Abstract
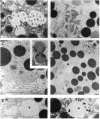
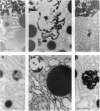
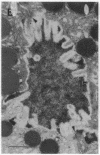
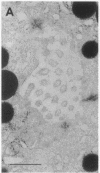
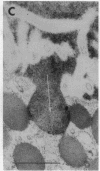
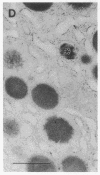
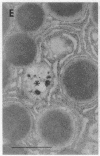
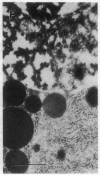
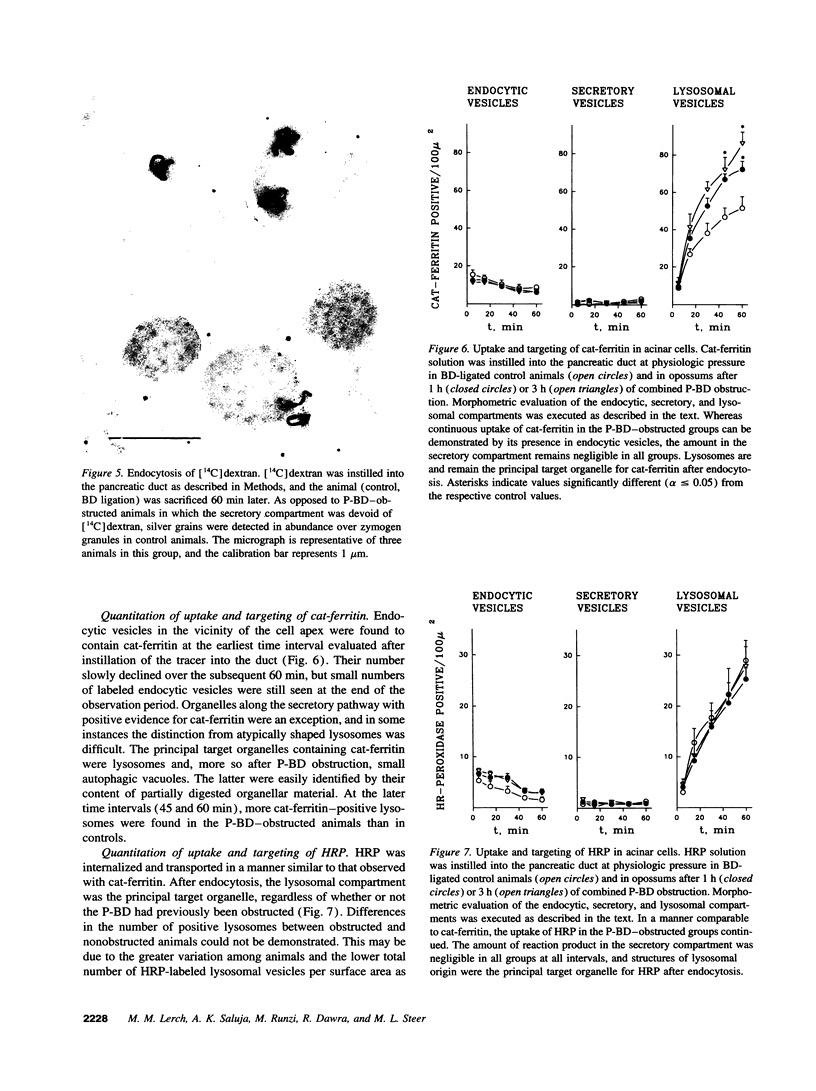
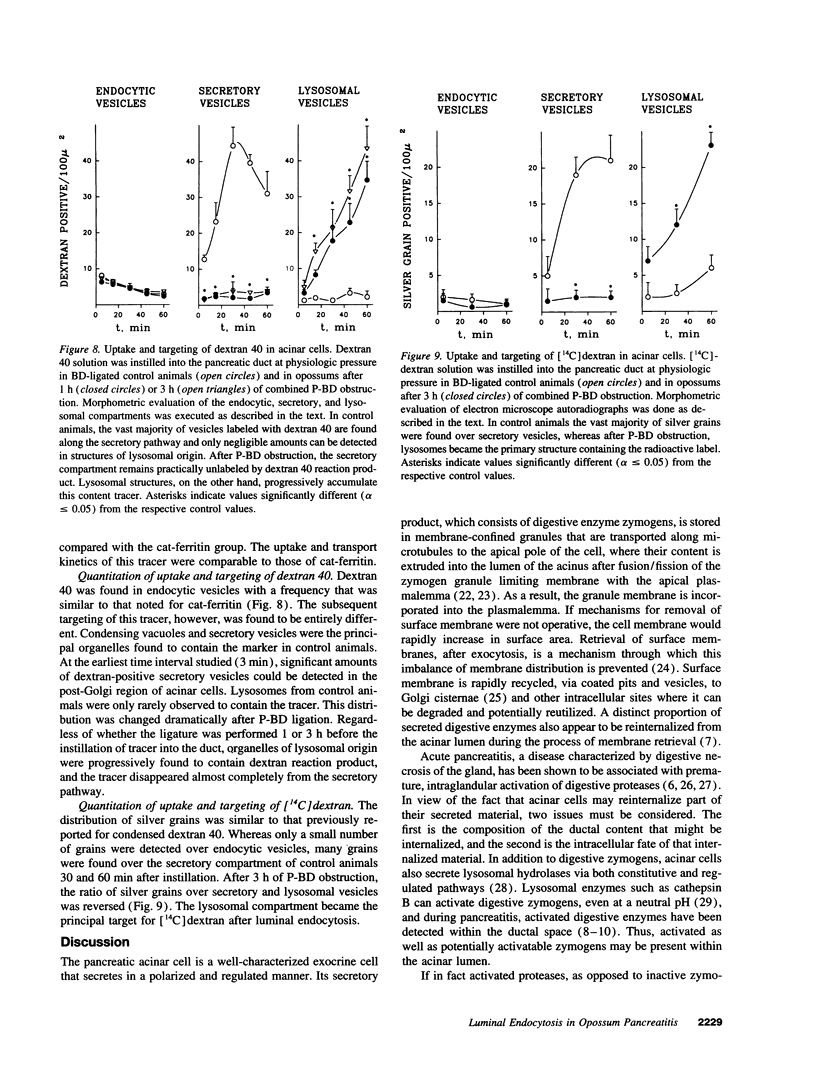
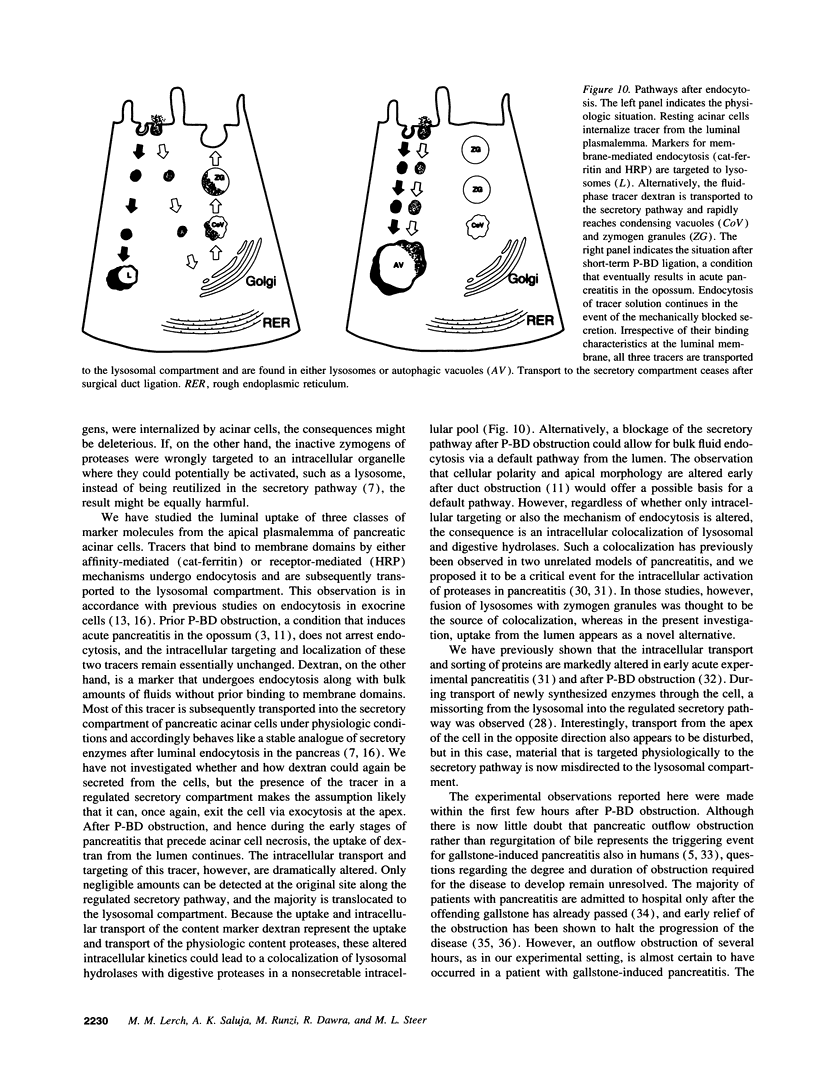
Cell necrosis in acute experimental pancreatitis is preceded by a redistribution of digestive enzymes into a lysosomal subcellular compartment. We have investigated whether endocytosis from the acinar cell lumen might contribute to this disturbance of intracellular compartmentation. In an animal model of pancreatitis involving pancreatic bile duct ligation in opossums, we have studied in vivo endocytosis of dextran 40 and [14C]dextran 70, cationized ferritin, and horseradish peroxidase from the apical surface of acinar cells before the onset of necrosis. Marker solutions were instilled into the pancreatic duct of anesthetized animals at physiological pressure. Tissue samples obtained at intervals of up to 60 min after instillation of markers were studied by electron microscopy and electron microscope autoradiography. All markers were taken up by acinar cells in control animals and in animals with obstructed pancreatic bile ducts. Markers for membrane-mediated endocytosis (cationated ferritin and horseradish peroxidase) were transported to lysosomes in both groups. In contrast, the fluid-phase tracer dextran was transported to the secretory pathway in controls but to lysosomes after duct ligation. Since dextran and luminally present secretory proteins can be expected to follow the same route after endocytosis, our findings suggest that altered intracellular targeting of endocytosed proteases might be one mechanism by which digestive zymogens reach an intracellular compartment in which premature activation can occur. This phenomenon may be a critical and early event in the pathogenesis of biliary pancreatitis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta J. M., Ledesma C. L. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974 Feb 28;290(9):484–487. doi: 10.1056/NEJM197402282900904. [DOI] [PubMed] [Google Scholar]
