Abstract
Purpose
To compare the effect on the retrobulbar hemodynamics and intraocular pressure (IOP) of dorzolamide 2% and brinzolamide 1%, each added to timolol 0.5% in patients with primary open-angle glaucoma (POAG).
Methods
146 POAG patients were prospectively randomized to receive either dorzolamide 2% or brinzolamide 1% BID, each added to timolol 0.5%, during a 60-month evaluator-masked study. At baseline and every 6 months for 60 months, we measured the retrobulbar hemodynamic parameters in the ophthalmic artery (OA), central retinal artery (CRA), and short posterior ciliary arteries (SPCA) using color Doppler imaging (CDI), intraocular pressure (IOP), and blood pressure measurements.
Results
Dorzolamide significantly increased the end-diastolic velocity (EDV) in the OA in 1.22 cm/s, 95% confidence interval (95% CI) 0.90–1.56 cm/s, P < 0.001 and reduced the resistivity index (RI) in the OA in 0.04 units, 95% CI 0.03–0.05, P < 0.001. None of the retrobulbar parameters changed significantly on therapy with brinzolamide when the results were analyzed at month 60. Both dorzolamide and brinzolamide significantly decreased IOP (−4.3, 95% CI −4.5 to −4.2 mmHg and −4.3, 95% CI −4.4 to −4.2 mmHg, respectively). Dorzolamide significantly reduced the RI in the OA from 0.74 (0.02) to 0.70 (0.02), CRA from 0.66 (0.02) to 0.62 (0.02), and SPCA from 0.66 (0.02) to 0.62 (0.02), P < 0.001, respectively.
Conclusions
Our results suggest augmented retrobulbar blood flow after 5 years of treatment with dorzolamide but not with brinzolamide, each added to timolol, in POAG patients.
Introduction
Glaucoma is a progressive optic neuropathy in which increased intraocular pressure (IOP) is a primary risk factor for progressive damage.1–3 Nevertheless, the assumption that IOP is the only relevant risk factor is changing.
There is increasing evidence that ocular blood flow (OBF) changes are involved both in the pathogenesis of glaucoma4–6 and in progression of glaucomatous damage.7–9 Carbonic anhydrase inhibitors (CAIs) have been used systematically to reduce IOP in patients with glaucoma for >50 years.10 Inhibition of ocular carbonic anhydrase (CA) decreases aqueous humor secretion, which in turn lowers IOP.
The mammalian metalloenzyme CA family has been reported to include at least 15 enzymatically active isoforms with different structural and catalytic properties.11 The CA isoenzymes differ in their kinetic properties, their tissue distribution, and subcellular localization.
Dorzolamide was the first topical CAI available on the market. In 1995, the United States Food and Drug Administration (FDA) approved dorzolamide for the treatment of elevated IOP. Brinzolamide, a second topically active CAI, became available in the United States in 1998 and in many European countries in 1999.
Previous evidences have reported the ocular hemodynamic effects of topical dorzolamide, not only upon the retina12–14 but also upon retrobulbar circulation.15–20 However, not all studies have reported a positive outcome.21–23
Relatively few studies have evaluated the effect of topical brinzolamide upon OBF in glaucoma patients.
Barnes and colleagues24 in an animal study, found that 1 week of twice-daily topical ocular treatment with brinzolamide significantly enhanced optic nerve head blood flow.
Kaup and colleagues 25 reported that topical brinzolamide accelerated the retinal arteriovenous passage time, while the retinal vessel diameters and retrobulbar hemodynamic parameters remain unaltered.
More recently, Siesky and colleagues26 have suggested that brinzolamide and dorzolamide significantly increased retinal oxygen tension saturation without modifications in retrobulbar hemodynamics.
Because antiglaucoma medications are usually prescribed for long-term use, it is important to know how they affect OBF.
In a prospective study recently published by our group20 using color Doppler ultrasound, we found that dorzolamide added to timolol showed increased blood flow velocities and decreased resistivity indices of the retrobulbar vessels in open-angle glaucoma (OAG) patients after 48 months of follow-up.
