Abstract
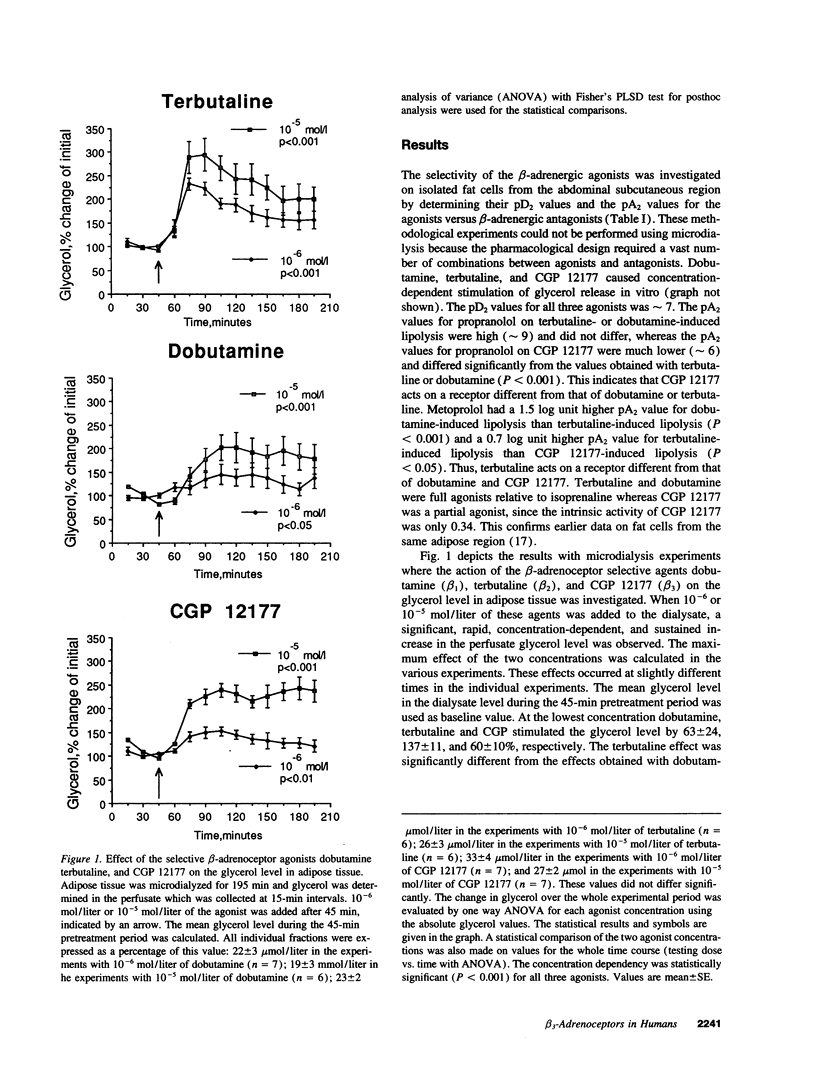
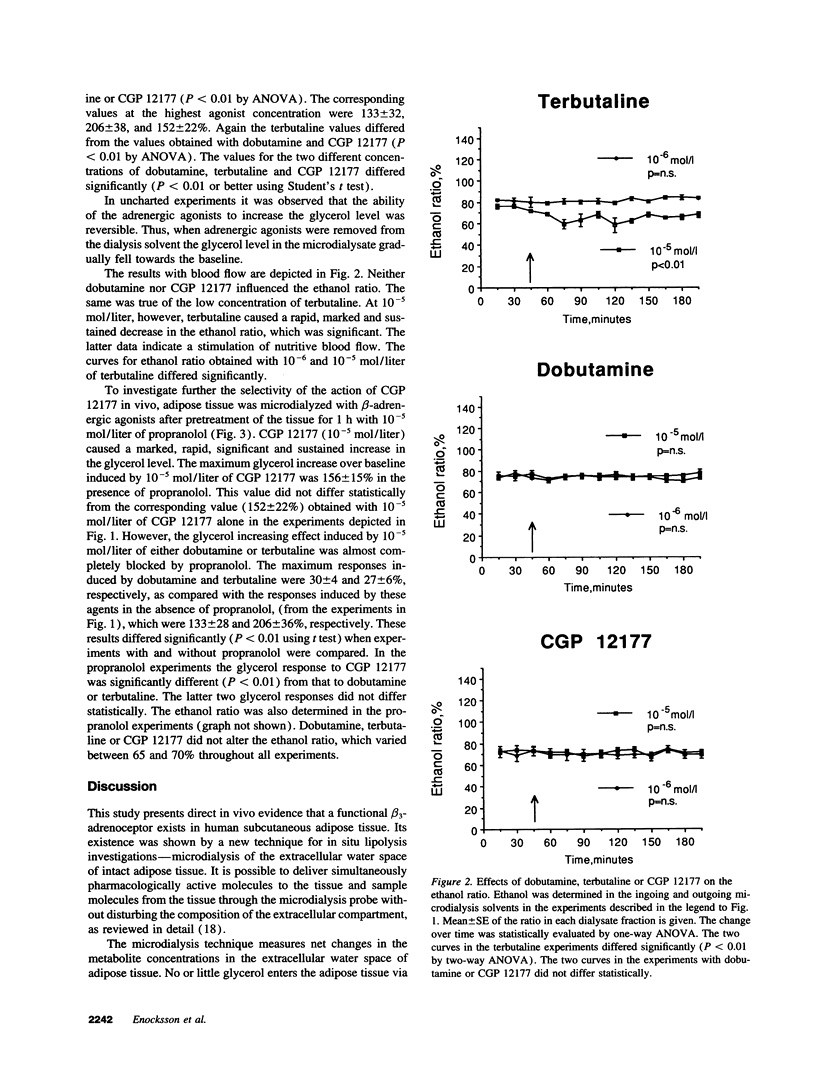
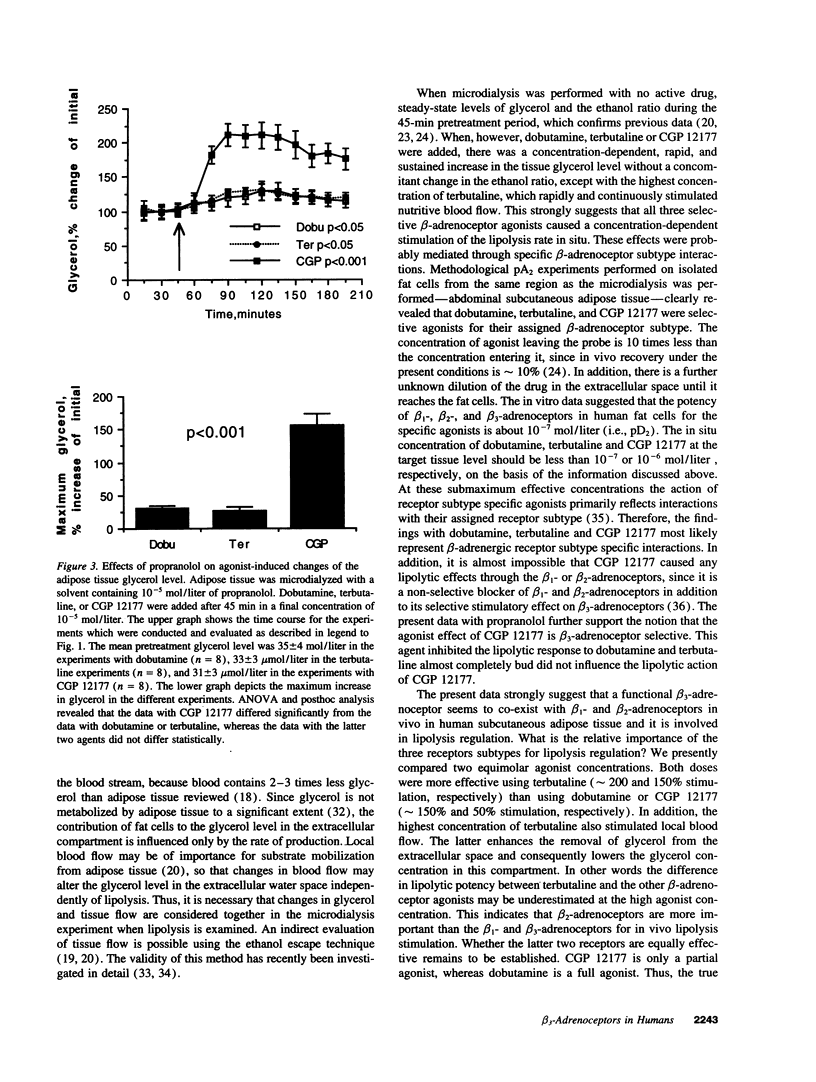
Although it is well established in several mammalian species that beta 3-adrenoceptors play a major role in regulating lipolysis and thermogenesis in adipose tissue, the functional existence and role of this receptor subtype in man has been controversial. We investigated whether the beta 3-adrenoceptor functionally co-exists with beta 1- and beta 2-adrenoceptors in vivo in human adipose tissue. Subcutaneous abdominal adipose tissue of healthy non-obese subjects was microdialyzed with equimolar concentrations of dobutamine (selective beta 1-adrenoceptor agonist), terbutaline (selective beta 2-adrenoceptor agonist), or CGP 12177 (selective beta 3-adrenoceptor agonist). All three agents caused a rapid, sustained, concentration-dependent and significant elevation of the glycerol level in the microdialysate (lipolysis index). However, only terbutaline stimulated the nutritive blood flow in adipose tissue, as measured by an ethanol escape technique. Dobutamine and CGP 12177 was equally effective in elevating the glycerol level (maximum effect 150% above baseline). Terbutaline was significantly more effective than the other two beta-agonists (maximum effect 200% above baseline). When adipose tissue was pretreated with the beta 1/beta 2-selective adrenoceptor blocker propranolol the glycerol increasing effect of dobutamine or terbutaline was inhibited by 80-85% but the glycerol response to CGP 12177 was not influenced. It is concluded that a functional beta 3-adrenoceptor is present in vivo in man. It co-exists with beta 1- and beta 2-adrenoceptors in adipose tissue and may therefore play a role in lipolysis regulation. It appears, however, that the beta 2-adrenoceptor is the most important beta-adrenoceptor subtype for the mobilization of lipids from abdominal subcutaneous adipose tissue because of its concomitant stimulatory effect on lipolysis and blood flow.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch J. R. The brown adipocyte beta-adrenoceptor. Proc Nutr Soc. 1989 Jul;48(2):215–223. doi: 10.1079/pns19890032. [DOI] [PubMed] [Google Scholar]
