis of 6,181 cases and 7,331 controls, the summary ORs were 1.04 (95% CI = 0.94–1.23) for Ser/Cys carriers and 1.22 (95% CI = 1.02–1.45) for Cys/Cys carriers. The Cys/Cys genotype was significantly associated with lung cancer risk in all populations combined. The summary ORs for the Cys/Cys genotype in Caucasians (mostly composed of Caucasians) and Asians were 1.24 (95% CI = 0.84–1.83) and 1.24 (95% CI = 1.00–1.55, P = .052), respectively. There was a marginally significant association between lung cancer risk and the OGG1 Ser326Cys polymorphism among Asians. Publication bias was absent in all analyses. Heterogeneity was present in the analyses of all studies combined and Caucasian studies combined.
Table 2.
Genetic polymorphisms in the BER pathway and lung cancer risk: OGG1 Ser326Cys polymorphism.
| Author, published year (reference no.) | Ethnicity | No. of Cases/Controls | Source of controls | OR (95% CI)* | Quality control of genotyping | ||
|---|---|---|---|---|---|---|---|
| Ser/Cys | Cys/Cys | ||||||
| Sugimura et al., 1999 [16] | Asian | 241/197 | Hospital | 0.80 (0.52–1.21) |
1.13 (0.63–2.02) |
Sequencing | |
| Wikman et al., 2000 [17] | Caucasian | 105/105 | Hospital | 0.66 (0.37–1.17) |
2.20 (0.41–11.8) |
Sequencing | |
| Ito et al., 2002 [18] | Asian | 138/240 | Hospital | 1.02 (0.63–1.67) |
0.85 (0.46–1.56) |
None | |
| Sunaga et al., 2002 [19] | Asian | 198/152 | Hospital | 1.49 (0.91–2.43) |
0.98 (0.54–1.77) |
None | |
| Le Marchand et al., 2002 [20] | Admixed population | 298/405 | Population | 0.90 (0.65–1.26) |
1.76 (1.15–2.71) |
Sequencing | |
| Lan et al., 2004 [21] | Asian | 118/109 | Population | 1.96 (1.10– 3.48) |
1.84 (0.83–4.06) |
None | |
| Park et al., 2004 [22] | Mostly composed of Caucasians | 179/350 | Screening | 1.89 (1.27–2.80) |
4.10 (1.65–10.2) |
Sequencing | |
| Vogel et al., 2004 [23] | Caucasian | 256/269 | Population | 1.09 (0.75– 1.60) |
0.78 (0.35–1.72) |
Replication (random samples) | |
| Liang et al., 2005 [24]‡ | Asian | 227/227 | Hospital | 0.94 (0.63–1.41) |
0.98 (0.33–2.87) |
Sequencing | |
| Hung et al., 2005 [25] | Mostly composed of Caucasians | 2,155/2,163 | Hospital | 0.90 (0.79–1.03) |
1.15 (0.84–1.57) |
Replication (random samples) | |
| Zienolddiny et al., 2006 [26]‡ | Caucasian | 326/386 | Population | 0.91 (0.64–1.29) |
0.63 (0.40–0.97) |
Replication (all samples) | |
| Matullo et al., 2006 [27] | Caucasian | 116/1094 | Population | 1.26 (0.83– 1.91) |
0.82 (0.21–2.33) |
Replication (random samples) | |
| Kohno et al., 2006 [28] | Asian | 1097/394 | Hospital | 1.24 (0.94–1.63) |
1.43 (1.02–2.01) |
None | |
| Sørensen et al., 2006 [29] | Caucasian | 431/796 | Population | 1.04 (0.80–1.35) |
1.18 (0.63–2.21) |
Replication (random samples) | |
| De Ruyck et al., 2007 [30] | Caucasian | 110/110 | Hospital | 0.58 (0.33–1.02) |
0.61 (0.13–2.82) |
None | |
| Karahlil et al., 2008 [31] | Turkish | 165/250 | Hospital | 0.82 (0.54–1.24) |
0.65 (0.32–1.29) |
None | |
| Miyaishi et al., 2009 [32] | Asian | 108/121 | Hospital | 1.47 (0.79–2.73) |
1.34 (0.65–2.77) |
None | |
| Chang et al., 2009 [33] | Latino | 112/296 | Population | 0.91 (0.56–1.47) |
1.05 (0.45–2.32) |
Replication (random samples) | |
| Chang et al., 2009 [33] | African-American | 254/280 | Population | 1.32 (0.89–1.98) |
0.89 (0.25–3.00) |
Replication (random samples) | |
|
| |||||||
| Summary** | No. of populations | Cochran Q test for heterogeneity | |||||
| Ser/Cys | Cys/Cys | ||||||
|
| |||||||
| All | 17 | 6,181/7,331 | 1.04 (0.94–1.23) |
1.22 (1.02–1.45) |
0.004 | 0.220 | |
| Caucasian (mostly composed of Caucasians) | 7 | 3,352/4,887 | 1.02 (0.81–1.29) |
1.24 (0.84–1.83) |
0.004 | 0.133 | |
| Asian | 6 | 1,900/1,213 | 1.23 (0.97–1.55) |
1.24 (1.00–1.55)† |
0.159 | 0.572 | |
*Crude odds ratio and 95% confidence interval.
**Based on random effects model.
† P = .052.
‡Excluded from the meta-analysis because genotype distribution of control population was not in Hardy-Weinberg equilibrium. NA, not available.
A further analysis on histological type was performed to assess whether the impact of the OGG1 Ser326Cys polymorphism between adenocarcinoma and squamous cell carcinoma cases (the two histological types present most often in the data set) was similar or not. Among the seven case-control studies (2,052 lung cancer cases and 3,032 controls), the summary OR for the Cys/Cys genotype in adenocarcinoma was 1.38 (95% CI = 1.12–1.75) (data not shown). Among both Caucasians (612 cases and 2,618 controls) [17, 22, 25] and Asians (1,440 cases and 864 controls) [16, 19, 28, 32], subjects with the Cys/Cys genotype were at increased risk of adenocarcinoma. Summary ORs for Caucasians and Asians were 1.90 (95% CI = 0.99–3.63, P = .054) ad 1.30 (95% CI = 1.00–1.29, P = .049), respectively (data not shown). It was found that increased risk associated with the Cys/Cys genotype was not evident for squamous cell lung cancer risk among Caucasians [17, 22, 25]. The available data on squamous cell carcinoma are insufficient for Asians.
3.2. XRCC1 Polymorphism
Table 3 shows that summary ORs of the XRCC1 Arg399Gln polymorphism on the basis of 8,684 cases and 10,913 controls [23, 25–27, 30, 33–49]. The summary OR for the 339Gln/Gln genotype among 24 different ethnic populations was 1.00 (95% CI = 0.86–1.17). The Cochran Q test for heterogeneity showed a statistical significance (P = .004). Both the Egger's and Begg's tests were not statistically significant, however. The summary ORs for the 339Gln/Gln genotype among Caucasians and Asians were 0.95 (95% CI = 0.83–1.10) and 1.08 (95% CI = 0.78–1.49), respectively. Evidence for publication bias was absent in subgroup analyses by ethnic. The Cochran Q test for heterogeneity showed a statistical significance among Asians.
Table 3.
Genetic polymorphisms in the BER pathway and lung cancer risk: XRCC1 Arg399Gln polymorphism.
