Abstract
Purpose
The two most widely investigated animal models for diabetic retinopathy (DR) are the rat and dog. In dogs, aldose reductase (AR) is present only in retinal capillary pericytes and their destruction has been linked to polyol accumulation and resulting apoptosis. Since both rat capillary pericytes and endothelial cells have been reported to contain AR, the role of polyol pathway activity in capillary cell destruction has been investigated in rat retinal capillary pericyte (TR-rPCT) and endothelial (TR-iBRB) cells.
Methods
TR-rPCT and TR-iBRB cell lines were recloned and their identities were reconfirmed by characteristic immunostaining. Cells were cultured up to 72 h in media containing 50 mM glucose or galactose with/without the AR inhibitors or a sorbitol dehydrogenase inhibitor (SDI) or with 30 mM 3-fluoro-3-deoxyglucose. Polyol levels were determined by HPLC or 19F-NMR. Apoptosis was detected with TUNEL/DAPI staining.
Results
Smooth muscle actin is present only in pericytes while only endothelial cells stain for von Willebrand factor and accumulate acetylated low-density lipoprotein. AR is present in both cells but AR levels are lower in endothelial cells. Aldehyde reductase is also present in both cells. Cells cultured in 50 mM glucose or galactose show significant polyol accumulation in pericytes but endothelial cells show little accumulation of galactitol and no accumulation of sorbitol. Sorbitol accumulation in pericytes resulted in increased cellular permeability and increased TUNEL staining, which was reduced by AR inhibition.
Conclusions
Although both rat retinal pericytes and endothelial cells contain AR, sorbitol accumulation and TUNEL staining primarily occur in pericytes and are inhibited by AR inhibitors.
Introduction
Retinopathy, the most common microvascular complication of diabetes mellitus, is characterized by vascular changes of the retinal capillary bed that include selective pericyte loss, capillary basement membrane thickening, dilations/endothelial hypertrophy, permeability/hard exudates, capillary nonperfusion and occlusion/acellularity, microaneurysms/intraretinal hemorrhages, intraretinal microvascular abnormalities (IRMAs)/shunts/dilated meshwork, cotton wool spots/ischemia, vessel–glial proliferation, extraretinal hemorrhages, glial–vitreal contraction, and macular edema. While some of these lesions are associated with other ocular or systemic disorders, diabetic retinopathy (DR) is the only disorder that elicits all of above described lesions.1
Retinal capillaries are composed of endothelial cells, which form the capillary lumen and pericytes (mural cells) that encircle the endothelial cells with their fine cytoplasmic structures. Pericytes contain smooth muscle actin and may play a role in regulating capillary blood flow, capillary permeability, phagocytosis, and endothelial cell growth through contact inhibition.2–4 With age, there is either a loss of retinal capillary endothelial cells or an equal loss of both pericytes and endothelial cells; however, with diabetes mellitus there is a selective loss of retinal capillary pericytes.5–7 This selective loss of pericytes is considered a hallmark of DR and precedes its clinical appearance.
Hyperglycemia is the central, underlying cause of DR and tight control of hyperglycemia has been established to reduce the progression of DR.8 Experimental animal studies suggest that hyperglycemia can be broadened to include the six-membered sugar galactose because similar retinal capillary lesions occur in both diabetic and galactosemic dogs and rats. The metabolism of glucose and galactose are linked by aldose reductase (AR), an enzyme that catalyzes the reduction of both sugars to their respective sugar alcohols sorbitol and galactitol. Inhibition of AR in diabetic or galactosemic rats and dogs delays the onset and progression of DR by preventing pericyte destruction, capillary basement membrane thickening, and the subsequent formation of acellular capillaries that result in areas of nonperfusion. This indicates that AR activity is important in the development of DR-associated vascular lesions.1,9 Further support for the importance of AR activity in the development of these vascular lesions comes from transgenic mouse studies where AR is either overexpressed or knocked out.10,11
In vitro cell cultures of retinal endothelial cells and pericytes have also been valuable tools for investigating the relationship between hyperglycemia, galactosemia, and AR in retinal cell degeneration. Studies using primary cultures from human, dog, and bovine retinal capillaries all indicate that pericytes contain AR and that AR activity is linked to the induction of apoptosis in retinal capillaries exposed to either hyperglycemia or galactosemia.12–15 However, reports using rat retinal capillary pericytes and endothelial cells have been minimal despite the fact that rats have been widely used to investigate the development of DR. This may in part be due to the difficulty of obtaining adequate amounts of retinal vascular tissue from the rat eye compared to the bovine eye, which is the most common source of retinal capillary cells. Here, we report the response of cell lines of rat retinal capillary pericytes and endothelial cells (TR-rPCT and TR-iBRB).16–18 These cells were developed from a transgenic rat harboring the temperature-sensitive simian virus 40 (SV40) large T-antigen gene (Tg rat).16
Methods
Chemicals
All reagents and solvents were commercially obtained from Acros Organics and Fisher Chemicals (Fairlawn, NJ) or Sigma-Aldrich Corporation (St Louis, MO) and utilized without further purification. 3-Fluoro-3-deoxyglucose (3FDG) was obtained from Omicron Biochemicals Inc. (South Bend, IN). All solvents were reagent or HPLC grade. The aldose reductase inhibitor (ARI) AL-1576 (2,4-difluorospirofluorene-9,5′-imidazolidine-2′,4′-dione) was obtained from Alcon Laboratories (Ft. Worth, TX) and the sorbitol dehydrogenase inhibitor (SDI) CP-166,572 (4-[4-(N,N-dimethylsulfamoyl) piperazino]-2-hydroxymethylpyrimidine) was obtained from Pfizer Central Research (Groton, CT). Antibodies utilized were as follows: mouse smooth muscle actin and von Willebrand rabbit polyclonal (Abcam Inc., Cambridge, MA); goat anti-rabbit IgG-FTIC and goat anti-rabbit IgG-HRP (Invitrogen Corp., Carlsbad, CA); donkey anti-mouse IgG-FTIC (Research Diagnostics Inc., Concord, MA); anti-rabbit IgG-FTIC (Zymed Laboratories Inc., South San Francisco, CA). Acetylated low-density lipoprotein from human plasma conjugated with Alexa Fluor® 488 was obtained from Molecular Probes, Inc., (Eugene, OR). DMEM cell culture media was obtained from Invitrogen Corp. (Carlsbad, CA). Rat tail collagen type 1, endothelial cell growth factor (ECGF), and the TUNEL staining kit were obtained from Roche Diagnostics (Indianapolis, IN). VECTA-SHIELD® containing 4′,6-diamino-2-phenylindole (DAPI) was obtained from Ventor Laboratories (Burlingame, CA). Block Ace blocking solution was obtained from Dainipppon Pharmaceutical Co. Ltd. (Osaka, Japan).