- Acosta J. M., Pellegrini C. A., Skinner D. B. Etiology and pathogenesis of acute biliary pancreatitis. Surgery. 1980 Jul;88(1):118–125. [PubMed] [Google Scholar]
- Allan P. J., Tournut R., White T. T. Intraductal activation of human pancreatic zymogens. N Engl J Med. 1973 Feb 1;288(5):266–266. [PubMed] [Google Scholar]
- Beaudoin A. R., Grondin G. Secretory pathways in animal cells: with emphasis on pancreatic acinar cells. J Electron Microsc Tech. 1991 Jan;17(1):51–69. doi: 10.1002/jemt.1060170107. [DOI] [PubMed] [Google Scholar]
- Bialek R., Willemer S., Arnold R., Adler G. Evidence of intracellular activation of serine proteases in acute cerulein-induced pancreatitis in rats. Scand J Gastroenterol. 1991 Feb;26(2):190–196. doi: 10.3109/00365529109025030. [DOI] [PubMed] [Google Scholar]
- Geokas M. C., Rinderknecht H. Free proteolytic enzymes in pancreatic juice of patients with acute pancreatitis. Am J Dig Dis. 1974 Jul;19(7):591–598. doi: 10.1007/BF01073012. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hernández C. A., Lerch M. M. Sphincter stenosis and gallstone migration through the biliary tract. Lancet. 1993 May 29;341(8857):1371–1373. doi: 10.1016/0140-6736(93)90942-a. [DOI] [PubMed] [Google Scholar]
- Herzog V., Farquhar M. G. Luminal membrane retrieved after exocytosis reaches most golgi cisternae in secretory cells. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5073–5077. doi: 10.1073/pnas.74.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog V., Reggio H. Pathways of endocytosis from luminal plasma membrane in rat exocrine pancreas. Eur J Cell Biol. 1980 Jun;21(2):141–150. [PubMed] [Google Scholar]
- Hirano T., Saluja A., Ramarao P., Lerch M. M., Saluja M., Steer M. L. Apical secretion of lysosomal enzymes in rabbit pancreas occurs via a secretagogue regulated pathway and is increased after pancreatic duct obstruction. J Clin Invest. 1991 Mar;87(3):865–869. doi: 10.1172/JCI115091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Synthesis, intracellular transport, and discharge of secretory proteins in stimulated pancreatic exocrine cells. J Cell Biol. 1971 Jul;50(1):135–158. doi: 10.1083/jcb.50.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Meldolesi J. Post-stimulation retrieval of luminal surface membrane in parotid acinar cells is calcium-dependent. Exp Cell Res. 1981 Aug;134(2):377–388. doi: 10.1016/0014-4827(81)90437-7. [DOI] [PubMed] [Google Scholar]