| Author, published year (reference no.) | Ethnicity | No. of Cases/Controls | Source of controls | OR (95% CI)* | Quality control of genotyping | ||
|---|---|---|---|---|---|---|---|
| Arg/Gln | Gln/Gln | ||||||
| Ratnasinghe et al., 2001 [34] | Asian | 107/208 | Population | 1.00 (0.60–1.60) |
1.40 (0.50–1.70) |
Replication (random sample) | |
| David-Beabes and London, 2001 [35] | African-American | 154/243 | Population | 1.03 (0.66–1.60) |
0.52 (0.14–1.97) |
Replication (random sample) | |
| David-Beabes and London , 2001 [35] | Caucasian | 180/461 | Population | 0.75 (0.52–1.08) |
0.63 (0.34–1.14) |
Replication (random sample) | |
| Divine et al., 2001 [36] | Caucasian | 172/143 | Hospital | 0.76 (0.47–1.22) |
1.64 (0.80–3.36) |
None | |
| Chen et al., 2002 [37] | Asian | 109/109 | Population | 1.02 (0.57–1.80) |
0.67 (0.20–2.26) |
None | |
| Park et al., 2002 [38] | Asian | 192/135 | Hospital visitors | 1.27 (0.81–2.04) |
2.30 (0.87–6.09) |
Sequencing | |
| Misra et al., 2003 [39] | Caucasian | 315/313 | Population | 1.10 (0.78–1.54) |
0.84 (0.45–1.58) |
Replication (random sample) | |
| Zhou et al., 2003 [40] | Caucasian | 1,091/1,240 | Hospital visitors | 1.00 (0.80–1.20) |
1.30 (1.00–1.70) |
Replication (random sample) | |
| Ito et al., 2004 [41] | Asian | 178/449 | Hospital | 1.01 (0.70–1.45) |
1.39 (0.70–2.76) |
None | |
| Popanda et al., 2004 [42] | Caucasian | 463/460 | Hospital | 0.89 (0.67–1.17) |
0.87 (0.58–1.29) |
Replication (random sample) | |
| Harms et al., 2004 [43] | Caucasian | 110/119 | Hospital | 0.73 (0.44–1.25) |
1.07 (0.39–2.96) |
Replication (all samples) | |
| Zhang et al., 2005 [44] | Asian | 1,000/1,000 | Hospital | 0.95 (0.79–1.14) |
1.14 (0.84–1.55) |
Replication (all samples) | |
| Hung et al., 2005 [25] | Mostly composed of Caucasians | 2,049/2,015 | Hospital | 1.12 (0.98–1.28) |
1.01 (0.83–1.23) |
Replication (random sample) | |
| Vogel et al., 2004 [23] | Caucasian | 256/269 | Population | 0.79 (0.54–1.17) |
0.81 (0.46–1.41) |
Replication (random sample) | |
| Schneider et al., 2005 [45] | Caucasian | 446/622 | Hospital | 0.94 (0.72–1.23) |
0.83 (0.54–1.26) |
None | |
| Shen et al., 2005 [46] | Asian | 116/109 | Population | 0.59 (0.33–1.05) |
0.75 (0.13–4.23) |
None | |
| Zienolddiny et al., 2006 [26] | Caucasian | 331/391 | Population | 1.08 (0.78–1.49) |
0.67 (0.39–1.14) |
Replication (all samples) | |
| Matullo et al., 2006 [27] | Caucasian | 116/1,094 | Population | 1.14 (0.75–1.73) |
0.52 (0.19-1.19) |
Replication (random sample) | |
| Yin et al., 2007 [47] | Asian | 205/193 | Hospital | 1.20 (0.77–1.85) |
0.21 (0.05–1.00) |
None | |
| López-Cima et al., 2007 [48] | Caucasian | 516/533 | Hospital | 0.91 (0.70–1.20) |
0.89 (0.61–1.31) |
Sequencing | |
| Pachouri et al., 2007 [49] | Asian | 103/122 | Population | 0.36 (0.20–0.64) |
0.47 (0.20–1.09) |
None | |
| De Ruyck et al., 2007 [30] | Caucasian | 109/109 | Hospital | 1.28 (0.69–2.28) |
1.68 (0.67–4.23) |
None | |
| Chang et al., 2009 [33] | Latino | 112/296 | Population | 1.30 (0.73–2.30) |
3.03 (1.11–7.83) |
Replication (random sample) | |
| Chang et al., 2009 [33] | African-American | 254/280 | Population | 1.02 (0.62–1.65) |
1.19 (0.24–5.13) | Replication (random sample) | |
|
| |||||||
| Summary** | No. of populations | Cochran Q test for heterogeneity | |||||
| Arg/Gln | Gln/Gln | ||||||
|
| |||||||
| All | 24 | 8,684/10,913 | 0.97 (0.89–1.05) |
1.00 (0.86–1.17) |
0.153 | 0.004 | |
| Caucasian (mostly composed of Caucasians) | 13 | 6,154/7,769 | 1.00 (0.92–1.08) |
0.95 (0.83–1.10) |
0.433 | 0.218 | |
| Asian | 8 | 2,010/2,325 | 0.90 (0.72–1.13) |
1.08 (0.78–1.49) |
0.024 | 0.030 | |
*Crude odds ratio and 95% confidence interval..
**Based on random effects model.
A further analysis on histological type (adenocarcinoma and squamous cell carcinoma) was carried out. Although available data were not sufficient, there were no statistically significant differences in risk associated with the XRCC1 Arg399Gln polymorphism and adenocarcinoma or squamous cell both Caucasians and Asians [25, 38, 42, 44, 45, 49].
Table 4 shows summary ORs of the XRCC1 Arg194Trp polymorphism [25–27, 30, 33–35, 37, 45–47, 49]. One study [49] was excluded from the meta-analysis because allelic frequency in control population is not in HWE. Based on 11 studies in 13 different ethnic populations on the basis of 4,431 cases and 6,320 controls, the summary ORs for the Arg/Trp genotype and Trp/Trp genotype were 0.89 (95% CI = 0.79–1.00, P = .047) and 1.15 (95% CI = 0.80–1.67), respectively. The ORs for the Trp/Trp genotype were 1.24 (95% CI = 0.50–3.11) in Caucasians and 1.18 (95% CI = 0.72–1.93) in Asians. This polymorphism was not associated with lung cancer risk among both Caucasians and Asians. Evidence for heterogeneity and publication bias was absent in any analysis.
Table 4.
Genetic polymorphisms in the BER pathway and lung cancer risk: XRCC1 Arg194Trp polymorphism.
| Author, published year (reference no.) | Ethnicity | No. of Cases/control |
Source of controls | OR (95% CI)* | Quality control of genotyping | ||
|---|---|---|---|---|---|---|---|
| Arg/Trp | Trp/Trp | ||||||
| Ratnasinghe et al., 2001 [34] | Asian | 108/216 | Population | 0.70 (0.40–1.20) |
0.70 (0.30–1.60) |
Replication (random sample) | |
| David-Beabes and London, 2001 [35] | African-American | 154/234 | Population | 0.40 (0.19–0.83) |
1.44 (0.20–10.37) |
Replication (random sample) | |
| David-Beabes and London, 2001 [35] | Caucasian | 180/461 | Population | 1.05 (0.62–1.78) |
— | Replication (random sample) | |
| Chen et al., 2002 [37] | Asian | 109/109 | Population | 1.31 (0.73–2.32) |
2.61 (0.85– 8.04) |
None | |
| Hung et al., 2005 [25] | Mostly composed of Caucasians | 2,147/2,132 | Hospital | 0.86 (0.72–1.03) |
0.81 (0.35–1.88) |
Replication (random sample) | |
| Schneider et al., 2005 [45] | Caucasian | 446/622 | Hospital | 0.99 (0.67–1.46) |
1.86 (0.31–12.8) |
None | |
| Shen et al., 2005 [46] | Asian | 118/112 | Population | 1.01 (0.56–1.83) |
1.48 (0.51–4.45) |
None | |
| Zienolddiny et al., 2006 [26] | Caucasian | 336/405 | Population | 0.88 (0.50–1.55) |
0.60 (0.01–11.5) |
Replication (all samples) | |
| Matullo et al., 2006 [27] | Caucasian | 116/1094 | Population | 1.10 (0.59–1.94) |
9.70 (0.69–134.6) |
Replication (random samples) | |
| Yin et al., 2007 [47] | Asian | 241/249 | Hospital | 0.89 (0.60–1.32) |
1.09 (0.54–2.18) |
None | |
| Pachouri et al., 2007 [49]‡ | Asian | 103/122 | Population | 0.97 (0.54–1.76) |
1.36 (0.67–2.75) |
None | |
| De Ruyck et al., 2007 [30] | Caucasian | 110/110 | Hospital | 0.43 (0.15–1.12) |
— | None | |
| Chang et al., 2009 [33] | Latino | 112/296 | Population | 0.73 (0.37–1.49) |
— | Replication (random samples) | |
| Chang et al., 2009 [33] | African-American | 254/280 | Population | 1.23 (0.64–2.30) |
— | Replication (random samples) | |
|
| |||||||
| Summary** | No. of populations | Cochran Q test for heterogeneity | |||||
| Arg/Trp | Trp/Trp | ||||||
|
| |||||||
| All | 13 | 4,431/6,320 | 0.89 (0.79–1.00)† |
1.15 (0.80–1.67) |
0.467 | 0.510 | |
| Caucasian (mostly composed of Caucasians) | 6 | 3,335/4,824 | 0.89 (0.77–1.03) |
1.24 (0.50–3.11) |
0.653 | 0.315 | |
| Asian | 4 | 576/686 | 0.93 (0.72–1.20) |
1.18 (0.72–1.93) |
0.476 | 0.305 | |
*Crude odds ratio and 95% confidence interval.
**Based on random effects model.
† P = .047.
‡Excluded from the meta-analysis because genotype distribution of control population was not in Hardy-Weinberg equilibrium.
Table 5 shows summary ORs of the XRCC1 Arg280His polymorphism [23, 25, 26, 30, 33, 34, 39, 45–47]. One study [26] was excluded from the meta-analysis because genotype distribution in control population does not fulfill HWE. The summary OR for the Arg/His versus the Arg/Arg genotype among 9 studies on the basis of 4,030 cases and 4,532 controls was 0.96 (95% CI = 0.83–1.11). The summary OR for the Arg/His and His/His genotypes combined versus the Arg/Arg genotype was 0.99 (95% CI = 0.83–1.19). The summary ORs for the Arg/His and His/His genotypes combined versus the Arg/Arg genotype in Caucasians and Asians were 0.96 (95% CI = 0.80–1.17) and 1.10 (95% CI = 0.66–1.84), respectively. There was no ethnic difference in the association between lung cancer risk and the XRCC1 Arg280His polymorphism. Evidence for heterogeneity and publication bias was absent in subgroup analyses by ethnic.
Table 5.