- Arner P., Bülow J. Assessment of adipose tissue metabolism in man: comparison of Fick and microdialysis techniques. Clin Sci (Lond) 1993 Sep;85(3):247–256. doi: 10.1042/cs0850247. [DOI] [PubMed] [Google Scholar]
- Arner P., Hellmér J., Hagström-Toft E., Bolinder J. Effect of phosphodiesterase inhibition with amrinone or theophylline on lipolysis and blood flow in human adipose tissue in vivo as measured with microdialysis. J Lipid Res. 1993 Oct;34(10):1737–1743. [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P., Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest. 1990 Mar;85(3):893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P. In vivo interactions between beta-1 and beta-2 adrenoceptors regulate catecholamine tachyphylaxia in human adipose tissue. J Pharmacol Exp Ther. 1991 Oct;259(1):317–322. [PubMed] [Google Scholar]
- Belfrage E. Comparison of beta-adrenoceptors mediating vasodilatation in canine subcutaneous adipose tissue and skeletal muscle. Acta Physiol Scand. 1978 Apr;102(4):469–476. doi: 10.1111/j.1748-1716.1978.tb06095.x. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Galitzky J., Lafontan M., Nordenström J., Arner P. Role of vascular alpha-2 adrenoceptors in regulating lipid mobilization from human adipose tissue. J Clin Invest. 1993 May;91(5):1997–2003. doi: 10.1172/JCI116421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman J. G., Lahners K. N., Chaudhry A. Characterization of the human beta 3-adrenergic receptor gene. Mol Pharmacol. 1993 Aug;44(2):264–270. [PubMed] [Google Scholar]
- Granneman J. G., Lahners K. N., Chaudhry A. Molecular cloning and expression of the rat beta 3-adrenergic receptor. Mol Pharmacol. 1991 Dec;40(6):895–899. [PubMed] [Google Scholar]