On the basis of this assumption, we evaluated and compared the effect upon the retrobulbar blood flow parameters (using color Doppler ultrasound) and IOP of topical dorzolamide 2% and brinzolamide 1%—both added to timolol 0.5%—over a 60-month period, in patients with primary open-angle glaucoma (POAG).
Methods
Study design
The study involved a randomized, evaluator-masked, parallel group design. Patients received either dorzolamide 2% or brinzolamide 1%, each added to timolol maleate 0.5%, twice-daily (BID) (separately rather than in a fixed-dose combination), for 5 years.
Unmasked personnel were responsible for study medication distribution and collection, whereas masked personnel performed all study-related examinations.
Patients and unmasked personnel were recommended not to reveal the study assignment to the masked evaluator.
Patient recruitment
The study was conducted on consecutive, referred or recruited patients who met the inclusion and exclusion criteria, seen in the outpatient service of the Ophthalmology Department (Public Foundation Barbanza Hospital, dependent on the Instituto Galego de Oftalmoloxia, Spain) between January 1999 and December 2001.
Recruitment was ongoing for a period of 3 years in order to collect a sufficiently large target sample.
All patients had previous treatment with beta-blockers (at least 6 months), with mean IOP values of ≥20 mmHg.
The study protocol was approved by the local ethics committee. All patients were fully informed about the details of the study protocol. Written informed consent was obtained from all subjects at the beginning of the study, in accordance with the Declaration of Helsinki.
Table 1 summarizes the main inclusion and exclusion criteria.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| ≥40 years of age | Presence of any form of glaucoma other than OAG |
| Clinical diagnosis of OAG in at least one eye | Any concurrent infectious/noninfectious conjunctivitis, keratitis, or uveitis in either eye |
| At screening visit: mean diurnal IOP ≥20 mmHg under treatment with beta-blockers (at least 6 months) in monotherapy | Any abnormality preventing reliable applanation tonometry in study eye(s) |
| At baseline visit: mean diurnal IOP ≥20 mmHg under treatment with timolol 0.5 BID in monotherapy | Patients previously treated with argon laser trabeculoplasty and/or ocular filtering surgical intervention |
| Early visual-field defect | Progressive retinal or optic nerve disease due to any cause |
| Willingness to comply with the investigator's and protocol indications | Asthma or other obstructive pulmonary disease |
| Visual acuity ≥0.3 (ETDRS scalea) in both eyes | Cardiogenic shock, 2° or 3° atrioventricular block, sinus bradycardia, or heart failure |
| Spherical refractive error between +3.00 and −6.00 diopters | Diabetes with any sign of retinopathy (hemorrhages, hard and/or cotton wool spots, macular edema) |
| Systemic medications that affect IOP or systemic blood pressure were ineligible unless the patients' medication dosages were stable ≥6 months before the screening visit | |
| Pregnant or nursing women |
Abbreviations: OAG, open-angle glaucoma; IOP, intraocular pressure; ETDRS, Early Treatment Diabetic Retinopathy Study.
See reference 27.
Definitions
Glaucoma was defined as either a visual field defect or as glaucomatous changes of the optic nerve head.
For glaucoma diagnosis, visual field examinations were performed with the program 24-2, full threshold strategy, on the Humphrey visual field analyzer. Glaucomatous field defects were defined as follows: a minimum of one location in the paracentral or nasal step regions corresponding to sectors 1 or 2, or to the inferior three location in sector 3 of the Glaucoma Hemifield Test28 exhibiting repeatable abnormality at the P < 0.5% level by pattern deviation probability analysis; or two or more locations in a cluster exhibiting repeatable abnormality at the P < 2% level or lower by pattern deviation probability analysis, excluding any location in the cluster situated in the opposite horizontal hemifield. Early glaucoma was defined in accordance with the criteria of Hodapp and colleagues29 which require the mean defect (MD) to be greater than −6 dB, with fewer than 18 points depressed below P < 0.05, fewer than 10 points depressed below P < 0.001, and no point in the central 5° with a sensitivity of <15 dB.
Patients with unreliable visual field assessment (false negatives, false positives, or fixation losses exceeding 20%) were excluded.