- Lang T., de Chastellier C. Fluid phase and mannose receptor-mediated uptake of horseradish peroxidase in mouse bone marrow-derived macrophages. Biochemical and ultrastructural study. Biol Cell. 1985;53(2):149–154. doi: 10.1111/j.1768-322x.1985.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Leach S. D., Modlin I. M., Scheele G. A., Gorelick F. S. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest. 1991 Jan;87(1):362–366. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch M. M., Hernández C. A., Adler G. Acute pancreatitis. N Engl J Med. 1994 Oct 6;331(14):948–949. doi: 10.1056/NEJM199410063311415. [DOI] [PubMed] [Google Scholar]
- Lerch M. M., Saluja A. K., Dawra R., Ramaraò P., Saluja M., Steer M. L. Acute necrotizing pancreatitis in the opossum: earliest morphological changes involve acinar cells. Gastroenterology. 1992 Jul;103(1):205–213. doi: 10.1016/0016-5085(92)91114-j. [DOI] [PubMed] [Google Scholar]
- Lerch M. M., Saluja A. K., Dawra R., Saluja M., Steer M. L. The effect of chloroquine administration on two experimental models of acute pancreatitis. Gastroenterology. 1993 Jun;104(6):1768–1779. doi: 10.1016/0016-5085(93)90658-y. [DOI] [PubMed] [Google Scholar]
- Lerch M. M., Saluja A. K., Rünzi M., Dawra R., Saluja M., Steer M. L. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology. 1993 Mar;104(3):853–861. doi: 10.1016/0016-5085(93)91022-a. [DOI] [PubMed] [Google Scholar]
- Lerch M. M., Weidenbach H., Hernandez C. A., Preclik G., Adler G. Pancreatic outflow obstruction as the critical event for human gall stone induced pancreatitis. Gut. 1994 Oct;35(10):1501–1503. doi: 10.1136/gut.35.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne E., Oliver C. Internalization of cationized ferritin by isolated pancreatic acinar cells. J Histochem Cytochem. 1986 Feb;34(2):167–176. doi: 10.1177/34.2.3944455. [DOI] [PubMed] [Google Scholar]
- Nadler N. J. The interpretation of grain counts in electron microscope radioautography. J Cell Biol. 1971 Jun;49(3):877–882. [PubMed] [Google Scholar]