Rat retinal capillary pericytes and endothelial cells
Rat retinal capillary pericytes and endothelial cells (TR-rPCT and TR-iBRB), obtained from Dr. Hosoya, were developed from a transgenic rat harboring the temperature-sensitive simian virus 40 (SV40) large T-antigen gene (Tg rat).17 After recloning these cells, each cell line was grown as follows:
Cell culture of rat TR-rPCT pericytes
Culture medium for the pericytes was prepared by dissolving 10 g of DMEM powder containing glutamine (Gibco-BRL, Bethesda, MD) with 1.5 g NaHCO3, 100 mg streptomycin sulfate, 6 mg fungizone, and cell culture grade water to make 1 L. The solution was sterilized by filtration (pore size 0.22 μm) and 10% v/v of heat-inactivated Fetal bovine serum (FBS; incubated at 56°C for 45 min) was then added to the medium.
Cryopreserved pericytes (1 × 106 cells/tube) stored under liquid nitrogen were thawed and washed three times with 13 mL DMEM media until all of the DMSO was removed. Fourteen milliliters of the DMEM culture media was then added to the cells and they were seeded onto 75-cm2 culture flasks coated with rat tail collagen type 1, and cultured in DMEM media containing glutamine, NaHCO3, and streptomycin at 33°C in humidified atmosphere of 5% CO2 and 95% air until subconfluent (2–4 days). The cells were then passaged by treatment with trypsin–EDTA when the cells became 80%–90% confluent. The passaged cells were then plated at a density of 2 × 104 onto 24-well plates or 75-cm2 culture flasks coated with rat tail collagen type 1. DMEM medium was replaced every 2 days. Only cells passaged less than seven times were utilized.
Cell culture of rat TR-iBRB retinal capillary endothelial cells
Retinal capillary endothelial cells were cultured using procedures similar to that employed for the pericytes with the exception that the culture medium contained 15 mg/L of ECGF.
Sugar analysis of cultured cells
Media was removed from each 75-cm2 culture flask and the culture flask was washed with 6 mL of phosphate-buffered saline (PBS). Trypsin solution (4.5 mL) was added to each flask and the cells were incubated at 37°C until all cells were released from the flasks. The trypsin solution-containing cells was then pipetted into 15 mL tubes and sonicated for 3 × 10 s. An aliquot of the homogenate was removed for colorimetric protein quantification using the DC Protein Assay (Bio-Rad Laboratories, Hercules, CA) and bovine serum albumin (BSA) protein standards. Three micromoles of xylitol, an internal standard, was then added to the remaining homogenate. Each sample was deproteinized by overnight centrifugation at 8°C through a Microcon YM-10 Centrifugal Filter Device and the filtrates were dried in a Speedvac. Each dried residue was dissolved in 900 μL of pyridine and then derivatized with 900 μL of phenyl isocyanate at 55°C for 60 min. After cooling in an ice bath, the reaction was halted with cold methanol. This was again followed by heating for 5 min. The derivatized samples were analyzed by HPLC on an automated Hewlet Packard 1100 Chemstation equipped with a diode array detector. Samples (5 μL) were injected onto a 150 × 4.6 mm Tosoh TSK-GEL ODS-80Tm column containing a 3.2 × 15 mm guard column at 35°C. Samples were eluted isocratically with 20 mM potassium phosphate/acetonitrile (35:65 v%), pH 7.0, at a flow rate of 1.0 mL/min and detected at 235 nm. Samples were quantified against standard curves of glucose, galactose, sorbitol, galactitol, myoinositol, xylose (0.008–6.0 μmol).
19F-NMR
Following 72-h culture in media containing 30 mM 3FDG as described earlier, the cells were washed with 2 mL of PBS, released from their flasks with trypsin, and homogenized in a glass homogenizer. After centrifugation, the supernatants were transferred to 5-mm NMR tubes containing 100 μL each of D2O and 0.5% trifluorotoluene. Each sample was run overnight on a 500-MHz Varian NMR spectrometer.