Genetic polymorphisms in the BER pathway and lung cancer risk: XRCC1 Arg280His polymorphism.
| Author, published year (reference no.) | Ethnicity | No. of Cases/ controls |
Source of controls | OR (95% CI)* | Quality control of genotyping | ||
|---|---|---|---|---|---|---|---|
| Arg/His | Arg/His or His/His | ||||||
| Ratnasinghe et al., 2001 [34] | Asian | 106/209 | Population | 1.40 (0.70–2.60) |
1.60 (0.90–2.90) |
Replication (random sample) | |
| Mirsa et al., 2003 [39] | Caucasian | 309/302 | Population | 1.12 (0.70–1.80) |
1.17 (0.73–1.87) |
Replication (random samples) | |
| Hung et al., 2005 [25] | Mostly composed of Caucasians | 2,088/2,092 | Hospital | 0.95 (0.77–1.18) |
0.95 (0.77–1.17) |
Replication (random samples) | |
| Vogel et al., 2004 [23] | Caucasian | 256/269 | Population | 0.98 (0.53–1.79) |
1.01 (0.56–1.85) |
Replication (random samples) | |
| Schneider et al., 2005 [45] | Caucasian | 446/622 | Hospital | 0.93 (0.59–1.44) |
0.97 (0.63–1.53) |
None | |
| Shen et al., 2005 [46] | Asian | 111/110 | Population | 1.14 (0.60–2.18) |
1.29 (0.69–2.41) |
None | |
| Zienolddiny et al., 2006 [26]‡ | Caucasian | 324/377 | Population | 1.53 (0.85–2.78) |
1.45 (0.82–2.56) |
Replication (All samples) | |
| Yin et al., 2007 [47] | Asian | 238/242 | Hospital | 0.73 (0.46–1.16) |
0.72 (0.46–1.12) |
None | |
| De Ruyck et al., 2007 [30] | Caucasian | 110/110 | Hospital | 0.26 (0.06–0.87) |
0.26 (0.06–0.87) |
None | |
| Chang et al., 2009 [33] | Latino | 112/296 | Population | 1.11 (0.53–2.20) |
1.08 (0.53–2.10) |
Replication (random samples) | |
| Chang et al., 2009 [33] | African-American | 254/280 | Population | — | — | Replication (random samples) | |
|
| |||||||
| Summary** | No. of populations | Cochran Q test for heterogeneity | |||||
| Arg/His | Arg/His or His/His | ||||||
|
| |||||||
| All | 10 | 4,030/4,532 | 0.96 (0.83–1.11) |
0.99 (0.83–1.19) |
0.525 | 0.281 | |
| Caucasian (mostly composed of Caucasians) | 5 | 3,209/3,395 | 0.95 (0.80–1.13) |
0.96 (0.80–1.17) |
0.400 | 0.357 | |
| Asian | 3 | 455/561 | 0.99 (0.66–1.48) |
1.10 (0.66–1.84) |
0.236 | 0.076 | |
*Crude odds ratio and 95% confidence interval.
**Based on random effects model.
‡Excluded from the meta-analysis because genotype distribution of control population was not in Hardy-Weinberg equilibrium.
3.3. XPA G23A Polymorphism
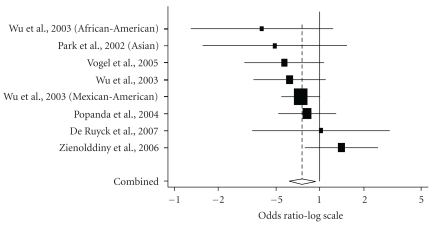
Table 6 shows summary ORs of the XPA G23A polymorphism on the basis of 2,025 cases and 1,991 controls [26, 30, 42, 50–52]. SNP alleles with higher frequencies are more likely to be ancestral than less frequently occurring alleles although there may be some exceptions. As the 23G allele was more prevalent than the 23A allele [53], we regarded the 23G allele as ancestral (wild-type or major) allele for descriptive purposes (the XPA 23 polymorphism caused by the G-to-A substitution is the XPA G23A polymorphism). Summary ORs for the G/A genotype and G/G genotype among 6 studies in 8 populations were 0.74 (95% CI = 0.61–0.90) and 0.76 (95% CI = 0.61–0.94), respectively (Table 6, Figure 1). Among Caucasian studies, the summary ORs for the G/A genotype and the A/A genotype were 0.73 (95% CI = 0.59–0.90) and 0.83 (95% CI = 0.64–1.08), respectively. The Cochran Q test for heterogeneity did not show a statistical significance. The Egger's test was statistically significant for publication bias in a subgroup analysis of Caucasians (P = .024, G/A genotype versus G/G genotype).
Table 6.
Genetic polymorphisms in the NER pathway and lung cancer risk: XPA G23A polymorphism.
| Author, published year (reference no.) | Ethnicity | No. of Cases /controls | Source of controls | OR (95% CI)* | Quality control of genotyping | ||
|---|---|---|---|---|---|---|---|
| G/A | G/G | ||||||
| Park et al., 2002 [50] | Asian | 265/185 | Population | 1.00 (0.62–1.62) |
0.62 (0.35–1.10) |
Sequencing | |
| Wu et al., 2003 [51] | Caucasian | 564/581 | Population | 0.65 (0.48–0.87) |
0.74 (0.55–1.01) |
None | |
| Wu et al., 2003 [51] | Mexican-American | 50/47 | Population | 0.31 (0.09–1.00) |
0.40 (0.13–1.25) |
None | |
| Wu et al., 2003 [51] | African-American | 71/67 | Population | 0.54 (0.16–1.68) |
0.49 (0.15–1.49) |
None | |
| Popanda et al., 2004 [42] | Caucasian | 461/457 | Hospital | 0.77 (0.48–1.21) |
0.82 (0.52–1.30) |
Replication (random samples) | |
| Vogel et al., 2005 [52] | Caucasian | 256/269 | Population | 0.78 (0.41–1.49) |
0.57 (0.30–1.06) |
None | |
| Zienolddiny et al., 2006 [26] | Caucasian | 248/276 | Population | 0.87 (0.48–1.57) |
1.41 (0.79–2.52) |
Replication (all samples) | |
| De Ruyck et al., 2007 [30] | Caucasian | 110/109 | Hospital | 1.00 (0.34–2.92) |
1.02 (0.34–3.03) |
None | |
|
| |||||||
| Summary** | No. of populations | Cochran Q test for heterogeneity | |||||
| G/A | G/G | ||||||
|
| |||||||
| All | 8 | 2,025/1,991 | 0.74 (0.61–0.90) |
0.76 (0.61–0.94) |
0.640 | 0.345 | |
| Caucasian | 5 | 1,639/1,692 | 0.73 (0.59–0.90) |
0.83 (0.64–1.08) |
0.855 | 0.266 | |
*Crude odds ratio and 95% confidence interval.
** Based on random effects model.
Figure 1.
Meta-analysis of 8 studies (5 Caucasian studies and 3 non-Caucasian studies) of lung cancer and theXPA G23A polymorphism (GG versus AA). The center of a box and the horizontal line (logarithm) indicate the odds ratio (OR) and the 95% confidence interval (CI) in each study, with the areas of the boxes representing the weight of each study. The summary OR based on random effects model is represented by the middle of a diamond whose width indicated the 95% CI. The summary OR is shown by the dotted vertical line. Statistical heterogeneity between studies was assessed with Cochran Q test (Q = 7.86, P = .35). Summary: OR = 0.76 (95% CI = 0.61–0.94).
3.4. ERCC2/XPD Polymorphism
Table 7 shows summary ORs of the ERCC2 Asp312Asn polymorphism on the basis of 6,346 cases and 7,792 controls [26, 27, 30, 39, 42, 48, 54–63]. The summary OR for the Asn/Asn genotype among 17 different ethnic populations was 1.19 (95% CI = 1.03–1.38). Caucasians with the Asn/Asn genotype and Asian with the Asn/Asn genotype had a marginal 1.15-fold (95% CI = 0.98–1.32, P = .079) and a significant 8.26-fold (95% CI = 1.50–45.6, P = .015) risk of developing lung cancer, respectively. No significant association between lung cancer and the heterozygous Asp/Asn genotype was found for all of the studies combined or by ethnicity. The impact of the heterozygous genotype on lung cancer was similar between Caucasians and Asians. The Cochran Q test for heterogeneity did not show a statistical significance in all analyses. Although no evidence of publication bias was found in overall analyses, both Begg's (P = .040) and Egger's (P = .010) tests showed a statistical significance in a subgroup analysis of Caucasians (Asn/Asn genotype versus Asp/Asp genotype).
Table 7.
Genetic polymorphisms in the NER pathway and lung cancer risk: ERCC2 Asp312Asn polymorphism.
| Author, published year (reference no.) | Ethnicity | No. of Cases/controls | Source of controls | OR (95% CI)* | Quality control of genotyping | ||
|---|---|---|---|---|---|---|---|
| Asp/Asn | Asn/Asn | ||||||
| Butkiewicz et al., 2001 [54] | Caucasian | 96/94 | Population | 0.49 (0.24–0.98) |
0.71 (0.29–1.74) |
Sequencing | |
| Spitz et al., 2001 [55] | Admixed population | 195/257 | Population | 0.92 (0.62–1.36) |
1.54 (0.78–3.05) |
None | |
| Hou et al., 2002 [56] | Caucasian | 184/162 | Population | 1.27 (0.78–2.05) |
0.88 (0.43–1.84) |
Replication (random samples) | |
| Zhou et al., 2002 [57] | Caucasian | 1,092/1,240 | Population | 0.98 (0.82–1.17) |
1.41 (1.06–1.86) |
Replication (random samples) | |
| Liang et al., 2003 [58] | Asian | 1,006/1,020 | Population | 0.98 (0.76–1.28) |
11.2 (1.45–87.2) |
Replication (random samples) | |
| Misra et al., 2003 [39] | Caucasian | 313/312 | Population | 0.76 (0.53–1.07) |
0.94 (0.56–1.59) |
Replication (random samples) | |
| Popanda et al., 2004 [42] | Caucasian | 463/460 | Hospital | 1.14 (0.77–1.68) |
1.03 (0.70–1.51) |
Replication (random samples) | |
| Vogel et al., 2004 [59] | Caucasian | 252/263 | Population | 1.27 (0.86–1.89) |
1.09 (0.63–1.86) |
None | |
| Shen et al., 2005 [60] | Asian | 118/113 | Population | 0.58 (0.21–1.52) |
— | Replication (random samples) | |
| Zienolddiny et al., 2006 [26] | Caucasian | 275/290 | Population | 0.85 (0.58–1.25) |
1.11 (0.68–1.81) |
Replication (all samples) | |
| Matullo et al., 2006 [27] | Caucasian | 116/1094 | Population | 0.81 (0.52– 1.26) |
0.95 (0.51–1.71) |
Replication (random samples) | |
| Hu et al., 2006 [61] | Asian | 970/986 | Hospital | 1.07 (0.81–1.43) |
4.11 (0.41–202.7) |
None | |
| López-Cima et al., 2007 [48] | Caucasian | 516/533 | Hospital | 1.04 (0.80–1.35) |
1.39 (0.88–2.20) |
Sequencing | |
| De Ruyck et al., 2007 [30] | Caucasian | 110/109 | Hospital | 1.28 (0.70–2.35) |
1.03 (0.40–2.66) |
None | |
| Chang et al.,2008 [62] | Latino | 108/297 | Population | 1.37 (0.83–2.26) |
2.13 (0.72–5.96) |
Replication (random samples) | |
| Chang et al.,2008 [62] | African-American | 247/277 | Population | 1.10 (0.71–1.70) |
0.68 (0.10–3.57) |
Replication (random samples) | |
| Yin et al., 2009 [63] | Asian | 285/285 | Hospital | 1.31 (0.77–2.77) |
— | Replication (random samples) | |
|
| |||||||
| Summary** | No. of populations | Cochran Q test for heterogeneity | |||||
| Asp/Asn | Asn/Asn | ||||||
|
| |||||||
| All | 17 (15) | 6,346/7,792 | 1.00 (0.92–1.10) |
1.19 (1.03–1.38) |
0.510 | 0.510 | |
| Caucasian | 10 | 3,417/4,557 | 0.98 (0.86–1.11) |
1.15 (0.98–1.34) |
0.257 | 0.770 | |
| Asian | 4 (2) | 2,379/2,404 | 1.02 (0.85–1.22) |
8.26 (1.50–45.6) |
0.565 | 0.597 | |
* Crude odds ratio and 95% confidence interval.