The glaucomatous optic nerve head changes included either notching of the rim, diffuse emaciation of the rim area, a cup-to-disc ratio of >0.6, or asymmetry of the cup-to-disc ratio of >0.2.30
Patient visits
This protocol includes one screening visit, one post-screening visit, and one baseline visit. At the screening visit, each subject underwent a standard ophthalmic examination, including a review of the medical history, best-corrected visual acuity (EDTRS scale), slit-lamp examination of the anterior segment with dilated pupils, IOP measurement in triplicate (at 9:00 am, 12:00 pm, and 3:00 pm) using Goldmann applanation tonometry (Goldmann tonometer, Haag Streit, Switzerland), gonioscopy, dilated funduscopic examination using a 78 diopter lens, stereoscopic optic disc photography, and automated perimetry using the 24-2 full threshold strategy on the Humphrey visual field analyzer (Carl Zeiss Meditec, Dublin, CA). A detailed medical history was obtained, paying special attention to vascular disease. Although subjects with systemic hypertension or cardiovascular disease were not excluded, patients receiving systemic medications that affect IOP or systemic blood pressure were noneligible unless medication dosages had remained stable for ≥6 months before the screening visit.
Potentially eligible patients started a 4-week run-in period under timolol maleate 0.5% (one drop in each eye twice each day).
The postscreening examination included measurement of blood pressure, heart rate (HR), IOP in triplicate (at 9:00 am, 12:00 pm, and 3:00 pm), and visual field. After completion of the timolol run-in period, patients reported to the clinic for baseline measurements.
The baseline examination included measurement of blood pressure, HR, IOP in triplicate (at 9:00 am, 12:00 pm, and 3:00 pm), visual fields, and color Doppler examinations.
Patients who met the IOP inclusion requirements were randomly assigned, using a computer-generated randomization sequence, to receive either dorzolamide 2% or brinzolamide 1%, each added to timolol maleate 0.5%, BID.
Although central corneal thickness (CCT) measurements were not included in the original protocol, such measurements were obtained during the study in a large proportion of patients: 61 patients in the dorzolamide/timolol group (87.1%) and 64 in the brinzolamide/timolol group (84.2%). CCT was measured using an ultrasonic pachymeter (Corneogage plus 2; Sonogage, Cleveland, OH).
Follow-up visits were scheduled every 6 months, and included a review of the medical history, best-corrected visual acuity (EDTRS scale), slit-lamp examination of the anterior segment with dilated pupils, IOP measurement in triplicate (at 9:00 am, 12:00 pm, and 3:00 pm), gonioscopy, dilated funduscopic examination using a 78 diopter lens, stereoscopic optic disc photography, automated perimetry, blood pressure, HR, and color Doppler examinations (at 9:00 am). The study measurements were made at ±1 h with respect to the stated times.
Follow-up visits included the assessment of compliance, checking for possible side effects, and the occurrence of adverse effects.
Compliance data were collected using a standardized questionnaire.
Color Doppler imaging
All color Doppler imaging (CDI) examinations (model SSA-340; Toshiba Medical Systems, Tustin, CA) were performed, on all study visits, by the same experienced observer (blinded to the treatment).
A 7.5 MHz vector-array transducer was applied to the closed eyelid using a coupling gel, and taking care to avoid any pressure to the eye itself. During the examination, subjects were in the supine position, with the head tilted forward at an angle of about 30°. The measurements were obtained according to the conventional technique,31 which is identical to the technique we have used in many previous studies.9,15,18,19,32,33 The ophthalmic artery (OA) was identified nasally and superiorly to the optic nerve, medially to the hyporeflective shadow representing it. Measurements of OA flow were performed ∼10–15 mm posterior to the globe, where the echographic signals are stronger. The short posterior ciliary artery (SPCA) images were taken temporally and nasally to the optic nerve just behind the posterior pole of the eye. The angle between transducer and orientation of the vessels was corrected. Central retinal artery (CRA) measurements were taken at the level of the optic nerve head.