Immunostaining of cells
Media was removed from the cultured cells and the cells were washed with PBS. This was followed by 30-min treatment with 4% paraformaldehyde and washing with PBS. The cells were then treated with an ice-cold methanol:acetone:water (68:39:3) for 10 min and again washed with PBS. This was followed by 1 h treatment at room temperature with Block Ace blocking solution. After removal of the blocking solution, the cells were washed with PBS and incubated for 1 h at room temperature with PBS containing selected primary antibodies, followed by overnight incubation at 4°C. The antibody solution was removed, the cells rinsed with PBS and then incubated with fluorescent-tagged secondary antibodies dissolved in blocking solution for 1.5 h. The antibody solutions were then removed, the cells rinsed with PBS and examined by fluorescent microscopy.
TUNEL and DAPI staining
Pericytes and endothelial cells (2 × 104 cells) were seeded onto eight-well/slide Lab-Tek™ Chamber Slide™ (Thermo Fisher Scientific, Rochester, NY). After 18–24 h the initial medium as described earlier was removed and replaced with FBS and antibiotics-free medium containing 5.5, 25, 50, or 100 mM glucose; however, for the epithelial cells media, the ECGF (15 mg/L) remained. After 24 h, the cells were washed with PBS and fixed in freshly prepared 4% paraformaldehyde in PBS. The cells were then treated for 2 min with an ice-cold solution of 0.1% Triton X-100 and 0.1% sodium citrate and then rinsed twice with PBS and incubated for 1 h at 37°C with the TUNEL reaction mixture (Roche Diagnostics, Indianapolis, IN). Each slide was mounted with VECTASHIELD® containing 4′6-diamino-2-phenylindole (DAPI). Image capture and analyses were performed using a NIKON fluorescence microscope equipped with the appropriate wavelength filters. Cell counts were obtained from three photographs for each well and the results were expressed as mean ± SEM. A minimum of three independent experiments were conducted.
Statistical analyses
The calculations and statistical analyses (t-test and ANOVA) were conducted using Origin® software (OriginLab Corp., Northampton, MA). Differences with a P < 0.05 were defined as significant.
Results
Cell lines of rat retinal capillary pericytes and endothelial cells (TR-rPCT and TR-iBRB) established from a transgenic rat harboring the temperature-sensitive simian virus 40 (SV40) large T-antigen gene (Tg rat) were recloned and each cell line was grown at 33°C on rat tail type I collagen-coated plates with DMEM medium containing 10% FBS. The identity of each cell line was reconfirmed by immunostaining with antibodies against smooth muscle actin, von Willebrand factor, and the uptake of Ac-LDLs. Characteristically, only the pericytes contained smooth muscle actin, while only the endothelial cells incorporated Ac-LDL into their cytoplasm and immunostained for von Willebrand factor (data not shown).
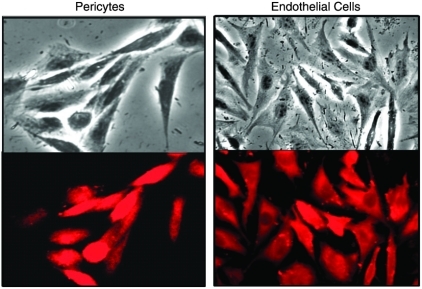
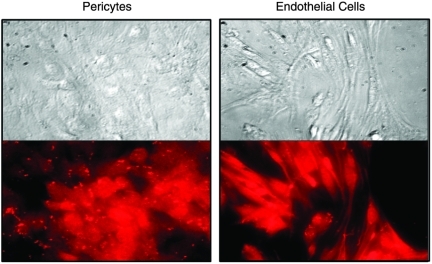
To establish the presence of AR in retinal pericytes versus endothelial cells, both retinal cells lines were probed with antibodies raised against rat lens AR.19 Using rhodamine-conjugated second antibodies to visualize the antigen–antibody complexes, fluorescence microscopy revealed the presence of AR in the cytoplasm of both rat pericytes and endothelial cells (Fig. 1). Fluorescence in endothelial cells was weaker, suggesting that qualitatively lower levels of AR are present in endothelial cells. Both cell lines were also probed for the presence of the closely related oxidoreductase aldehyde reductase (ALR, hexonate dehydrogenase). Using antibodies raised against rat kidney ALR,20 immunohistochemisty revealed that the cytoplasm of both cell types contain ALR (Fig. 2).
FIG. 1.
Immunohistochemical presence of aldose reductase (AR) in the cytoplasm of rat retinal capillary pericytes (left) and endothelial cells (right). The cells were first treated with antibodies against rat lens AR prepared in goat and then visualized with rhodamine-coupled anti-goat IgG. Top represents phase-contrast imaging while the bottom represents fluorescent imaging of the same cells.
FIG. 2.
Immunohistochemical presence of oxidoreductase aldehyde reductase (ALR) in rat retinal capillary pericytes (left) and endothelial cells (right). The cells were first treated with antibodies against rat kidney ALR prepared in goat and then visualized with rhodamine-coupled anti-goat IgG. Top represents phase-contrast imaging while the bottom represents fluorescent imaging of the same cells.