** Based on random effects model.
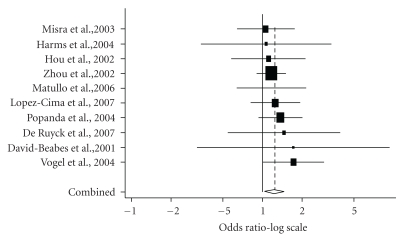
Table 8 shows summary ORs of the ERCC2 Lys751Gln polymorphism [26, 27, 30, 37, 39, 42, 43, 48, 55–65]. One study [26] was excluded from the meta-analysis because genotype distribution in control population significantly deviates from HWE. Based on 6,941 cases and 8,595 controls, summary ORs for the Gln/Gln genotype and Lys/Gln genotype were 1.09 (95% CI = 1.04–1.18) and 1.27 (95% CI = 1.10–1.46), respectively. The Gln/Gln genotype was significantly associated with an increased risk of lung cancer in Caucasians (OR = 1.24, 95% CI = 1.06–1.45) (Figure 2) but not in Asians (OR = 1.16, 95% CI = 0.48–2.80). The Cochran Q test for heterogeneity showed a statistical significance among Asian studies. Evidence of publication bias was absent in all of the analyses.
Table 8.
Genetic polymorphisms in the NER pathway and lung cancer risk: ERCC2 Lys751Gln polymorphism.
| Author, published year (reference no.) | Ethnicity | No. of Cases/controls |
Source of controls | OR (95% CI)** | Quality control of genotyping | ||
|---|---|---|---|---|---|---|---|
| Lys/Gln | Gln/Gln | ||||||
| David-Beabes et al., 2001 [64] | Caucasian | 178/453 | Population | 1.14 (0.77–1.71) |
1.72 (1.00–2.94) |
Replication (random samples) | |
| David-Beabes et al., 2001 [64] | African-American | 153/234 | Population | 1.14 (0.73–1.78) |
1.39 (0.54–3.55) |
Replication (random samples) | |
| Spitz et al., 2001 [55] | Admixed population | 341/360 | Population | 1.07 (0.78–1.46) |
1.36 (0.84–2.20) |
None | |
| Chen et al., 2002 [37] | Asian | 109/109 | Population | 0.79 (0.17–1.11) |
0.44 (0.17–1.11) |
None | |
| Hou et al., 2002 [56] | Caucasian | 185/162 | Population | 1.22 (0.75–2.00) |
1.11 (0.58–2.13) |
Replication (random samples) | |
| Zhou et al., 2002 [57] | Caucasian | 1,092/1,240 | Population | 1.01 (0.84–1.21) |
1.17 (0.90–1.51) |
Replication (random samples) | |
| Park et al., 2002 [65] | Asian | 250/163 | Population | 1.06 (0.55–2.11) |
— | None | |
| Liang et al., 2003 [58] | Asian | 1,006/1,020 | Population | 0.93 (0.73–1.18) |
2.36 (0.90–6.17) |
Replication (random samples) | |
| Misra et al., 2003 [39] | Caucasian | 310/302 | Population | 0.87 (0.60–1.26) |
1.06 (0.64–1.76) |
Replication (random samples) | |
| Popanda et al., 2004 [42] | Caucasian | 463/459 | Hospital | 1.14 (0.86–1.52) |
1.37 (0.93–2.02) |
Replication (random samples) | |
| Harms et al., 2004 [43] | Caucasian | 110/119 | Population | 1.34 (0.79–2.49) |
1.07 (0.34–3.38) |
Replication (all samples) | |
| Vogel et al., 2004 [59] | Caucasian | 256/269 | Population | 1.57 (1.05–2.34) |
1.73 (1.01–2.96) |
None | |
| Shen et al., 2005 [60] | Asian | 118/108 | Population | 0.44 (0.18–1.03) |
— | Replication (random samples) | |
| Zienolddiny et al., 2006 [26]‡ | Caucasian | 317/386 | Population | 1.20 (0.84–1.73) |
1.56 (1.06–2.31) |
Replication (all samples) | |
| Matullo et al., 2006 [27] | Caucasian | 116/1094 | Population | 1.23 (0.78–1.96) |
1.17 (0.63–2.11) |
Replication (random samples) | |
| Hu et al., 2006 [61] | Asian | 975/997 | Hospital | 1.16 (0.89–1.52) |
1.46 (0.40–5.87) |
None | |
| De Ruyck et al., 2007 [30] | Caucasian | 110/109 | Hospital | 1.07 (0.58–1.97) |
1.46 (0.55–3.94) |
None | |
| López-Cima et al., 2007 [48] | Caucasian | 516/533 | Hospital | 1.08 (0.83–1.41) |
1.25 (0.80–1.95) |
Sequencing | |
| Chang et al.,2008 [62] | Latino | 113/299 | Population | 1.01 (0.61–1.66) |
2.89 (1.20–6.91) |
Replication (random samples) | |
| Chang et al., 2008 [62] | African American | 255/280 | Population | 1.20 (0.83–1.74) |
1.01 (0.41–2.43) |
Replication (random samples) | |
| Yin et al., 2009 [63] | Asian | 285/285 | Hospital | 1.68 (1.06–2.67) |
1.47 (0.24–10.1) |
Replication (random samples) | |
|
| |||||||
| Summary** | No. of populations | Cochran Q test for heterogeneity | |||||
| Lys/Gln | Gln/Gln | ||||||
|
| |||||||
| All | 20 (18) | 6,941/8,595 | 1.09 (1.04–1.18) |
1.27 (1.10–1.46) |
0.613 | 0.727 | |
| Caucasian | 10 | 3,336/4,740 | 1.10 0.99–1.22)† |
1.24 (1.06–1.45) |
0.702 | 0.973 | |
| Asian | 6 (4) | 2,743/2,682 | 1.04 (0.81–1.35) | 1.16 (0.48–2.80) | 0.085 | 0.096 | |
* Crude odds ratio and 95% confidence interval. ** Based on random effects model. † P = .051
‡ Excluded from the meta-analysis because genotype distribution of control population was not in Hardy-Weinberg equilibrium.
Figure 2.
Meta-analysis of 10 Caucasian studies of lung cancer and the ERCC2 Lys751Gln polymorphism (Gln/Gln versus Lys/Lys). The center of a box and the horizontal line (logarithm) indicate the odds ratio (OR) and the 95% confidence interval (CI) in each study, with the areas of the boxes representing the weight of each study. The summary OR based on random effects model is represented by the middle of a diamond whose width indicated the 95% CI. The summary OR is shown by the dotted vertical line. Statistical heterogeneity between studies was assessed with Cochran Q test(Q = 2.75, P = .97). Summary: OR = 1.24 (95% CI = 1.06–1.45).
4. Discussion
Epidemiological studies of common polymorphisms in DNA repair genes, if large and unbiased, can provide insight into the in vivo relationships between DNA repair genes and lung cancer risk. Such studies may identify empirical associations which indicate that a polymorphism in a gene of interest has an impact on lung cancer, independent of metabolic regulatory mechanisms and other genetic and environmental variability. Findings from epidemiological studies can complement in vitro analyses of the various polymorphisms, genes, and pathways. In addition, epidemiological studies of common polymorphisms can lead to an increased understanding of the public health dimension of DNA-repair variation.