- Hellmér J., Arner P., Lundin A. Automatic luminometric kinetic assay of glycerol for lipolysis studies. Anal Biochem. 1989 Feb 15;177(1):132–137. doi: 10.1016/0003-2697(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Hickner R. C., Bone D., Ungerstedt U., Jorfeldt L., Henriksson J. Muscle blood flow during intermittent exercise: comparison of the microdialysis ethanol technique and 133Xe clearance. Clin Sci (Lond) 1994 Jan;86(1):15–25. doi: 10.1042/cs0860015. [DOI] [PubMed] [Google Scholar]
- Hickner R. C., Rosdahl H., Borg I., Ungerstedt U., Jorfeldt L., Henriksson J. Ethanol may be used with the microdialysis technique to monitor blood flow changes in skeletal muscle: dialysate glucose concentration is blood-flow-dependent. Acta Physiol Scand. 1991 Nov;143(3):355–356. doi: 10.1111/j.1748-1716.1991.tb09243.x. [DOI] [PubMed] [Google Scholar]
- Hickner R. C., Rosdahl H., Borg I., Ungerstedt U., Jorfeldt L., Henriksson J. The ethanol technique of monitoring local blood flow changes in rat skeletal muscle: implications for microdialysis. Acta Physiol Scand. 1992 Sep;146(1):87–97. doi: 10.1111/j.1748-1716.1992.tb09396.x. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P., Linde B. Influence of circulating NE and Epi on adipose tissue vascular resistance and lipolysis in humans. Am J Physiol. 1983 Sep;245(3):H447–H452. doi: 10.1152/ajpheart.1983.245.3.H447. [DOI] [PubMed] [Google Scholar]
- Hollenga C., Brouwer F., Zaagsma J. Differences in functional cyclic AMP compartments mediating lipolysis by isoprenaline and BRL 37344 in four adipocyte types. Eur J Pharmacol. 1991 Aug 6;200(2-3):325–330. doi: 10.1016/0014-2999(91)90590-m. [DOI] [PubMed] [Google Scholar]
- Hollenga C., Haas M., Deinum J. T., Zaagsma J. Discrepancies in lipolytic activities induced by beta-adrenoceptor agonists in human and rat adipocytes. Horm Metab Res. 1990 Jan;22(1):17–21. doi: 10.1055/s-2007-1004839. [DOI] [PubMed] [Google Scholar]
- Kenakin T. P. The classification of drugs and drug receptors in isolated tissues. Pharmacol Rev. 1984 Sep;36(3):165–222. [PubMed] [Google Scholar]