Peak systolic velocity (PSV) and end-diastolic velocity (EDV) were measured in the OA, CRA, and medial and lateral SPCAs. Although medial and lateral PCA were individually assessed, the mean value of both was used for the statistical analysis.
PSV and EDV were used to calculate the Pourcelot resistivity index (RI) according to the following equation: RI = PSV – EDV/PSV.34
Evaluations of blood pressure and radial pulse were obtained in a supine position after 10 min of rest. Systolic (SBP) and diastolic blood pressure (DBP) were measured in the upper right arm using a mercury sphygmomanometer and HR was measured by palpation of the radial pulse. These parameters were obtained every 10 min, during Doppler examination. Mean arterial pressure (MAP) was calculated as: DBP + ⅓ (SBP – DBP).
Ocular perfusion pressure (OPP) was calculated as: ⅔MAP – IOP.
Statistical analysis
Before the study, it was determined that a sample of at least 40 patients per treatment group was required to detect a difference of 0.05 units in mean RI in the OA, SPCA, and CRA increase between the two treatment groups, at a significance level of 0.01, with a power of 0.95, and assuming a standard deviation of 0.05 units. The adjusted power of the study was 0.86 (0.95′ 0.95′ 0.95).
The primary efficacy variables were the mean change between baseline and month 60 in RI in the OA, CRA, and SPCA.
Intent-to-treat (ITT) efficacy analyses included all patients who received study medication and had at least a valid month 12 visit. For the purpose of statistical analysis, only a randomly chosen eye was included for patients with bilateral involvement by intent-to-treat analyses.
The last-observation-carried-forward method was used to impute missing data.
Per protocol analyses, which excluded patients who did not complete the study or who had major protocol violations, were also conducted to confirm the ITT results.
Descriptive statistics [mean (standard deviation)] and 95% confidence intervals (95% CIs) were used to report demographic and ocular baseline characteristics. Data were tested for normal distribution using a Kolmogorov–Smirnov test. As data were normally distributed, one-way analysis of variance (ANOVA) was used to compare means between treatment groups for quantitative variables at baseline. A two-way ANOVA was used to evaluate the effect of treatments and the time factor.
Because of the large number of tests, simultaneous inference using the Bonferroni correction was used to correct the P-value from individual time points (α/11). Statistical significance was accepted for P < 0.0045.
Adverse events were evaluated by a Fisher exact test. The power of the Fisher exact test was 70.1% in detecting differences in proportions of adverse events of 15%, if there was a proportion of an event of 15%.
Statistical analyses were performed using Stata 9.0 (Statacorp. Lakeway Drive, TX).
Results
Of 182 screened patients, 146 met the inclusion/exclusion criteria. Their main characteristics are shown in Table 2.
Table 2.
Baseline Demographic Characteristics
| |
DT |
BT |
|
|---|---|---|---|
| Number | 70 | 76 | P value |
| Age (years) | |||
| Mean (SD) | 64.0 (8.2) | 63.7 (7.4) | 0.821 |
| 95% CI | 62.1–66.0 | 62.1–65.4 | |
| Min | 50 | 51 | |
| Max | 70 | 76 | |
| MAPa (mmHg) | |||
| Mean (SD) | 89.6 (6.8) | 89.0 (6.2) | 0.619 |
| 95% CI | 87.9–91.2 | 87.6–90.4 | |
| Min | 78.3 | 77.6 | |
| Max | 100.0 | 100.0 | |
| Mean IOP (mmHg) | |||
| Mean (SD) | 22.8 (1.2) | 22.7 (1.2) | 0.631 |
| 95% CI | 22.5–23.1 | 22.5–23.0 | |
| Min | 20.7 | 20.5 | |
| Max | 25.7 | 25.7 | |
| Systemic medicationsb | |||
| High blood pressure | 12 (17.1%) | 16 (21.1%) | 0.675 |
| Cardiovascular disease | 3 (4.3%) | 5 (6.6%) | 0.721 |
| Arthrosis | 9 (12.9%) | 5 (6.6%) | 0.263 |
Abbreviations: DT, dorzolamide/timolol; BT, brinzolamide/timolol; SD, standard deviation; 95% CI, 95% confidence interval; MAP, mean arterial pressure; IOP, intraocular pressure.