We conducted a systematic literature review to evaluate the associations between sequence variants in DNA repair genes and lung cancer risk. We found an increased risk of lung cancer among subjects carrying the ERCC2 751 Gln/Gln genotype in Caucasians (OR = 1.24, 95% CI = 1.06–1.45). The meta-analysis by Hu et al., showed that the Gln/Gln genotype had a significant 23% (95% CI = 3%–47%) increased risk of lung cancer compared with individuals with the Lys/Lys genotype among Caucasians [66]. The meta-analysis by Benhamou and Sarasin reported that the summary OR for the Gln/Gln genotype was 1.25 (95% CI = 1.03–1.52) in the United States (stratified by geographic region) [67]. Both of the meta-analyses were based on the same published data from 8 individual case-control (five Caucasian and three Asians) studies [37, 39, 55–58, 64, 65]. These meta-analyses also indicate that the excess lung cancer risk from the Gln/Gln genotype may be about 20%. The Gln allele of the ERCC2 Lys751Gln polymorphism is associated with a higher DNA adduct level or lower DNA repair efficiency [56, 68, 69], except in research published by Duell et al., who found no correlation between the ERCC2 Lys751 Gln polymorphism and the level of polyphenol-DNA adducts in human blood samples [70]. Thus, it is biologically plausible that subjects with the Gln/Gln genotype are at increased risk of lung cancer. As with the two meta-analyses, in our meta-analysis the Gln/Gln genotype was not associated with an increased risk of lung cancer among Asians. The Cochran Q test for heterogeneity showed a statistical significance among Asian studies. The presence of heterogeneity may compromise the interpretation of meta-analyses and result in erroneous and potentially misleading conclusions [71, 72]. The presence of significant heterogeneity suggests that the estimated OR in each study is not homogeneous and the estimated ORs are close to 1.0 in the larger studies. Possible sources of heterogeneity are ethnicity (the prevalence of the “at risk" allele, ethnic differences in roles of the polymorphism), study design, and so on. Another possible reason for heterogeneity is linkage disequilibrium, with additional allelic variants of this gene that modulate overall enzyme activity. Furthermore, it is possible that interaction with polymorphisms at other genes may be important. Heterogeneity can be taken into account by applying the random effects model, however. This discrepancy between Caucasian studies and Asian studies may only be due to a difference in sample sizes. Reasons for this difference in risk among different ethnic populations are as yet unknown but, if real, may be related to other genetic or environmental factors.
In contrast to the Lys751Gln polymorphism, the Asp312Asn polymorphism was not associated with an increased risk of lung cancer among Caucasians. Both Begg's and Egger's tests were statistically significant for publication bias in a subgroup analysis of Caucasians. Publication bias may be always a possible limitation of combining data from various sources as in a meta-analysis. The idea of adjusting the results of meta-analyses for publication bias and imputing “fictional" studies into a meta-analysis is controversial at the moment [73]. Although publication bias is always a possible limitation of combining data from various sources as in a meta-analysis, Sutton et al., concluded that publication or related biases did not affect the conclusions in most meta-analyses because missing studies changed the conclusions in less than 10% of meta-analyses [73]. Two meta-analyses have been published in 2004 [66] and 2005 [67], respectively. Both of them are based on the same published data from 6 individual case-control (five Caucasian and one Asian) studies [54–58, 74]. The first meta-analysis showed that individuals with the Asn/Asn genotype were associated with an increased risk of lung cancer among Caucasians (OR = 1.22, 95% CI = 0.99–1.49). The second meta-analysis was somewhat different from the first one, because unadjusted ORs were summarized in the first one. A significantly increased risk of lung cancer associated with the Asn/Asn genotype of the ERCC2 Asp312Asn polymorphism in the United States was found (OR = 1.43, 95% CI = 1.11–1.83) [67]. The study of Zhou et al., [57], which was based on a large sample size and observed the significant result (OR = 1.5, 95 % CI = 1.1–2.0), made a significant influence on the summary OR of the United States. In this study, a significant 8.26-fold (95% CI = 1.50–45.6) risk of developing lung cancer was observed among Asians. This finding was entirely due to the study of Liang et al. [58]. Although no clear association between ERCC2 Asp312Asn polymorphism and lung cancer can be found, the Asn allele of the ERCC2 Asp312Asn polymorphism has been reported to be associated with a higher DNA adduct level or lower DNA repair efficiency [56]. Therefore, it is plausible that the Asn allele is associated with an increased risk of lung cancer. The Lys751Gln polymorphism has been more studied than the Asp312Asn polymorphism because the frequency of the 751Gln allele is more prevalent than the 312Asn allele. Moreover, the Asp312Asn polymorphism is in linkage disequilibrium with the Lys751Gln polymorphism [54, 55, 58]. As absence of association with lung cancer risk and Asp312Asn polymorphism may be partly due to the low prevalence of the 312Asn allele (low statistical power), the finding on the ERCC2 Asp312Asn polymorphism should be interpreted with caution before being confirmed in future studies.
In contrast, we found a protective effect of the XPG G23A G/G genotype (OR = 0.76, 95% CI = 0.61–0.94) on lung cancer risk. The XPA G23A polymorphism itself may alter the transcription and/or translation of the gene. Because this polymorphism is located in the vicinity of the translation initiation codon, it may alter translation efficiency. The nearby proximal nucleotides to the AUG initiation codon are important for the initiation of translation because the 40S ribosomal subunit binds initially at the 5′-end of the mRNA [75]. The consensus sequence around the start codon is GCCRCCAUGG, which is known as the Kozak consensus sequence [76]. The R at position −3 and the G just downstream of the start codon are especially important, and the lack of these bases leads to read-through of the start codon [77]. However, there has been no precise explanation of the mechanism by which the recognition of the start codon is aided by a purine at position −3 [76], which is the core nucleotide of the Kozak consensus. The polymorphism XPA G23A is a G/A transversion occurring four nucleotides upstream of the start codon of XPA and possibly improving the Kozak sequence [50]. The sequences (CCAGAGAUGG) around the predicted initiator methionine codon of the XPA gene agree with the Kozak's consensus sequence at positions −3 and +4 [78]. Although both the A and polymorphic variant G nucleotides at the −4 position of the XPA gene do not correspond to the original consensus Kozak sequence containing the nucleotide C at position −4, it is possible that a nucleotide substitution of A to G at position-4 preceding the AUG codon may affect ribosomal binding and thus alter the efficiency of XPA protein synthesis. To investigate whether the transition from G to A changes the translation efficiency, an in vitro transcription/translation analysis and a primer extension assay of the initiation complex will be necessary in the future. Furthermore, a functional association between the G23A polymorphism and DRC was reported [51], which showed significantly higher repair efficiency in healthy subjects with at least one G allele. An alternative explanation could be that the protective XPA 23G allele is in linkage disequilibrium with an allele from an adjacent gene which is the true susceptibility gene. The XPA G23A polymorphism may be a promising SNP for lung cancer. It is thought that cigarette smoking modifies the association between DNA repair polymorphisms, as well as metabolic polymorphisms, and lung cancer risk. Since interactions between the XPA G23A polymorphism and smoking have not been fully elucidated, further studies are needed to better understand the associations between the XPA G23A polymorphism and lung cancer risk.
The Cys/Cys genotype of the OGG1 Ser326Cys polymorphism was significantly associated with lung cancer risk in all of the studies combined (OR = 1.22, 95% CI = 1.02–1.45) and was marginally associated with lung cancer risk in Asian populations (OR = 1.24, 95% CI = 1.00–1.55, P = .052). In the stratified analysis by histological type of lung cancer, a significant association was found for adenocarcinoma. In a narrative review, the Ser326Cys polymorphism has inconsistently been associated with risk of lung cancer [79]. There was an increased risk of lung cancer among subjects with the OGG1 326Cys/Cys genotype, which is consistent with experimental evidence that this isoform exhibits decreased the BER activity [80, 81]. The meta-analysis of Hung et al. showed that the summary OR was 1.37 (95% CI = 1.02–1.82) for the Cys/Cys genotype in various ethnic populations combined [82]. The meta-analysis of Li et al. showed that individuals carrying the Cys/Cys genotype did not have significantly increased risk of lung cancer in all populations combined but, in the stratified analysis by ethnicity, a significantly increased risk was found among Asians (OR = 1.18, 95% CI = 1.01–1.38) [83]. Ethnic difference in the association between lung cancer risk and the OGG1 Ser326Cys polymorphism was suggested. Large studies including different ethnic groups with a careful matching between cases and controls should be considered in future association studies to confirm results from the meta-analyses.
None of the XRCC1 polymorphisms was associated with an increased risk of lung cancer among both Caucasians and Asians. Our result for the XRCC1 Arg280His and Arg399Gln polymorphisms replicated the results of the meta-analysis by Hung et al. [82]. Results of previous studies that examined the association between the XRCC1 polymorphisms and lung cancer risk were inconsistent, possibly owing to the large random error in several small studies. This inconsistency might be due, in part, to differences in the prevalence of smokers. Lunn et al., [84] measured higher levels of aflatoxin B1 adducts in the XRCC1 Arg399Gln polymorphism and suggested that this might result in a deficient DRC. Two other XRCC1 polymorphisms, Arg194Trp and Arg280His, have been also determined and the functional effect of these polymorphisms is also unclear, even though some studies have revealed that amino acid changes at the evolutionary conserved regions can alter its function [85]. Although these polymorphisms result in amino acid substitutions, there is no direct evidence on its functional consequences. The XRCC1 Arg399Gln polymorphism has been associated with risk of breast cancer among African Americans, but not among Caucasians [86, 87], indicating that the XRCC1 Arg399Gln polymorphism may be linked to another biologically effective mutation. Further investigations of the combined effects of polymorphisms within these DNA repair genes, smoking, and other risk factors may help to clarify the influence of genetic variation in the carcinogenic process.
