Abstract
Ultraviolet (UV) irradiation is one of the significant risk factors in the genesis of cataracts. Pathogenetically, the process can be triggered by the intraocular generation of various reactive species of oxygen that are well known to be initiated by the penetration of light, especially of the UV frequencies. The contribution of UV exposure in the etiology of this disease is likely to increase further due to ozone depletion in the upper atmosphere. The present studies were undertaken to examine if the UV effects can be attenuated with the xanthine-based alkaloids primarily present in tea and coffee. We have examined this possibility by in vitro lens culture studies with caffeine. As expected, mice lenses incubated in Tyrode solution exposed to UV at 302 nm are physiologically damaged, as evidenced by the inhibition of the active transport of 86Rb+, an ion acting as a surrogate of the K+. There was a simultaneous decrease in the levels of adenosine triphosphate and glutathione. The addition of caffeine to the medium prevented such deleterious effects. That caffeine and perhaps other xanthinoids have a protective effect against cataract formation induced by UV has hence been demonstrated for the first time.
Introduction
It is well known that the pathogenesis of cataracts is multifactorial in nature. Among the well-known metabolic and nonmetabolic confounding factors, including aging, are diabetes, galactosemia, nutritional deficiencies, and constant penetration of light into the eye, especially during the photopic vision. The eventual damage-inflicting event in most cases, apart from the initial metabolic effects, consists of oxidative stress due to the intraocular generation of reactive oxygen species (ROS) in the aqueous and the lens. In the case of metabolic disturbances caused by various diseases, including that with aging, the inherent inhibition of normal oxygen utilization leads to the diversion of molecular oxygen to abnormal autooxidative reactions that progress with the simultaneous generation of the ROS, namely superoxide, hydrogen peroxide, singlet oxygen, and hydroxyl radicals, and so on. These species are also produced by the leakage of the monovalently reduced oxygen during electron transport in the cytochrome pathway of the reduction of the respired oxygen to water. However, in the case of the eye, the generation of these species is greatly amplified by the continued intraocular penetration of light during the photopic vision and consequent initiation of cyclically continued pseudocatalytic photochemical reactions.1 This phenomenon helps to explain the higher incidence of cataracts in areas of the world with a relatively higher dose of solar radiation reaching the earth.2–4 Such a photocatalytic generation of reactive oxygen can be initiated by exposure to visible, as well as the ultraviolet (UV) frequencies, of the solar radiation. The quantum yield of such species is expected to be much higher on irradiation with UV frequencies of relatively lower wavelengths because of their higher photon energy, which is considered adequate to elevate the molecular oxygen from its ground triplet state to the singlet state by direct energy transfer.5 While this species is itself a potent oxidant,6 in the presence of reducing equivalents, it can give rise to hydroxyl radicals through the formation of oxene ions (O·) followed by its protonation.7 Indeed, of the 20 million people affected with cataracts, its genesis in ∼20% of this population has been attributable to UV exposure.9 In addition, UV irradiation at the earth surface is likely to increase further by depletion of ozone (O3) in the stratosphere. This is caused by fluorohydrocarbon-induced free-radical reactions and the consequent degradation of this allotrope to its molecular and atomic forms. Since the ozone layer in the stratosphere serves to filter out the ultraviolet frequency of light from reaching the earth, its depletion therein is likely to further enhance UV penetration and the incidence of cataracts. This would also be the case with the induction of skin cancer and the formation of ocular melanomas. It is estimated that each 1% decrease in the atmospheric ozone would increase the incidence of cataracts by 0.5%.* In addition to the flourohydrocarbons, the ozone depletion can also be caused by free-radical reactions induced by nitrogen oxides emitted by the internal combustion engines burning fossil fuels.