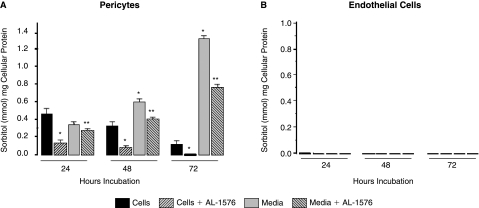
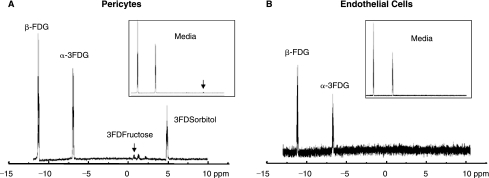
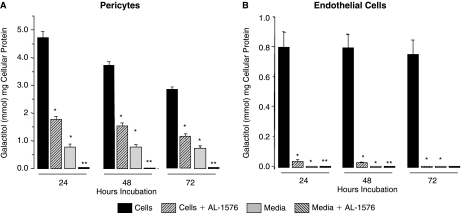
Since AR is present in both retinal capillary pericytes and endothelial cells, the rate of polyol formation in these cells was compared by culturing each cell line for up to 72 h in media containing 50 mM glucose. Surprisingly, sorbitol was only detected by HPLC in pericytes but not in endothelial cells (Fig. 3A, B), despite the immunohistochemical presence of AR in both capillary cells. Sorbitol formation in the pericytes was reduced by the presence of the ARI AL-1576. In the pericytes, intracellular sorbitol formation was accompanied by a time-dependent increase of sorbitol in the medium, suggesting that sorbitol accumulation in pericytes may be associated with permeability changes. Sorbitol in endothelial cells was also not observed when equal amounts of pericytes or endothelial cells were cultured for 72 h with 3-fluoro-3-deoxyglucose (3FDG), a more sensitive glucose substrate for AR.21 The 19F-NMR spectra (Fig. 4 A, B) clearly show the presence of 3-fluoro-3-deoxy sorbitol (3FDS) in both pericytes and pericyte culture media, but not in either endothelial cells or endothelial cell culture medium.
FIG. 3.
Accumulation of sorbitol in (A) rat retinal capillary cells and (B) rat capillary endothelial cells cultured up to 72 h in DMEM media containing 50 mM glucose. Media was changed every 24 h. Sorbitol accumulation was only observed in pericytes. The increase in media sorbitol concentrations at 24 and 48 h suggests that pericyte membrane permeability had increased with prolonged culture in high glucose. Mean ± SD, n = 4. *P < 0.05 versus untreated cells; **P < 0.05 versus AL-1576-treated cells.
FIG. 4.
(A) 19F-NMR spectrum obtained from pericytes cultured for 72 h in media where glucose was replaced with 30 mM 3FDG. Note the accumulation of 3FDS in both the cells and media (insert). (B) 19F-NMR spectrum obtained from endothelial cells similarly cultured for 72 h in 30 mM 3FDG confirming the absence of sorbitol production in either cells or media.
Galactose is a better substrate for AR than glucose. In the presence of 50 mM galactose, galactitol formation was observed in both pericytes and endothelial cells and this formation was reduced by AL-1576 (Fig. 5A, B). In pericytes, galactitol formation was 10-fold higher compared to sorbitol; however, the media galactitol levels were lower suggesting that sorbitol- and galactitol-associated permeability differs. In the presence of galactose, galactitol formation was observed in endothelial cells; however, this formation was significantly less than in pericytes. Galactitol formation remained constant with time and did not induce increased membrane permeability as evidenced by the lack of galactitol in the culture medium.
FIG. 5.
Accumulation of galactitol in rat retinal capillary cells cultured up to 72 h in DMEM media containing 50 mM galactose. Media was changed every 24 h. Galactitol production was observed in both (A) pericytes and (B) endothelial cells; however, galactitol accumulation in the endothelial cells was 5-fold less. In contrast to sorbitol, media galactitol did not increase with time. Mean ± SD, n = 4. *P < 0.05 versus untreated cells; **P < 0.05 versus AL-1576-treated cells.
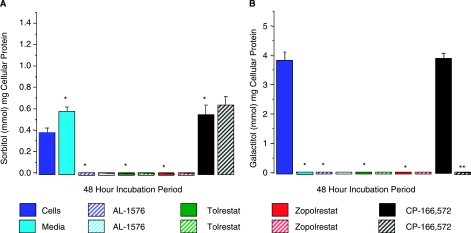
Since previous studies report that ALR in addition to AR can contribute to sorbitol/galactitol (polyol) formation,22 the potential contribution of ALR versus AR to polyol accumulation was investigated by comparing the effects of the more specific ARIs tolrestat and zopolrestat to AL-1576, a nonspecific inhibitor of both AR and ALR (Fig. 6). In pericytes cultured in either 50 mM glucose or galactose, polyol formation was equally reduced by 10 μM of each ARI, suggesting that the polyols are primarily formed by AR rather than ALR. Sorbitol levels also increased when the SDI CP-166,572 was added to pericytes cultured in glucose, confirming that the sorbitol pathway is the primary source of sorbitol formation.
FIG. 6.