Several DNA repair pathways are involved in the maintenance of genetic stability. The most versatile and important one is the NER pathway, which detects and removes bulky DNA adducts, including those induced by cigarette smoking [88]. However, there are several conflicting reports on the association between this polymorphism and lung cancer risk among various populations. Although the reasons for the inconsistencies in the studies are not clear, possible explanations are (1) low frequency of the “at-risk" genotype, which would reduce the statistical power of the studies and (2) small size of the studies. Ethnic differences in the roles of the polymorphism may be caused by gene-gene interactions, different linkages to the polymorphisms determining lung cancer risk, and different lifestyles.
The most important problems facing lung cancer research are identifying “at-risk" individuals and implementing clinical surveillance, prevention practices, and follow-up care. Repair pathways play an important role in lung cancer risk, and genetic variations may contribute to decreased DRC and lung cancer susceptibility. Although the increased/decreased risk associated with individual DNA repair SNPs may be small compared to that conferred by high-penetrance cancer genes, their public health implication may be large because of their high frequency in the general population. It is thus essential that epidemiological investigations of DNA repair polymorphisms are adequately designed. Unfortunately a fairly good number of studies are limited by their sample size and subsequently suffer from too low power to detect effects that may truly exist. Also, given the borderline significance of some associations and multiple comparisons that have been carried out, there is a possibility that one or more findings are false-positives [89]. Large and combined analyses may be preferred to minimize the likelihood of both false-positive and false-negative results. In addition, controls should be chosen in such a way that, if they were cases, they would be included in the case group; when controls are matched to cases, it is essential to account for matching in the analysis. When appropriate, confounding factors should be controlled for, with particular consideration of race and ethnicity. An additional major concern is the grouping of genotypes for calculation of ORs. Without functional data to dictate genotype groupings, it seems prudent to present two ORs per polymorphism (one for heterozygotes versus common-allele homozygotes and one for rare-allele homozygotes versus common-allele homozygotes) so that dominant, codominant, or recessive patterns may be elucidated.
Continued advances in SNP maps and in high-throughput genotyping methods will facilitate the analysis of multiple polymorphisms within genes and the analysis of multiple genes within pathways. The effects of polymorphisms are best represented by their haplotypes. Data from multiple polymorphisms within a gene can be combined to create haplotypes, the set of multiple alleles on a single chromosome. None of the studies reviewed here reported haplotype associations, although several studies analyzed multiple polymorphisms within a gene, sometimes with inconsistent results. The analysis of haplotypes can increase the power to detect disease associations because of higher heterozygosity and tighter linkage disequilibrium with disease-causing mutations. In addition, haplotype analysis offers the advantage of not assuming that any of the genotyped polymorphisms is functional; rather, it allows for the possibility of an ungenotyped functional variant to be in linkage disequilibrium with the genotyped polymorphisms [90]. An analysis of data from multiple genes within the same DNA-repair pathway (particularly those known to form complexes) can provide more comprehensive insight into the studied associations. Such an analysis may shed light on the complexities of the many pathways involved in DNA repair and lung cancer development, providing hypotheses for future functional studies. Because of concerns over inflated type I error rates in pathway-wide or genome-wide association studies, methods of statistical analysis seeking to obviate this problem are under development [91]. The ability to include haplotype information and data from multiple genes, and to model their interactions, will provide more powerful and more comprehensive assessments of the DNA repair pathways.
This review, which is limited by the bias against publication of null findings, highlights the complexities inherent in epidemiological research and, particularly, in molecular epidemiological research. There is evidence that some polymorphisms in DNA repair genes play a role in carcinogenesis, most notably the ERCC2 Lys751Gln and XPA G23A polymorphisms. The variant allele of each of the three polymorphisms was associated with about a 30% decrease or increase in lung cancer risk. Although the summary risk for developing lung cancer in individuals of each genotype may not be large, lung cancer is such a common malignancy that even a small increase in risk can translate to a large number of excess lung cancer cases. Therefore, polymorphisms, even those not strongly associated with lung cancer, should be considered as potentially important public health issues. In addition, it is important to keep in mind that a susceptibility factor in one population may not be a factor in another. There are differences in the prevalence of DNA repair polymorphisms across populations. In a population where the prevalence of an “at-risk" genotype in a given polymorphism is very low, the “at-risk" allele or “at-risk" genotype may be too infrequent to assess its associated risk. At a population level, the attributable risk must be small simply because it is an infrequent allele. Finally, the major burden of lung cancer in the population probably results from the complex interaction between many genetic and environmental factors over time. Most environmental carcinogens first require metabolic activation by Phase I enzymes to their ultimate forms which then bind to DNA, forming aromatic-DNA adducts that are thought to be an early step in tumorigenesis. On the other hand, these activated forms are detoxified by Phase II enzymes. Thus, genetically determined susceptibility to lung cancer may depend on the metabolic balance among Phase I enzymes, Phase II enzymes, and DNA repair enzymes [92]. Further investigations of the combined effects of polymorphisms between DNA repair genes and drug-metabolizing genes may also help to clarify the influence of genetic variation in the carcinogenic process. Consortia and international collaborative studies, which may be a way to maximize study efficacy and overcome the limitations of individual studies, are needed to help further illuminate the complex landscape of lung cancer risk and genetic variations.
Acknowledgment
This study was funded in part by a Grant-in-Aid for Scientific Research (B) (21390190) from the Ministry of Education, Science, Sports and Culture, Japan.
Conflict of Interests
The authors have declared that no conflict of interests exists.
References
- 1.Pharoah PDP, Dunning AM, Ponder BAJ, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nature Reviews Cancer. 2004;4(11):850–860. doi: 10.1038/nrc1476. [DOI] [PubMed] [Google Scholar]
- 2.Hecht SS. Tobacco smoke carcinogens and lung cancer. Journal of the National Cancer Institute. 1999;91(14):1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 3.Livneh Z. DNA damage control by novel DNA polymerases: translesion replication and mutagenesis. Journal of Biological Chemistry. 2001;276(28):25639–25642. doi: 10.1074/jbc.R100019200. [DOI] [PubMed] [Google Scholar]
- 4.Berwick M, Vineis P. Markers of DNA repair and susceptibility to cancer in humans: an epidemiologic review. Journal of the National Cancer Institute. 2000;92(11):874–897. doi: 10.1093/jnci/92.11.874. [DOI] [PubMed] [Google Scholar]
- 5.Wei Q, Cheng L, Amos CI, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. Journal of the National Cancer Institute. 2000;92(21):1764–1772. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 6.Wei Q, Cheng L, Hong WK, Spitz MR. Reduced DNA repair capacity in lung cancer patients. Cancer Research. 1996;56(18):4103–4107. [PubMed] [Google Scholar]
- 7.Rajaee-Behbahani N, Schmezer P, Risch A, et al. Altered DNA repair capacity and bleomycin sensitivity as risk markers for non-small cell lung cancer. International Journal of Cancer. 2001;95(2):86–91. doi: 10.1002/1097-0215(20010320)95:2<86::aid-ijc1015>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Tomkinson AE, Mackey ZB. Structure and function of mammalian DNA ligases. Mutation Research. 1998;407(1):1–9. doi: 10.1016/s0921-8777(97)00050-5. [DOI] [PubMed] [Google Scholar]
- 9.Hanawalt PC. Subpathways of nucleotide excision repair and their regulation. Oncogene. 2002;21(58):8949–8956. doi: 10.1038/sj.onc.1206096. [DOI] [PubMed] [Google Scholar]