The physiologic significance of the oxygen free radicals in lens damage with implications in the genesis of actual cataracts has now been proven by several studies showing the induction of cataracts by UV irradiation, as well as its prevention by nutritional and metabolically produced scavengers.8,9 Soderberg's laboratory has directly demonstrated that the UV induction of cataract is preventable by vitamin E.9 Hence, further studies on possible strategies to prevent lens damage by UV are considered important therapeutically. In a previous in vitro study, lens damage caused by UV irradiation has been shown to be preventable by pyruvate, a compound that acts as a potent scavenger of ROS, in addition to its effect of directly supporting the tissue metabolically.8 In view of the possibility that pyruvate's effect may become limited at lower wavelengths of UV because of its photodecarboxylation, further studies are in progress with alkaloids present in tea and coffee. The present studies have been conducted with caffeine, an alkaloid, which is present at relatively high concentrations in many beverages. These alkaloids, in addition to their property of possibly providing metabolic support to the tissue by maintaining adequate levels of cyclic AMP through the inhibition of phosphodiesterase, are also effective scavengers of reactive oxygen, particularly the hydroxyl radicals.10 The background of this study is provided also by a previous report showing the inhibition of selenite-induced cataract formation by green tea, which is known to be richer in caffeine.11
Material and Methods
CD-1 mice (weighing ∼25 g) were obtained from Charles River Laboratories (Wilmington, MA) and were used to obtain fresh lenses. The nonradioactive chemicals were obtained from Sigma Chemical Company (St. Louis, MO), and 86rubidium chloride (RbCl) was obtained from Perkin Elmer (Boston, MA).
The effect of caffeine against UV (302 nm) damage was studied by culturing mice lenses in 1 mL of Tyrode solution mixed with trace amounts of 86RbCl, with the gamma disintegration rate adjusted to about 1000 cpm/100 μL of the medium. The weight of the lenses was 7 ± 1 mg. Incubations were done in contralateral pairs, without or with caffeine (5 mM). The irradiation intensity was adjusted to 0.6 milliwatts/sq.cm (UVLMS38, EL series 3 UV lamp; UVP, Cambridge, UK). The period of incubation was 5 h. Incubations were done in glass tubes, with light entering the medium through the open end. Subsequent to incubations, the lenses were removed from the medium and briefly rinsed with physiologic saline to get rid of the adherent radioactivity. The radioactivity in the lens was then determined by gamma counting. The results were expressed as the distribution ratio (CL/CM) of the counts between the lens water (CL) and the equivalent volume of the medium (CM). The lens water was taken to be 60% of the tissue weight. The number of lenses in each group was 8.
Subsequent to the determination of the distribution ratio, an aqueous extract of the tissue was prepared by homogenizing it with 0.5 mL of distilled water and centrifugation for adenosine triphosphate (ATP) determination (n = 8 in each group). An aliquot of the supernatant (∼50 μL) was then reacted with 100 μL of a firefly lantern extract containing a luciferin-luciferase mixture housed in a luminometer.12 The luminescence generated was compared with ATP standards. An aliquot of 100% trichloroacetic acid (TCA) was then added to the residual volume of homogenate to a final concentration of 5% and centrifugation repeated to get a protein-free, acid-soluble extract. Glutathione (GSH) was determined by adding 100 μL of this extract to a cuvette containing 0.5 mL of 0.6 M Na2HPO4. Addition of the TCA extract brings the pH down to ∼7.0. Then 100 μL of a DTNB [5,5′-dithiobis(2-nitrobenzoic acid)] reagent, prepared by dissolving 4 mg of DTNB in 1% trisodium citrate, was added.13 The yellow color representing GSH was then read spectrophotometrically at 412 nm, along with standards prepared by dissolving pure GSH in 5% TCA. Thiol leakage in the medium was determined by directly reacting it with the DTNB reagent. Six (6) lenses in each group were used for this assay.
In separate incubation experiments, postincubation lenses were also examined for their transparency by photographing them after placing them on a metal grid.