Polyol accumulation in rat retinal pericytes cultured for 48 h with/without 10 μM of the aldose reductase inhibitors (ARIs), AL-1576, tolrestat, and zopolrestat; or the sorbitol dehydrogenase inhibitor (SDI), CP-166,572. Similar inhibitions of (A) sorbitol and (B) galactitol were observed with all three ARIs. The SDI increased sorbitol levels by inhibiting its conversion to fructose but had no effect on galactitol concentrations because galactitol is not further metabolized. Mean ± SD, n = 4. *P < 0.05 versus untreated cells; **P < 0.05 versus CP-166,572-treated cells.
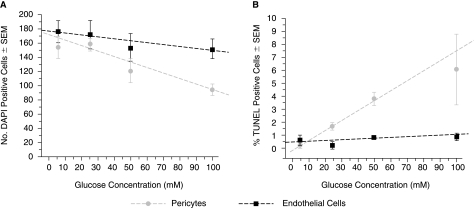
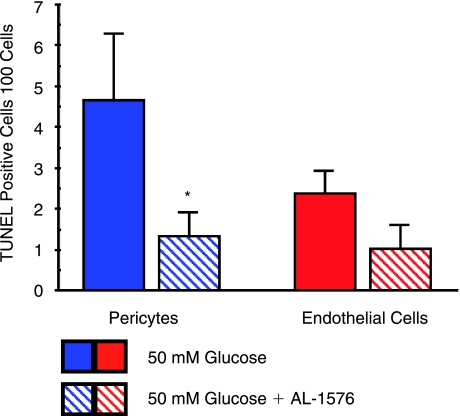
To evaluate the potential toxic effects of hyperglycemia and sorbitol formation on these cells, equal numbers of both retinal capillary pericytes and endothelial cells were cultured for 24 h with media containing increasing concentrations of glucose. As summarized in Figure 7, increasing concentrations of glucose from 5.5 to 100 mM reduced cell viability in both cell types as indicated by the number of DAPI-stained cells; however, the decrease was greater with pericytes where DAPI staining was reduced ∼42% compared to 15% for endothelial cells when cultured with media containing 100 versus 5.5 mM of glucose. This decrease in DAPI staining was accompanied by a concentration-dependent increase of TUNEL staining indicative of apoptosis. Similar TUNEL staining was not observed in retinal capillary endothelial cells. TUNEL staining was reduced by the presence of the ARI AL-1576 (Fig. 8).
FIG. 7.
Effect of increased glucose concentrations on (A) DAPI staining and (B) TUNEL staining in 24-h cultured pericytes and endothelial cells. Mean ± SD, n = 4. The lines in each figure were generated from least-square calculations to illustrate progressions.
FIG. 8.
Effect of presence of aldose reductase inhibitor AL-1576 on TUNEL staining in pericytes and endothelial cells cultured for 48 h with 50 mM glucose. Numbers represent TUNEL-positive cells counted per 100 cells in three separate experiments normalized against staining in similar cells cultured in 5 mM glucose. Mean ± SD. *P < 0.05 versus untreated cells.
Discussion
Rat TR-rPCT pericyte and TR-iBRB endothelial cells demonstrate the common characteristics of all retinal capillary vascular cells; that is, the pericytes contain smooth muscle actin while the rat endothelial cells stain for von Willebrand factor and incorporate acetylated low-density lipoprotein (Ac-LDL). However, in contrast to dog where only the pericytes express AR,14,23 both rat vascular cells contain immunoreactive AR. Nevertheless, despite the presence of AR in both cells, culture of both rat cells in 50 mM glucose results in only significant polyol accumulation in pericytes and no detectable accumulation of sorbitol in endothelial cells despite the presence of AR (Figs. 3, 4). This suggests that the levels of AR are too low in endothelial cells for significant sorbitol production under hyperglycemic conditions. When cultured in 50 mM galactose, a better substrate for AR, galactitol formation in both cells types was observed (Fig. 5). The levels of galactitol obtained after 24-h culture in endothelial cells, however, was significantly less (0.8 mmol/mg cellular protein for endothelial cells versus 4.8 mmol/mg cellular proteins for pericytes). In both cells, sorbitol and/or galactitol formation was reduced by the presence of the AR inhibitor AL-1576.
In addition to AR, both retinal capillary pericytes and endothelial cells contain aldehyde reductase, ALR, an oxidoreductase that generally does not significantly contribute to polyol formation. In early studies Kennedy and colleagues24 reported that AR “reductase” activity is present in isolated rat capillary endothelial cells. Careful examination of substrate specificities utilized in that report, however, suggests that this reductase activity is more consistent with ALR than AR. Our results confirm that ALR is present in endothelial cells.
In vitro and in vivo sugar cataract studies, spearheaded by Kinoshita's laboratory, demonstrate that excess intracellular sorbitol or galactitol accumulation leads to osmotic changes that alter lens cell permeability.25–30 Consistent with this hypothesis is the observation that galactosemia results in a more rapid and severe cataract formation than hyperglycemia because galactitol accumulation causes greater hyperosmotic effects than sorbitol accumulation. Animal studies in rats, dogs, and transgenic mice clearly demonstrate that galactosemia, compared to diabetes, accelerates AR-linked diabetic complications including retinal changes in both rats and dogs. In rat pericytes, exposure to 50 mM glucose resulted in an increased accumulation of sorbitol in both the cells and culture medium, consistent with apparent osmotic-linked permeability changes (Fig. 3). However, with galactose only a small, constant amount of galactitol was observed in pericyte culture medium and no galactitol was detected in endothelial cell culture medium (Fig. 5). The higher accumulation of galactitol without increased permeability contradicts the premise of osmotic-linked permeability changes as observed in other systems. The absence of observed vacuoles in the cytoplasm of rat pericytes further suggests that osmotic stress in rat pericytes is lower than in dog pericytes. The observed differences between glucose and galactose and their relationship to the lack of osmotic changes may be linked to differences in myoinositol transport by low- and high-affinity myoinositol transport sites.31 Further studies are required to elucidate the apparent permeability and osmotic stress differences between sorbitol and galactitol accumulation in these cells.