- 10.Bootsma D, Kraemer KH, Cleaver JE, Hoeijmakers JH. Nucleotide excision repair syndromes: xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy. In: Vogelstein B, Kinzler KW, editors. The Genetic Basis of Human Cancer. New York, NY, USA: McGraw-Hill; 1998. pp. 245–274. [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 13.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Statistics in Medicine. 1991;10(11):1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugimura H, Kohno T, Wakai K, et al. hOGG1 Ser326Cys polymorphism and lung cancer susceptibility. Cancer Epidemiology, Biomarkers and Prevention. 1999;8(8):669–674. [PubMed] [Google Scholar]
- 17.Wikman H, Risch A, Klimek F, et al. hOGG1 polymorphism and loss of heterozygosity (LOH): significance for lung cancer susceptibility in a caucasian population. International Journal of Cancer. 2000;88(6):932–937. doi: 10.1002/1097-0215(20001215)88:6<932::aid-ijc15>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.Ito H, Hamajima N, Takezaki T, et al. A limited association of OGG1 Ser326Cys polymorphism for adenocarcinoma of the lung. Journal of Epidemiology. 2002;12(3):258–265. doi: 10.2188/jea.12.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunaga N, Kohno T, Yanagitani N, et al. Contribution of the NQO1 and GSTT1 polymorphisms to lung adenocarcinoma susceptibility. Cancer Epidemiology, Biomarkers and Prevention. 2002;11(8):730–738. [PubMed] [Google Scholar]
- 20.Le Marchand L, Donlon T, Lum-Jones A, Seifried A, Wilkens LR. Association of the hOGG1 Ser326Cys polymorphism with lung cancer risk. Cancer Epidemiology, Biomarkers and Prevention. 2002;11(4):409–412. [PubMed] [Google Scholar]
- 21.Lan Q, Mumford JL, Shen M, et al. Oxidative damage-related genes AKR1C3 and 0GG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions. Carcinogenesis. 2004;25(11):2177–2181. doi: 10.1093/carcin/bgh240. [DOI] [PubMed] [Google Scholar]
- 22.Park J, Chen L, Tockman MS, Elahi A, Lazarus P. The human 8-oxoguanine DNA N-glycosylase 1 (hOGG1) DNA repair enzyme and its association with lung cancer risk. Pharmacogenetics. 2004;14(2):103–109. doi: 10.1097/00008571-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Vogel U, Nexø BA, Wallin H, Overvad K, Tjønneland A, Raaschou-Nielsen O. No association between base excision repair gene polymorphisms and risk of lung cancer. Biochemical Genetics. 2004;42(11-12):453–460. doi: 10.1023/b:bigi.0000043957.03420.7e. [DOI] [PubMed] [Google Scholar]
- 24.Liang G, Pu Y, Yin L. Rapid detection of single nucleotide polymorphisms related with lung cancer susceptibility of Chinese population. Cancer Letters. 2005;223(2):265–274. doi: 10.1016/j.canlet.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Hung RJ, Brennan P, Canzian F, et al. Large-scale investigation of base excision repair genetic polymorphisms and lung cancer risk in a multicenter study. Journal of the National Cancer Institute. 2005;97(8):567–576. doi: 10.1093/jnci/dji101. [DOI] [PubMed] [Google Scholar]
- 26.Zienolddiny S, Campa D, Lind H, et al. Polymorphisms of DNA repair genes and risk of non-small cell lung cancer. Carcinogenesis. 2006;27(3):560–567. doi: 10.1093/carcin/bgi232. [DOI] [PubMed] [Google Scholar]
- 27.Matullo G, Dunning AM, Guarrera S, et al. DNA repair polymorphisms and cancer risk in non-smokers in a cohort study. Carcinogenesis. 2006;27(5):997–1007. doi: 10.1093/carcin/bgi280. [DOI] [PubMed] [Google Scholar]
- 28.Kohno T, Kunitoh H, Toyama K, et al. Association of the OGG1-Ser326Cys polymorphism with lung adenocarcinoma risk. Cancer Science. 2006;97(8):724–728. doi: 10.1111/j.1349-7006.2006.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sørensen M, Raaschou-Nielsen O, Hansen RD, Tjoønneland A, Overvad K, Vogel U. Interactions between the OGG1 Ser326Cys polymorphism and intake of fruit and vegetables in relation to lung cancer. Free Radical Research. 2006;40(8):885–891. doi: 10.1080/10715760600733129. [DOI] [PubMed] [Google Scholar]
- 30.De Ruyck K, Szaumkessel M, De Rudder I, et al. Polymorphisms in base-excision repair and nucleotide-excision repair genes in relation to lung cancer risk. Mutation Research. 2007;631(2):101–110. doi: 10.1016/j.mrgentox.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Karahalil B, Emerce E, Koçer B, Han S, Alkiş N, Karakaya AE. The association of OGG1 Ser326Cys polymorphism and urinary 8-OHdG levels with lung cancer susceptibility: a hospital-based case-control study in Turkey. Arhiv za Higijenu Rada i Toksikologiju. 2008;59(4):241–250. doi: 10.2478/10004-1254-59-2008-1924. [DOI] [PubMed] [Google Scholar]
- 32.Miyaishi A, Osawa K, Osawa Y, et al. MUTYH Gln324His gene polymorphism and genetic susceptibility for lung cancer in a Japanese population. Journal of Experimental and Clinical Cancer Research. 2009;28(1, article 10) doi: 10.1186/1756-9966-28-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang JS, Wrensch MR, Hansen HM, et al. Base excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African-Americans. Carcinogenesis. 2009;30(1):78–87. doi: 10.1093/carcin/bgn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratnasinghe D, Yao S-X, Tangrea JA, et al. Polymorphisms of the DNA repair gene XRCC1 and lung cancer risk. Cancer Epidemiology, Biomarkers and Prevention. 2001;10(2):119–123. [PubMed] [Google Scholar]
- 35.David-Beabes GL, London SJ. Genetic polymorphism of XRCC1 and lung cancer risk among African-Americans and Caucasians. Lung Cancer. 2001;34(3):333–339. doi: 10.1016/s0169-5002(01)00256-2. [DOI] [PubMed] [Google Scholar]
- 36.Divine KK, Gilliland FD, Crowell RE, et al. The XRCC1 399 glutamine allele is a risk factor for adenocarcinoma of the lung. Mutation Research. 2001;461(4):273–278. doi: 10.1016/s0921-8777(00)00059-8. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Tang D, Xue K, et al. DNA repair gene XRCC1 and XPD polymorphisms and risk of lung cancer in a Chinese population. Carcinogenesis. 2002;23(8):1321–1325. doi: 10.1093/carcin/23.8.1321. [DOI] [PubMed] [Google Scholar]
- 38.Park JY, Lee SY, Jeon H-S, et al. Polymorphism of the DNA repair gene XRCC1 and risk of primary lung cancer. Cancer Epidemiology, Biomarkers and Prevention. 2002;11(1):23–27. [PubMed] [Google Scholar]
- 39.Misra RR, Ratnasinghe D, Tangrea JA, et al. Polymorphisms in the DNA repair genes XPD, XRCC1, XRCC3, and APE/ref-1, and the risk of lung cancer among male smokers in Finland. Cancer Letters. 2003;191(2):171–178. doi: 10.1016/s0304-3835(02)00638-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhou W, Liu G, Miller DP, et al. Polymorphisms in the DNA repair genes XRCC1 and ERCC2, smoking, and lung cancer risk. Cancer Epidemiology, Biomarkers and Prevention. 2003;12(4):359–365. [PubMed] [Google Scholar]
- 41.Ito H, Matsuo K, Hamajima N, et al. Gene-environment interactions between the smoking habit and polymorphisms in the DNA repair genes, APE1 Asp148Glu and XRCC1 Arg399Gln, in Japanese lung cancer risk. Carcinogenesis. 2004;25(8):1395–1401. doi: 10.1093/carcin/bgh153. [DOI] [PubMed] [Google Scholar]
- 42.Popanda O, Schattenberg T, Phong CT, et al. Specific combinations of DNA repair gene variants and increased risk for non-small cell lung cancer. Carcinogenesis. 2004;25(12):2433–2441. doi: 10.1093/carcin/bgh264. [DOI] [PubMed] [Google Scholar]
- 43.Harms C, Salama SA, Sierra-Torres CH, Cajas-Salazar N, Au WW. Polymorphisms in DNA repair genes, chromosome aberrations, and lung cancer. Environmental and Molecular Mutagenesis. 2004;44(1):74–82. doi: 10.1002/em.20031. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Miao X, Liang G, et al. Polymorphisms in DNA base excision repair genes ADPRT and XRCC1 and risk of lung cancer. Cancer Research. 2005;65(3):722–726. [PubMed] [Google Scholar]
- 45.Schneider J, Classen V, Bernges U, Philipp M. XRCC1 polymorphism and lung cancer risk in relation to tobacco smoking. International Journal of Molecular Medicine. 2005;16(4):709–716. [PubMed] [Google Scholar]
- 46.Shen M, Berndt SI, Rothman N, et al. Polymorphisms in the DNA base excision repair genes APEX1 and XRCC1 and lung cancer risk in Xuan Wei, China. Anticancer Research. 2005;25(1B):537–542. [PubMed] [Google Scholar]
- 47.Yin J, Vogel U, Ma Y, Qi R, Sun Z, Wang H. The DNA repair gene XRCC1 and genetic susceptibility of lung cancer in a northeastern Chinese population. Lung Cancer. 2007;56(2):153–160. doi: 10.1016/j.lungcan.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 48.López-Cima MF, González-Arriaga P, García-Castro L, et al. Polymorphisms in XPC, XPD, XRCC1, and XRCC3 DNA repair genes and lung cancer risk in a population of Northern Spain. BMC Cancer. 2007;7, article 162 doi: 10.1186/1471-2407-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pachouri SS, Sobti RC, Kaur P, Singh J. Contrasting impact of DNA repair gene XRCC1 polymorphisms Arg399Gln and Arg194Trp on the risk of lung cancer in North-Indian population. DNA and Cell Biology. 2007;26(3):186–191. doi: 10.1089/dna.2006.9999. [DOI] [PubMed] [Google Scholar]
- 50.Park JY, Park SH, Choi JE, et al. Polymorphisms of the DNA repair gene xeroderma pigmentosum group A and risk of primary lung cancer. Cancer Epidemiology, Biomarkers and Prevention. 2002;11(10, part 1):993–997. [PubMed] [Google Scholar]
- 51.Wu X, Zhao H, Wei Q, et al. XPA polymorphism associated with reduced lung cancer risk and a modulating effect on nucleotide excision repair capacity. Carcinogenesis. 2003;24(3):505–509. doi: 10.1093/carcin/24.3.505. [DOI] [PubMed] [Google Scholar]