- Neoptolemos J. P., Carr-Locke D. L., London N. J., Bailey I. A., James D., Fossard D. P. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988 Oct 29;2(8618):979–983. doi: 10.1016/s0140-6736(88)90740-4. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Mori M., Quintana N., Yam A. Studies of the secretory process in the mammalian exocrine pancreas. I. The condensing vacuoles. J Cell Biol. 1977 Oct;75(1):148–165. doi: 10.1083/jcb.75.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H. Activation of pancreatic zymogens. Normal activation, premature intrapancreatic activation, protective mechanisms against inappropriate activation. Dig Dis Sci. 1986 Mar;31(3):314–321. doi: 10.1007/BF01318124. [DOI] [PubMed] [Google Scholar]
- Romagnoli P., Herzog V. Reinternalization of secretory proteins during membrane recycling in rat pancreatic acinar cells. Eur J Cell Biol. 1987 Oct;44(2):167–175. [PubMed] [Google Scholar]
- Rünzi M., Saluja A., Lerch M. M., Dawra R., Nishino H., Steer M. L. Early ductal decompression prevents the progression of biliary pancreatitis: an experimental study in the opossum. Gastroenterology. 1993 Jul;105(1):157–164. doi: 10.1016/0016-5085(93)90021-4. [DOI] [PubMed] [Google Scholar]
- Saluja A., Saluja M., Villa A., Leli U., Rutledge P., Meldolesi J., Steer M. Pancreatic duct obstruction in rabbits causes digestive zymogen and lysosomal enzyme colocalization. J Clin Invest. 1989 Oct;84(4):1260–1266. doi: 10.1172/JCI114293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja M., Saluja A., Lerch M. M., Steer M. L. A plasma protease which is expressed during supramaximal stimulation causes in vitro subcellular redistribution of lysosomal enzymes in rat exocrine pancreas. J Clin Invest. 1991 Apr;87(4):1280–1285. doi: 10.1172/JCI115130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Permeability of intestinal capillaries. Pathway followed by dextrans and glycogens. J Cell Biol. 1972 May;53(2):365–392. doi: 10.1083/jcb.53.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. The role of the mannose/N-acetylglucosamine receptor in the pinocytosis of horseradish peroxidase by mouse peritoneal macrophages. J Cell Physiol. 1983 Jul;116(1):21–25. doi: 10.1002/jcp.1041160105. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Baccino F. M., Steer M. L., Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984 Apr;246(4 Pt 1):G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Kimura T., Mimura K., Nawata H. Activation of proteases in cerulein-induced pancreatitis. Pancreas. 1989;4(5):565–571. doi: 10.1097/00006676-198910000-00007. [DOI] [PubMed] [Google Scholar]