Results
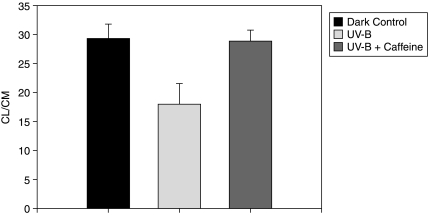
The physiologic effectiveness of caffeine against UV-induced damage to the lens was determined on the basis of its ability to maintain its active transport activity, as measured by the uptake of rubidium ions, and to maintain the levels of ATP and GSH. Data on the status of the active transport are summarized in Figure 1. As expected, the incubation of lenses in media exposed to UV led to a substantial decrease in its ability to transport rubidium, as compared to the dark controls. In the presence of caffeine, this inhibition was significantly minimized. As compared to the dark controls (CL/CM ∼29), the ratio decreased to a mean value of 18 in the lenses incubated with UV. Such a significant decrease under UV was not noted when the medium was fortified with caffeine. Indeed, the uptake of rubidium in the latter case was close to the dark controls. The protective effect of this xanthinoid against the pump damage was, hence, highly significant.
FIG. 1.
Distribution ratios of Rubidium ions between the lens water (CL) and the medium of incubation (CM). Incubation period was 5 hours. The distribution ratio (CL/CM) was determined by dividing the radioactivity present in the lens water at the end of incubation by the counts present in equivalent volume of the medium of incubation. The values are expressed as mean ± S.D. n ≥ 8 in each group. P values between UV and UV + caffeine group is at least <0.001. The values between the dark controls and the UV + caffeine are similar, indicating that inclusion of caffeine in the medium abolished the effect of UV.
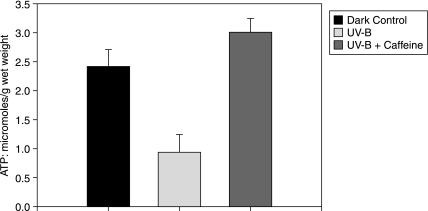
In addition to the direct damage to the pump caused by UV irradiation, the content of ATP, the energy source required for the pump activity, is also decreased. The susceptibility of the lens to oxidants has previously been attributed to a deactivation of the Na+-K+-ATPase itself.14 However, the observed damage could be attributable to a direct damage to the cell membranes exposed to ROS, as well as damage to the intracellular metabolic pathways generating ATP. As shown in Figure 2, the ATP content in the lenses incubated with UV is also significantly decreased (0.95 μmoles/g), as compared to the control value of 2.4 μmoles/g. The value in the presence of caffeine was very close to the controls, with no decrease being apparent.
FIG. 2.
Levels of ATP. The values are expressed as μmoles/g wet weight ± S.D as indicated on the ordinate. The values in presence of UV are significantly depressed as compared to the dark controls, P < 0.001. The depressive effect of UV is completely prevented in the presence of caffeine, P < 0.001. (n = 8)
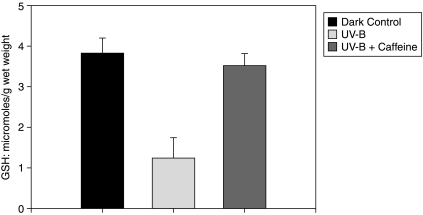
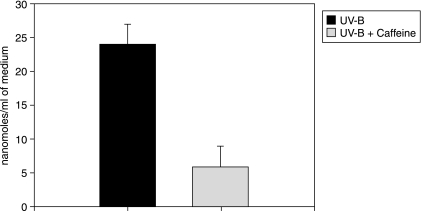
As shown in Figure 3, the incubation of lenses exposed to UV also results in a decrease of GSH known to be highly susceptible to oxidation by ROS. In addition to oxidation, its decrease can also be attributed to the overall inhibition of tissue metabolism, with an adverse effect on the regeneration of GSH from glutathione disulfide. Additionally, it leaks out of the lens on incubation with ROS. The presence of caffeine in the medium protected the lens against such losses of GSH. As shown in Figure 3, in the case of lenses incubated in the dark, the content of this –SH tripeptide was maintained at a level of 3.8 μmoles/g tissue wet weight, a value close to that of the fresh lenses (∼4 μmoles/g). On incubation under UV, it decreased to 1.25 μmoles/g. However, such a decrease was substantially prevented in the presence of caffeine, with the level in the lenses incubated with UV + caffeine being 3.5 μmoles/g. That the decrease in lens GSH could be attributed also to its leakage was confirmed by measuring the thiol content in the medium of incubation. As apparent in Figure 4, it was significantly higher in the medium where the lens was incubated and exposed to UV in the absence of caffeine, as compared to that in the medium wherein the lens was incubated with caffeine.