- 52.Vogel U, Overvad K, Wallin H, Tjønneland A, Nexø BA, Raaschou-Nielsen O. Combinations of polymorphisms in XPD, XPC and XPA in relation to risk of lung cancer. Cancer Letters. 2005;222(1):67–74. doi: 10.1016/j.canlet.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 53.Kiyohara C, Yoshimasu K. Genetic polymorphisms in the nucleotide excision repair pathway and lung cancer risk: a meta-analysis. International Journal of Medical Sciences. 2007;4(2):59–71. doi: 10.7150/ijms.4.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butkiewicz D, Rusin M, Enewold L, Shields PG, Chorazy M, Harris CC. Genetic polymorphisms in DNA repair genes and risk of lung cancer. Carcinogenesis. 2001;22(4):593–597. doi: 10.1093/carcin/22.4.593. [DOI] [PubMed] [Google Scholar]
- 55.Spitz MR, Wu X, Wang Y, et al. Modulation of nucleotide excision repair capacity by XPD polymorphisms in lung cancer patients. Cancer Research. 2001;61(4):1354–1357. [PubMed] [Google Scholar]
- 56.Hou S-M, Fält S, Angelini S, et al. The XPD variant alleles are associated with increased aromatic DNA adduct level and lung cancer risk. Carcinogenesis. 2002;23(4):599–603. doi: 10.1093/carcin/23.4.599. [DOI] [PubMed] [Google Scholar]
- 57.Zhou W, Liu G, Miller DP, et al. Gene-environment interaction for the ERCC2 polymorphisms and cumulative cigarette smoking exposure in lung cancer. Cancer Research. 2002;62(5):1377–1381. [PubMed] [Google Scholar]
- 58.Liang G, Xing D, Miao X, et al. Sequence variations in the DNA repair gene XPD and risk of lung cancer in a Chinese population. International Journal of Cancer. 2003;105(5):669–673. doi: 10.1002/ijc.11136. [DOI] [PubMed] [Google Scholar]
- 59.Vogel U, Laros I, Jacobsen NR, et al. Two regions in chromosome 19q13.2-3 are associated with risk of lung cancer. Mutation Research. 2004;546(1-2):65–74. doi: 10.1016/j.mrfmmm.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Shen M, Berndt SI, Rothman N, et al. Polymorphisms in the DNA nucleotide excision repair genes and lung cancer risk in Xuan Wei, China. International Journal of Cancer. 2005;116(5):768–773. doi: 10.1002/ijc.21117. [DOI] [PubMed] [Google Scholar]
- 61.Hu Z, Xu L, Shao M, et al. Polymorphisms in the two helicases ERCC2/XPD and ERCC3/XPB of the transcription factor IIH complex and risk of lung cancer: a case-control analysis in a Chinese population. Cancer Epidemiology, Biomarkers and Prevention. 2006;15(7):1336–1340. doi: 10.1158/1055-9965.EPI-06-0194. [DOI] [PubMed] [Google Scholar]
- 62.Chang JS, Wrensch MR, Hansen HM, et al. Nucleotide excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African Americans. International Journal of Cancer. 2008;123(9):2095–2104. doi: 10.1002/ijc.23801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin Z, Su M, Li X, et al. ERCC2, ERCC1 polymorphisms and haplotypes, cooking oil fume and lung adenocarcinoma risk in Chinese non-smoking females. Journal of Experimental and Clinical Cancer Research. 2009;28(1, article 153) doi: 10.1186/1756-9966-28-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.David-Beabes GL, Lunn RM, London SJ. No association between the XPD (Lys751G1n) polymorphism or the XRCC3 (Thr241Met) polymorphism and lung cancer risk. Cancer Epidemiology, Biomarkers and Prevention. 2001;10(8):911–912. [PubMed] [Google Scholar]
- 65.Park JY, Lee SY, Jeon H-S, et al. Lys751Gln polymorphism in the DNA repair gene XPD and risk of primary lung cancer. Lung Cancer. 2002;36(1):15–16. doi: 10.1016/s0169-5002(01)00447-0. [DOI] [PubMed] [Google Scholar]
- 66.Hu Z, Wei Q, Wang X, Shen H. DNA repair gene XPD polymorphism and lung cancer risk: a meta-analysis. Lung Cancer. 2004;46(1):1–10. doi: 10.1016/j.lungcan.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 67.Benhamou S, Sarasin A. ERCC2/XPD gene polymorphisms and lung cancer: a HuGE review. American Journal of Epidemiology. 2005;161(1):1–14. doi: 10.1093/aje/kwi018. [DOI] [PubMed] [Google Scholar]
- 68.Matullo G, Peluso M, Polidoro S, et al. Combination of DNA repair gene single nucleotide polymorphisms and increased levels of DNA adducts in a population-based study. Cancer Epidemiology, Biomarkers and Prevention. 2003;12(7):674–677. [PubMed] [Google Scholar]
- 69.Palli D, Russo A, Masala G, et al. DNA adduct levels and DNA repair polymorphisms in traffic-exposed workers and a general population sample. International Journal of Cancer. 2001;94(1):121–127. doi: 10.1002/ijc.1433. [DOI] [PubMed] [Google Scholar]
- 70.Duell EJ, Wiencke JK, Cheng T-J, et al. Polymorphisms in the DNA repair genes XRCC1 and ERCC2 and biomarkers of DNA damage in human blood mononuclear cells. Carcinogenesis. 2000;21(5):965–971. doi: 10.1093/carcin/21.5.965. [DOI] [PubMed] [Google Scholar]
- 71.Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. British Medical Journal. 1994;309(6965):1351–1355. doi: 10.1136/bmj.309.6965.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faddy SC. Significant statistical heterogeneity in a meta-analysis of the usefulness of acetylcysteine for prevention of contrast nephropathy. American Journal of Cardiology. 2004;94(3):p. 414. doi: 10.1016/j.amjcard.2004.02.079. [DOI] [PubMed] [Google Scholar]
- 73.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. British Medical Journal. 2000;320(7249):1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Misra RR, Ratnasinghe D, Tangrea JA, et al. Polymorphisms in the DNA repair genes XPD, XRCC1, XRCC3, and APE/ref-1, and the risk of lung cancer among male smokers in Finland. Cancer Letters. 2003;191(2):171–178. doi: 10.1016/s0304-3835(02)00638-9. [DOI] [PubMed] [Google Scholar]
- 75.Kozak M. Role of ATP in binding and migration of 40S ribosomal subunits. Cell. 1980;22(2, part 2):459–467. doi: 10.1016/0092-8674(80)90356-6. [DOI] [PubMed] [Google Scholar]
- 76.Kozak M. Initiation of translation in prokaryotes and eukaryotes. Gene. 1999;234(2):187–208. doi: 10.1016/s0378-1119(99)00210-3. [DOI] [PubMed] [Google Scholar]
- 77.Kozak M. Adherence to the first-AUG rule when a second AUG codon follows closely upon the first. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(15):p. 7134. doi: 10.1073/pnas.92.15.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanaka K, Miura N, Satokata I, et al. Analysis of a human DNA excision repair gene involved in group A xeroderma pigmentosum and containing a zinc-finger domain. Nature. 1990;348(6296):73–76. doi: 10.1038/348073a0. [DOI] [PubMed] [Google Scholar]
- 79.Weiss JM, Goode EL, Ladiges WC, Ulrich CM. Polymorphic variation in hOGG1 and risk of cancer: a review of the functional and epidemiologic literature. Molecular Carcinogenesis. 2005;42(3):127–141. doi: 10.1002/mc.20067. [DOI] [PubMed] [Google Scholar]
- 80.Kohno T, Shinmura K, Tosaka M, et al. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene. 1998;16(25):3219–3225. doi: 10.1038/sj.onc.1201872. [DOI] [PubMed] [Google Scholar]
- 81.Dhénaut A, Boiteux S, Radicella JP. Characterization of the hOGG1 promoter and its expression during the cell cycle. Mutation Research. 2000;461(2):109–118. doi: 10.1016/s0921-8777(00)00042-2. [DOI] [PubMed] [Google Scholar]
- 82.Hung RJ, Hall J, Brennan P, Boffetta P. Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review. American Journal of Epidemiology. 2005;162(10):925–942. doi: 10.1093/aje/kwi318. [DOI] [PubMed] [Google Scholar]
- 83.Li H, Hao X, Zhang W, Wei Q, Chen K. The hOGG1 Ser326Cys polymorphism and lung cancer risk: a meta-analysis. Cancer Epidemiology, Biomarkers and Prevention. 2008;17(7):1739–1745. doi: 10.1158/1055-9965.EPI-08-0001. [DOI] [PubMed] [Google Scholar]
- 84.Lunn RM, Langlois RG, Hsieh LL, Thompson CL, Bell DA. XRCC1 polymorphisms: effects on aflatoxin B1-DNA adducts and glycophorin A variant frequency. Cancer Research. 1999;59(11):2557–2561. [PubMed] [Google Scholar]
- 85.Savas S, Kim DY, Ahmad MF, Shariff M, Ozcelik H. Identifying functional genetic variants in DNA repair pathway using protein conservation analysis. Cancer Epidemiology, Biomarkers and Prevention. 2004;13(5):801–807. [PubMed] [Google Scholar]
- 86.Duell EJ, Millikan RC, Pittman GS, et al. Polymorphisms in the DNA repair gene XRCC1 and breast cancer. Cancer Epidemiology, Biomarkers and Prevention. 2001;10(3):217–222. [PubMed] [Google Scholar]
- 87.Nexø BA, Vogel U, Olsen A, et al. A specific haplotype of single nucleotide polymorphisms on chromosome 19q13.2-3 encompassing the gene RAI is indicative of post-menopausal breast cancer before age 55. Carcinogenesis. 2003;24(5):899–904. doi: 10.1093/carcin/bgg043. [DOI] [PubMed] [Google Scholar]
- 88.Sarasin A. An overview of the mechanisms of mutagenesis and carcinogenesis. Mutation Research. 2003;544(2-3):99–106. doi: 10.1016/j.mrrev.2003.06.024. [DOI] [PubMed] [Google Scholar]
- 89.Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. Journal of the National Cancer Institute. 2004;96(6):434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khoury M, Beaty TH, Cohen BH. Fundamentals of Genetic Epidemiology. New York, NY, USA: Oxford University Press; 1993. (Monographs in Epidemiology and Biostatistics). [Google Scholar]
- 91.Hoh J, Wille A, Ott J. Trimming, weighting, and grouping SNPs in human case-control association studies. Genome Research. 2001;11(12):2115–2119. doi: 10.1101/gr.204001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kiyohara C, Otsu A, Shirakawa T, Fukuda S, Hopkin JM. Genetic polymorphisms and lung cancer susceptibility: a review. Lung Cancer. 2002;37(3):241–256. doi: 10.1016/s0169-5002(02)00107-1. [DOI] [PubMed] [Google Scholar]