P values were calculated comparing the parameters at baseline between the two study groups (one-way ANOVA test); P values were considered statistically significant if lower than 0.05.
MAP = DBP + ⅓ (SBP – DBP).
Fisher's exact test. P values were considered statistically significant if lower than 0.05.
The mean follow-up was 47.9 (45.3–50.7) months, with 85 (58%) patients completing five or more years, 102 (70%) completing 3 years of follow-up, and 146 (100%) completing 1 year of follow-up.
Of the 61 patients (42%) not completing 5 years of follow-up, all of them withdrew because of visual field progression (20 [28%] in the DT group and 41 [54%] in the BT group, P = 0.003).
There was no statistically significant difference among study groups in terms of sex distribution, 37 men and 33 women in the dorzolamide/timolol (DT) group versus 41 men and 35 women in the brinzolamide/timolol (BT) group, P = 0.974, respectively.
The mean baseline IOP values (22.8 [1.2]mmHg in the DT group, versus 22.7 [1.2] mmHg in the BT group) showed no significant differences between groups, P = 0.631.
Mean visual field damage expressed as MD was −3.08 (0.93), 95% CI −3.29 to −2.89 dB in the DT group and −3.02 (0.92), 95% CI −3.23 to −2.80 in the BT group, P = 0.686.
Mean (standard deviation) systolic blood pressure levels in the two treatment groups were similar at baseline: 131.7 (6.8) mmHg in the DT group and 130.0 (6.9)mmHg in the BT group, P = 0.134. In addition, there was no statistically significant difference between groups in terms of DBP (68.5 [7.8] mmHg in the DT group and 68.6 [6.6] mmHg in the BT group, P = 0.965).
Systemic blood pressure did not change significantly during treatment in either group.
At baseline, there were no significant differences in retrobulbar blood flow velocities or OPP between the groups (Table 3).
Table 3.
Baseline Hemodynamic Parameters in Dorzolamide/Timolol and Brinzolamide/Timolol Treatment Groups
| Number | DT 70 | BT 76 | P value |
|---|---|---|---|
| PSV OA (cm/s) | |||
| Mean (SD) | 34.1 (2.8) | 35.2 (3.0) | 0.068 |
| 95% CI | 34.2–34.8 | 34.5–35.9 | |
| EDV OA (cm/s) | |||
| Mean (SD) | 8.8 (0.9) | 9.0 (0.9) | 0.119 |
| 95% CI | 8.5–9.0 | 8.8–9.2 | |
| RI OA | |||
| Mean (SD) | 0.74 (0.05) | 0.74 (0.02) | 0.658 |
| 95% CI | 0.74–0.75 | 0.74–0.75 | |
| PSV PCA (cm/s) | |||
| Mean (SD) | 13.6 (1.0) | 13.2 (1.4) | 0.074 |
| 95% CI | 13.3–13.8 | 12.9–13.5 | |
| EDV PCA (cm/s) | |||
| Mean (SD) | 4.7 (0.5) | 4.5 (0.5) | 0.068 |
| 95% CI | 4.6–4.8 | 4.4–4.7 | |
| RI PCA | |||
| Mean (SD) | 0.66 (0.02) | 0.66 (0.03) | 0.612 |
| 95% CI | 0.65–0.66 | 0.66–0.67 | |
| PSV CRA (cm/s) | |||
| Mean (SD) | 12.2 (1.5) | 12.4 (1.6) | 0.296 |
| 95% CI | 11.8–12.5 | 12.1–12.8 | |
| EDV CRA (cm/s) | |||
| Mean (SD) | 4.1 (0.7) | 4.2 (0.7) | 0.347 |
| 95% CI | 4.0–4.3 | 4.1–4.4 | |
| RI CRA (cm/s) | |||
| Mean (SD) | 0.66 (0.03) | 0.66 (0.03) | 0.703 |
| 95% CI | 0.66–0.67 | 0.65–0.67 | |
| OPPa (mmHg) | |||
| Mean (SD) | 36.9 (4.9) | 36.6 (4.3) | 0.730 |
| 95% CI | 35.7–38.0 | 35.6–37.6 | |
| Min | 26.9 | 26.1 | |
| Max | 46.0 | 44.7 | |
Abbreviations: DT, dorzolamide/timolol; BT, brinzolamide/timolol; SD, standard deviation; 95% CI, 95% confidence interval; PSV, peak systolic velocity; EDV, end diastolic velocity; RI, resistivity index; OA, ophthalmic artery; PCA, posterior ciliary arteries; CRA, central retinal artery; OPPa, ocular perfusion pressure.