FIG. 3.
Levels of GSH (μmoles/g wet wt). As apparent in the ordinate, the level is significantly lowered on incubation under UV as compared to the dark controls, P < 0.001. Addition of caffeine abolished this lowering, the level in this case being closer to the dark controls, (n = 6); P < 0.001 between UV and UV + caffeine groups
FIG. 4.
Leakage of thiol from the lenses incubated as indicated above. Aliquots of the post-incubation medium were directly reacted with the DTNB reagent and the resulting formation of yellow color measured spectrophotometrically at 412nm. Incubation under UV led to detectable leakage of the thiol from the lens. This was significantly reduced in presence of caffeine; n = 4.
Figure 5 depicts the status of transparency of the lens incubated under UV with or without caffeine. As apparent, the lenses incubated with caffeine retained their transparency to a significant extent, as compared to the lenses incubated without caffeine, which appear cloudy and obscure the underlying grid pattern substantially.
FIG. 5.

State of transparency of the lenses post-incubation. The lenses were placed on a grid and photographed by trans-illumination with white light. Transparency is indicated by the clarity of the holes which were much more obscured in the lenses incubated under UV (left) as compared to that in the lenses incubated with UV + caffeine (right).
Discussion
Exposure to UV is an important risk factor in the genesis of cataracts all over the world—albeit with greater implications in areas of the world with excessive solar irradiation, such as in the tropics.2–4 It is also high in mountainous regions with excessive UV irradiation as well as in populations in the temperate regions exposed occupationally to excessive sunlight. Several previous studies have suggested that an excessive incidence of cataracts in such areas is due to the photo-oxidation of the tryptophan residues in the lens proteins. This has been explained on the basis of a strong UV absorption of tryptophan and its consequent oxidation.15,16 However, cataract is also associated with the oxidation of GSH, which is UV transparent. Hence, several studies now suggest that cataractogenesis is actually initiated by the intraocular generation of ROS, such as superoxide, singlet oxygen, hydrogen peroxide, and hydroxyl radical. These species are known to set up aberrant oxidations. The hypothesis suggesting the involvement of the oxyradicals in cataractogenesis was initially based on lens-culture experiments showing that the active transport of rubidium into the lens is adversely affected by conducting the culture in medium generating the above species photochemically.17 Similar results are also obtained in experiments conducted even in the dark but generating various ROS enzymatically.18 Since then, many scavengers of these oxygen species have been found to actually inhibit cataract formation in vivo in animal models.19 Several recent studies in humans also suggest that the use of multivitamin supplements may be beneficial in the prevention of the onset and progression of cataracts.20–22
In a previous study, we have shown that photochemical damage induced by ROS generated by UV can also be effectively prevented by pyruvate.8,17 Its preventive effect is attributed to its action as a potent oxyradical scavenger, as well as to its action as a metabolic agonist. The latter effect is attributed to a stimulation of glycolysis as well as the citrate cycle. However, information on compounds that might be effective in preventing UV-B-induced damage is very limited, what with pyruvate being relatively unstable when exposed to such wavelengths. In view of the increasing interest in alternative medicine, we hypothesized that some of the tea alkaloids might be effective in preventing UV-induced lens damage and cataract formation.