- Krief S., Lönnqvist F., Raimbault S., Baude B., Van Spronsen A., Arner P., Strosberg A. D., Ricquier D., Emorine L. J. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest. 1993 Jan;91(1):344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontan M., Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993 Jul;34(7):1057–1091. [PubMed] [Google Scholar]
- Lands A. M., Arnold A., McAuliff J. P., Luduena F. P., Brown T. G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967 May 6;214(5088):597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Langin D., Portillo M. P., Saulnier-Blache J. S., Lafontan M. Coexistence of three beta-adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharmacol. 1991 Jul 9;199(3):291–301. doi: 10.1016/0014-2999(91)90492-9. [DOI] [PubMed] [Google Scholar]
- Linde B., Hjemdahl P., Freyschuss U., Juhlin-Dannfelt A. Adipose tissue and skeletal muscle blood flow during mental stress. Am J Physiol. 1989 Jan;256(1 Pt 1):E12–E18. doi: 10.1152/ajpendo.1989.256.1.E12. [DOI] [PubMed] [Google Scholar]
- Lönnqvist F., Krief S., Strosberg A. D., Nyberg S., Emorine L. J., Arner P. Evidence for a functional beta 3-adrenoceptor in man. Br J Pharmacol. 1993 Nov;110(3):929–936. doi: 10.1111/j.1476-5381.1993.tb13902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnqvist F., Nyberg B., Wahrenberg H., Arner P. Catecholamine-induced lipolysis in adipose tissue of the elderly. J Clin Invest. 1990 May;85(5):1614–1621. doi: 10.1172/JCI114612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnqvist F., Wahrenberg H., Hellström L., Reynisdottir S., Arner P. Lipolytic catecholamine resistance due to decreased beta 2-adrenoceptor expression in fat cells. J Clin Invest. 1992 Dec;90(6):2175–2186. doi: 10.1172/JCI116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzin P., Revelli J. P., Kuhne F., Gocayne J. D., McCombie W. R., Venter J. C., Giacobino J. P., Fraser C. M. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem. 1991 Dec 15;266(35):24053–24058. [PubMed] [Google Scholar]