Hyperglycemia and associated glucose toxicity has been established to initiate retinopathy.32,33 Moreover, AR activity associated with hyperglycemia or galactosemia has been linked to apoptosis in retinal capillary pericytes from dog, rat, and bovine retinas by a number of laboratories.10,13–15,34,35 The present studies agree with previous observations that hyperglycemia-associated sorbitol accumulation is linked to retinal capillary pericyte apoptosis and that this apoptosis is reduced by the presence of AR inhibitor (Figs. 7, 8).
The present results support the observation that AR is present in both rat endothelial and pericyte cells.34,36 However, they differ from reports that only cultured dog capillary cells express AR and that retinal capillaries isolated by trypsin digestion from dog37 and human38 retinas demonstrate by immunohistochemisty that AR is only present in the pericytes. Another difference is the apparent osmotic changes and permeability differences between rat and dog capillary pericytes cells. These differences may explain why the development of retinal lesions in rat and dog differ despite a similar link to AR. In both diabetic and galactosefed rats, retinal capillary changes are characterized by an increase in periodic acid Schiff (PAS) staining suggestive of basement membrane changes and rapid retinal capillary basement membrane thickening. Both PAS staining and basement membrane thickening is prevented by several structurally distinct AR inhibitors,39–41 confirming that this basement membrane thickening is directly related to the polyol pathway activity and not to glycation, since ARIs do not significantly reduce glycation levels.42,43 Through their fingerlike actin-containing projections that encompass the endothelial cells, pericytes regulate capillary tonicity and flow. The link between basement membrane thickening and tonicity is illustrated by the fact that similar basement membrane thickening occurs with hypertension. Sorbitol accumulation in the rat pericyte may sufficiently alter its ability to regulate capillary tonicity without inducing rapid cell death. Hence the marked basement membrane thickening observed in rats that is subsequently followed weeks to months later by the sporadic appearance of capillary ghosts. In contrast, pericyte ghost formation is the first pathological lesion observed in the dog. This is shortly followed by the clinical appearance of microaneurysms linked to focal endothelial cell proliferation associated with the absence of pericytes. In galactose-fed beagles, pericyte ghosts have been observed before significant basement membrane thickening is present.44,45
Caution must be used in extrapolating in vitro biochemical results on cultured cells or tissues to in vivo observations of AR-linked lesions. Capillaries are composed of both pericytes and endothelial cells and the in vivo interaction of these cells and how together they relate to hyperglycemia to date remain unknown. While prevention studies with AR inhibitors support the premise that similar biochemical changes occur in vivo, time lines for these biochemical changes are clearly different. For example, while cataract formation occurs within 24–48 h in culture under hyperglycemic conditions, it takes weeks to months for similar lens changes to occur in vivo in a diabetic rat. Similarly, it takes at least 4–6 months to demonstrate significant basement membrane thickening, 6–12 months to demonstrate sporadic pericyte ghost formation in rats, and 18 months to observe pericyte degeneration in dog while apoptosis of cultured dog retinal pericytes occurs within days. Nevertheless, the present studies suggest that the TR-rPCT and TR-iBRB cell lines of rat retinal capillary pericytes and endothelial cells may be useful in vitro tools for elucidating the biochemical changes associated with DR observed in vivo in the rat. Furthermore, these cell lines may serve as valuable tools in the identification of new drugs for the protection of retinal capillaries during the development of DR.
Acknowledgment
This work was supported by NIH grant EY016730.