The results described in this communication are supportive of this possibility. This was apparent initially by the ability of caffeine to protect the lens against UV-induced membrane damage. Since the lens is avascular, it derives most of its nutrients by transport processes residing in its cell membrane exposed to the aqueous humor. Its damage is, therefore, highly injurious. That caffeine can prevent this from happening was strongly evident by its protective effect against damage to the lens-transport activity, an active process dependent upon Na+-K+-ATPase. A significant inhibition of this activity was noticed within 5 h of incubation with UV with a dose rate of 0.6 mW/sq.cm, known to be quite cataractogenic. Caffeine at a millimolar concentration was highly effective. The damage to the pump, as indicated by the distribution ratio (CL/CM), was nearly fully inhibited by caffeine at a 5-mM concentration. The pump damage indeed is also associated with intracellular metabolic damage, as apparent by the loss of ATP. Apparently, the generation of ATP is inhibited by the ROS-dependent oxidative deactivation of enzymes involved in tissue metabolism. In the presence of caffeine, the level of ATP remained well maintained, the level in this case being similar to the levels in the dark controls. That the damaging process is oxidative in nature was apparent by the loss of GSH. This was also prevented by caffeine.
Conclusions
The investigations, therefore, demonstrate unequivocally that caffeine has the potential of protecting the lens against UV-induced damage. In view of the fact that even ambient oxidation also contributes to cataract formation in the long run, it is possible that caffeine might be useful against lens damage caused by ROS generation under nonphotochemical conditions also. It is also relatively nontoxic when given in small doses. Hence, the findings are considered pharmacologically significant, especially when used as an eye drop. Other possible mechanisms of caffeine action in preventing cataractogenesis, such as its ability to maintain cyclic-AMP level through inhibiting phosphodiesterase, also remains a good possibility. Such studies are under progress. The finding is also supported by our earlier observation, showing that the administration of green tea inhibits the formation of oxidation-induced selenite cataracts.11
Footnotes
www.who.int/mediacentre/factsheets/fs227/en. Ultraviolet radiation: solar radiation and human health. Too much sun is dangerous.
Acknowledgments
This study was supported by a grant through NEI, NIH, and Research to Prevent Blindness Inc. (New York, NY).
References
- 1.Varma S. D. Chand D. Sharma Y. R. Kuck J. F., Jr Richards R. D. Oxidative stress on lens and cataract formation: role of light and oxygen. Curr. Eye Res. 1984;3:35–57. doi: 10.3109/02713688408997186. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee A. Milton R. C. Thyle S. Prevalence and etiology of cataract in Punjab. Br. J. Ophthalmol. 1982;66:35–44. doi: 10.1136/bjo.66.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor H. R. West S. K. Rosenthal F. S. Munoz B. Newland H. S. Abbey H. Emmett E. A. Effect of ultraviolet radiation on cataract formation. N. Engl. J. Med. 1988;319:1429–1433. doi: 10.1056/NEJM198812013192201. [DOI] [PubMed] [Google Scholar]
- 4.West S. K. Duncan D. D. Munoz B. Rubin G. S. Fried L. P. Bandeen-Roche K. Schein O. D. Sunlight exposure and risk of lens opacities in a population-based study: the Salisbury Eye Evaluation project. JAMA. 1998;280:714–718. doi: 10.1001/jama.280.8.714. [DOI] [PubMed] [Google Scholar]