- Nahmias C., Blin N., Elalouf J. M., Mattei M. G., Strosberg A. D., Emorine L. J. Molecular characterization of the mouse beta 3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J. 1991 Dec;10(12):3721–3727. doi: 10.1002/j.1460-2075.1991.tb04940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostman J., Arner P., Kimura H., Wahrenberg H., Engfeldt P. Influence of fasting on lipolytic response to adrenergic agonists and on adrenergic receptors in subcutaneous adipocytes. Eur J Clin Invest. 1984 Oct;14(5):383–391. doi: 10.1111/j.1365-2362.1984.tb01199.x. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Revelli J. P., Muzzin P., Paoloni A., Moinat M., Giacobino J. P. Expression of the beta 3-adrenergic receptor in human white adipose tissue. J Mol Endocrinol. 1993 Apr;10(2):193–197. doi: 10.1677/jme.0.0100193. [DOI] [PubMed] [Google Scholar]
- Rosell S., Belfrage E. Blood circulation in adipose tissue. Physiol Rev. 1979 Oct;59(4):1078–1104. doi: 10.1152/physrev.1979.59.4.1078. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M., Malbon C. C., Hirsch J., Leibel R. L. Lack of beta 3-adrenergic effect on lipolysis in human subcutaneous adipose tissue. J Clin Endocrinol Metab. 1993 Aug;77(2):352–355. doi: 10.1210/jcem.77.2.8393882. [DOI] [PubMed] [Google Scholar]
- Tallarida R. J., Cowan A., Adler M. W. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979 Aug 20;25(8):637–654. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]
- Tossman U., Ungerstedt U. Microdialysis in the study of extracellular levels of amino acids in the rat brain. Acta Physiol Scand. 1986 Sep;128(1):9–14. doi: 10.1111/j.1748-1716.1986.tb07943.x. [DOI] [PubMed] [Google Scholar]
- Van Liefde I., van Witzenburg A., Vauquelin G. Isoproterenol and selective agonists stimulate similar atypical beta-adrenoceptors in rat adipocytes. Biochem Pharmacol. 1993 Feb 24;45(4):974–977. doi: 10.1016/0006-2952(93)90184-x. [DOI] [PubMed] [Google Scholar]
- Zaagsma J., Nahorski S. R. Is the adipocyte beta-adrenoceptor a prototype for the recently cloned atypical 'beta 3-adrenoceptor'? Trends Pharmacol Sci. 1990 Jan;11(1):3–7. doi: 10.1016/0165-6147(90)90032-4. [DOI] [PubMed] [Google Scholar]