References
- 1.Robison W.G., editor; Laver N.M., editor; Lou M.F., editor. The Role of Aldose Reductase in Diabetic Retinopathy: Prevention and Intervention Studies. Oxford: Pergamon; 1995. pp. 593–639. [Google Scholar]
- 2.Antonelli-Orlidge A. Smith S.R. D'Amore P.A. Influence of pericytes on capillary endothelial cell growth. Am. Rev. Respir. Dis. 1989;140:1129–1131. doi: 10.1164/ajrccm/140.4.1129. [DOI] [PubMed] [Google Scholar]
- 3.Betsholtz C. Lindblom P. Gerhardt H. Role of pericytes in vascular morphogenesis. Exs. 2005;94:115–125. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 4.Chan L.S. Li W.Y. Khatami M., et al. Actin in cultured bovine retinal capillary pericytes: morphological and functional correlation. Exp. Eye Res. 1986;43:41–54. doi: 10.1016/s0014-4835(86)80044-6. [DOI] [PubMed] [Google Scholar]
- 5.Kuwabara T. Carroll J.M. Cogan D.G. Retinal vascular patterns. III. Age, hypertension, absolute glaucoma, injury. Arch Ophthalmol. 1961;65:708–716. doi: 10.1001/archopht.1961.01840020710019. [DOI] [PubMed] [Google Scholar]
- 6.Yanoff M. Diabetic retinopathy. N. Engl. J. Med. 1966;274:1344–1349. doi: 10.1056/NEJM196606162742403. [DOI] [PubMed] [Google Scholar]
- 7.Cogan D.G. Kuwabara T. The mural cell in perspective. Arch Ophthalmol. 1967;78:133–139. doi: 10.1001/archopht.1967.00980030135005. [DOI] [PubMed] [Google Scholar]
- 8.Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Ophthalmology. 1995;102:647–661. doi: 10.1016/s0161-6420(95)30973-6. [DOI] [PubMed] [Google Scholar]
- 9.Robison W.G., editor. Galactosemic Animal Models. Amsterdam: Harwood Academic Publishers; 2000. pp. 273–308. [Google Scholar]
- 10.Cheung A.K. Fung M.K. Lo A.C., et al. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 11.Yamaoka T. Nishimura C. Yamashita K., et al. Acute onset of diabetic pathological changes in transgenic mice with human aldose reductase cDNA. Diabetologia. 1995;38:255–261. doi: 10.1007/BF00400627. [DOI] [PubMed] [Google Scholar]
- 12.Hohman T.C. Nishimura C. Robison W.G., Jr. Aldose reductase and polyol in cultured pericytes of human retinal capillaries. Exp. Eye Res. 1989;48:55–60. doi: 10.1016/0014-4835(89)90018-3. [DOI] [PubMed] [Google Scholar]
- 13.Miwa K. Nakamura J. Hamada Y., et al. The role of polyol pathway in glucose-induced apoptosis of cultured retinal pericytes. Diabetes Res. Clin. Pract. 2003;60:1–9. doi: 10.1016/s0168-8227(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 14.Murata M. Ohta N. Fujisawa S., et al. Selective pericyte degeneration in the retinal capillaries of galactose-fed dogs results from apoptosis linked to aldose reductase-catalyzed galactitol accumulation. J. Diabetes Complications. 2002;16:363–370. doi: 10.1016/s1056-8727(01)00171-4. [DOI] [PubMed] [Google Scholar]
- 15.Naruse K. Nakamura J. Hamada Y., et al. Aldose reductase inhibition prevents glucose-induced apoptosis in cultured bovine retinal microvascular pericytes. Exp. Eye Res. 2000;71:309–315. doi: 10.1006/exer.2000.0882. [DOI] [PubMed] [Google Scholar]
- 16.Hosoya K. Tomi M. Advances in the cell biology of transport via the inner blood-retinal barrier: establishment of cell lines and transport functions. Biol. Pharm. Bull. 2005;28:1–8. doi: 10.1248/bpb.28.1. [DOI] [PubMed] [Google Scholar]
- 17.Hosoya K. Tomi M. Ohtsuki S., et al. Conditionally immortalized retinal capillary endothelial cell lines (TR-iBRB) expressing differentiated endothelial cell functions derived from a transgenic rat. Exp. Eye Res. 2001;72:163–172. doi: 10.1006/exer.2000.0941. [DOI] [PubMed] [Google Scholar]
- 18.Kondo T. Hosoya K. Hori S., et al. Establishment of conditionally immortalized rat retinal pericyte cell lines (TR-rPCT) and their application in a co-culture system using retinal capillary endothelial cell line (TR-iBRB2) Cell Struct. Funct. 2003;28:145–153. doi: 10.1247/csf.28.145. [DOI] [PubMed] [Google Scholar]
- 19.Shiono T. Sato S. Reddy V.N., et al. Rapid purification of rat lens aldose reductase. Prog. Clin. Biol. Res. 1987;232:317–324. [PubMed] [Google Scholar]
- 20.Sato S., editor; Kador P., editor; Kinoshita J., editor. Rat Kidney Aldehyde Reductase: Purification and Comparison with Rat Lens Aldose Reductase. I. Amsterdam, The Netherlands: Excerpa Medica; 1988. pp. 72–81. [Google Scholar]
- 21.Mori K. Lizak M.J. Ceckler T.L., et al. Magnetic resonance imaging of the galactosemic dog eye using magnetization transfer contrast. Curr. Eye Res. 1995;14:1035–1040. doi: 10.3109/02713689508998527. [DOI] [PubMed] [Google Scholar]
- 22.Sato S. Rat kidney aldose reductase and aldehyde reductase and polyol production in rat kidney. Am. J. Physiol. 1992;263:F799–F805. doi: 10.1152/ajprenal.1992.263.5.F799. [DOI] [PubMed] [Google Scholar]