- 5.Foote C. S. Photosensitized oxidation and singlet oxygen: Consequences in biological systems. In: Pryor WA, editor. “Free Radicals in Biology Vol II”. Academic Press; New York New York: pp. 85–133. [Google Scholar]
- 6.Zigler J. S. Goosey J. D. Singlet oxygen as a possible factor in human senile cataract development. Curr. Eye Res. 1984;3:59–65. doi: 10.3109/02713688408997187. [DOI] [PubMed] [Google Scholar]
- 7.Valko M. Izakovic M. Mazur M. Rhodes C. J. Tesler J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 8.Hegde KR. Kovtun S. Varma SD. Induction of ultraviolet cataracts in vitro: prevention by pyruvate. J Ocul. Pharmacol. Ther. 2007;23:492–502. doi: 10.1089/jop.2007.0038. [DOI] [PubMed] [Google Scholar]
- 9.Ayala MN. Söderberg PG. Vitamin E can protect against ultraviolet radiation-induced cataract in albino rats. Ophthalmic Res. 2004;36:264–269. doi: 10.1159/000081206. [DOI] [PubMed] [Google Scholar]
- 10.Shi X. Dalal NS. Jain AC. Antioxidant behaviour of caffeine: efficient scavenging of hydroxyl radicals. Food Chem Toxicol. 1991;29:1–6. doi: 10.1016/0278-6915(91)90056-d. [DOI] [PubMed] [Google Scholar]
- 11.Gupta SK. Halder N. Srivastava S. Trivedi D. Joshi S. Varma SD. Green tea (Camellia sinensis) protects against selenite-induced oxidative stress in experimental cataractogenesis. Ophthalmic Res. 2002;34:258–263. doi: 10.1159/000063881. [DOI] [PubMed] [Google Scholar]
- 12.Strehler B. J. Totter J. K. Determination of ATP and related compounds: Firefly luminescence and other methods. In: Glick D., editor. Methods of Biochemical Analysis. Vol. 1. Interscience Publishers; New York: 1954. 341 pp. [DOI] [PubMed] [Google Scholar]
- 13.Ellman G. L. Tissue sulphydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 14.Epstein D. L. Kinoshita J. H. The effect of diamide on lens glutathione and lens membrane function. Invest. Ophthalmol. Vis. Sci. 1970;9:629–638. [PubMed] [Google Scholar]
- 15.Zigman S. Yulo T. Schultz J. B. Cataract induction in mice exposed to near-UV light. Ophthalmic Res. 1974;6:259–270. [Google Scholar]
- 16.Dillon J. Photolytic changes in lens proteins. Curr. Eye Res. 1984;3:145–150. doi: 10.3109/02713688408997196. [DOI] [PubMed] [Google Scholar]
- 17.Varma S. D. Devamanoharan P. S. Morris S.M. Photoin-duction of cataracts in rat lens in vitro. Preventive effect of pyruvate. Exp. Eye Res. 1990;50:805–812. doi: 10.1016/0014-4835(90)90131-d. [DOI] [PubMed] [Google Scholar]
- 18.Varma S. D. Hegde K. Henein M. Oxidative damage to mouse lens in culture. Protective effect of pyruvate. Biochim Biophys Acta. 2003;1621:246–252. doi: 10.1016/s0304-4165(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 19.Varma S.D. Ramachandran S. Devamanoharan P. S. Morris S. M. Ali A. H. Prevention of oxidative damage to rat lens by pyruvate in vitro: possible attenuation in vivo. Curr. Eye Res. 1995;14:643–649. doi: 10.3109/02713689508998491. [DOI] [PubMed] [Google Scholar]
- 20.Kuzniarz M. Mitchell P. Cumming RG. Flood VM. Use of vitamin supplements and cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2001;132:19–26. doi: 10.1016/s0002-9394(01)00922-9. [DOI] [PubMed] [Google Scholar]
- 21.Chong E. W. Wong T. Y. Multivitamin Supplements and Cataract Prevention. Ophthalmology. 2008;115:597–598E. doi: 10.1016/j.ophtha.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Clinical Trial of Nutritional Supplements Age-Related Cataract Study Group. Maraini G. Sperduto RD. Ferris F. Clemons TE. Rosmini F. Ferrigno L. A randomized, double-masked, placebo-controlled clinical trial of multivitamin supplementation for age-related lens opacities. Clinical trial of nutritional supplements and age-related cataract report no. 3. Ophthalmology. 2008;115:599–607.e1. doi: 10.1016/j.ophtha.2008.01.005. [DOI] [PubMed] [Google Scholar]