- 23.Sato S. Secchi E.F. Lizak M.J., et al. Polyol formation and NADPH-dependent reductases in dog retinal capillary pericytes and endothelial cells. Invest. Ophthalmol. Vis. Sci. 1999;40:697–704. [PubMed] [Google Scholar]
- 24.Kennedy A. Frank R.N. Varma S.D. Aldose reductase activity in retinal and cerebral microvessels and cultured vascular cells. Invest. Ophthalmol. Vis. Sci. 1983;24:1250–1258. [PubMed] [Google Scholar]
- 25.Lin L.R. Reddy V.N. Giblin F.J., et al. Polyol accumulation in cultured human lens epithelial cells. Exp. Eye Res. 1991;52:93–100. doi: 10.1016/0014-4835(91)90132-x. [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita J.H. Cataracts in galactosemia. The Jonas, S. Friedenwald Memorial Lecture. Invest. Ophthalmol. 1965;4:786–799. [PubMed] [Google Scholar]
- 27.Kinoshita J.H. Mechanisms initiating cataract formation. Proctor Lecture. Invest. Ophthalmol. 1974;13:713–724. [PubMed] [Google Scholar]
- 28.Kawaba T. Cheng H.M. Kinoshita J.H. The accumulation of myoinositol and rubidium ions in galactose-exposed rat lens. Invest. Ophthalmol. Vis. Sci. 1986;27:1522–1526. [PubMed] [Google Scholar]
- 29.Obazawa H. Merola L.O. Kinoshita J.H. The effects of xylose on the isolated lens. Invest. Ophthalmol. 1974;13:204–209. [PubMed] [Google Scholar]
- 30.Reddy V.N. Lin L.R. Giblin F.J., et al. Study of the polyol pathway and cell permeability changes in human lens and retinal pigment epithelium in tissue culture. Invest. Ophthalmol. Vis. Sci. 1992;33:2334–2339. [PubMed] [Google Scholar]
- 31.Cammarata P.R. Chen H.Q. Osmoregulatory alterations in myo-inositol uptake by bovine lens epithelial cells. Part 1: A hypertonicity-induced protein enhances myo-inositol transport. Invest. Ophthalmol. Vis. Sci. 1994;35:1223–1235. [PubMed] [Google Scholar]
- 32.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp. Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inokuchi Y. Nakajima Y. Shimazawa M., et al. Effect of an inducer of BiP, a molecular chaperone, on endoplasmic reticulum (ER) stress-induced retinal cell death. Invest. Ophthalmol. Vis. Sci. 2009;50:334–344. doi: 10.1167/iovs.08-2123. [DOI] [PubMed] [Google Scholar]
- 34.Dagher Z. Park Y.S. Asnaghi V., et al. Studies of rat and human retinas predict a role for the polyol pathway in human diabetic retinopathy. Diabetes. 2004;53:2404–2411. doi: 10.2337/diabetes.53.9.2404. [DOI] [PubMed] [Google Scholar]
- 35.Sun W. Oates P.J. Coutcher J.B., et al. A selective aldose reductase inhibitor of a new structural class prevents or reverses early retinal abnormalities in experimental diabetic retinopathy. Diabetes55:275. 2006:7–2762. doi: 10.2337/db06-0138. [DOI] [PubMed] [Google Scholar]
- 36.Vinores S.A. Van Niel E. Swerdloff J.L., et al. Electron microscopic immunocytochemical evidence for the mechanism of blood-retinal barrier breakdown in galactosemic rats and its association with aldose reductase expression and inhibition. Exp. Eye Res. 1993;57:723–735. doi: 10.1006/exer.1993.1180. [DOI] [PubMed] [Google Scholar]
- 37.Akagi Y. Terubayashi H. Millen J., et al. Aldose reductase localization in dog retinal mural cells. Curr. Eye Res. 1986;5:883–886. doi: 10.3109/02713688609029241. [DOI] [PubMed] [Google Scholar]
- 38.Akagi Y. Kador P.F. Kuwabara T., et al. Aldose reductase localization in human retinal mural cells. Invest. Ophthalmol. Vis. 1983;Sci.24:1516–1519. [PubMed] [Google Scholar]
- 39.Frank R.N. Keirn R.J. Kennedy A., et al. Galactose-induced retinal capillary basement membrane thickening: prevention by Sorbinil. Invest. Ophthalmol. Vis. Sci. 1983;24:1519–1524. [PubMed] [Google Scholar]
- 40.Robison W.G., Jr. Kador P.F. Akagi Y., et al. Prevention of basement membrane thickening in retinal capillaries by a novel inhibitor of aldose reductase, tolrestat. Diabetes. 1986;35:295–299. doi: 10.2337/diab.35.3.295. [DOI] [PubMed] [Google Scholar]
- 41.Robison W.G., Jr. Kador P.F. Kinoshita J.H. Retinal capillaries: basement membrane thickening by galactosemia prevented with aldose reductase inhibitor. Science. 1983;221:1177–1179. doi: 10.1126/science.6612330. [DOI] [PubMed] [Google Scholar]
- 42.Robison W.G., Jr. Laver N.M. Jacot J.L., et al. Efficacy of treatment after measurable diabetic like retinopathy in galactosefed rats. Invest. Ophthalmol. Vis. Sci. 1997;38:1066–1073. [PubMed] [Google Scholar]
- 43.Kador P.F. Lee J.W. Fujisawa S., et al. Relative importance of aldose reductase versus nonenzymatic glycosylation on sugar cataract formation in diabetic rats. J. Ocul. Pharmacol. Ther. 2000;16:149–160. doi: 10.1089/jop.2000.16.149. [DOI] [PubMed] [Google Scholar]
- 44.Robison W.G., Jr. Laver N.M. Lou M.F. The role of aldose reductase in diabetic retinopathy: prevention and intervention studies. Prog. Retin. Eye Res. 1995;14:593–640. [Google Scholar]
- 45.Kador P.F. Chapter 18: Ocular Pathology of Diabetes Mellitus. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 84. [Google Scholar]