P values were calculated comparing the parameters at baseline between the two study groups (one-way ANOVA test); P values were considered statistically significant if lower than 0.05.
OPP = ⅔ (MAP – IOP).
At the end of the study, the IOP values were significantly reduced in the two study groups, P < 0.001, respectively (Table 4).
Table 4.
Overview of the Values of Color Doppler Imaging Measurements, Ocular Perfusion Pressure, and Intraocular Pressure and Their Changes to Baseline for Study Treatment Groups
| |
Dorzolamide/timolol treatment |
Brinzolamide/timolol treatment |
Difference between treatment groups |
|||
|---|---|---|---|---|---|---|
| Variable | Mean (95% CI) difference from timolol baseline | P value | Mean (95% CI) difference from timolol baseline | P value | Mean (95% CI) | P value |
| Ophthalmic artery | ||||||
| PSV | −0.08 (−1.01 to 0.84) | 0.861 | −0.01 (−0.98 to 0.98) | 0.995 | −1.21 (−2.73 to 0.31) | 0.093 |
| EDV | 1.22 (0.90 to 1.56) | <0.001 | 0.02 (−0.26 to 0.30) | 0.865 | 0.96 (0.64 to 1.27) | <0.001 |
| RI | −0.04 (−0.05 to −0.03) | <0.001 | 0.00 (−0.01 to 0.01) | 0.713 | −0.04 (−0.05 to −0.03) | <0.001 |
| Short posterior ciliary arteries | ||||||
| PSV | 1.18 (0.82 to 1.53) | <0.001 | 0.37 (−0.09 to 0.79) | 0.101 | 0.79 (0.36 to 1.21) | <0.001 |
| EDV | 0.53 (0.36 to 0.69) | <0.001 | 0.08 (−0.09 to 0.25) | 0.359 | 0.45 (0.26 to 0.65) | <0.001 |
| RI | −0.04 (−0.05 to −0.03) | <0.001 | 0.00 (−0.01 to 0.01) | 0.379 | −0.04 (−0.05 to −0.03) | <0.001 |
| Central retinal artery | ||||||
| PSV | 1.47 (0.94 to 1.99) | <0.001 | 0.38 (−0.14 to 0.89) | 0.155 | 0.83 (0.28 to 1.36) | 0.003 |
| EDV | 0.67 (0.43 to 0.91) | <0.001 | 0.10 (−0.13 to 0.34) | 0.409 | 0.47 (0.38 to 0.55) | <0.001 |
| RI | −0.04 (−0.05 to −0.03) | <0.001 | 0.00 (−0.01 to 0.01) | 0.643 | −0.04 (−0.05 to −0.03) | 0.002 |
| OPP | 4.2 (3.5 to 4.8) | <0.001 | 4.1 (3.4 to 4.8) | <0.001 | 0.1 (−0.8 to 1.4) | 0.555 |
| IOP | −4.3 (−4.5 to −4.2) | <0.001 | −4.3 (−4.4 to −4.2) | <0.001 | 0.0 (−0.28 to 0.30) | 0.841 |
Abbreviations: PSV, peak systolic velocity (cm/s); EDV, end diastolic velocity (cm/s); RI, resistance index; OPP, ocular perfusion pressure (mmHg); IOP, intraocular pressure (mmHg).
P values are calculated comparing the parameters at baseline and at the end of the study (two-way ANOVA test); P values were considered statistically significant if lower than 0.005 (Bonferroni correction).
The differences observed in PSV, EDV, and RI in the OA, CRA, and SPCA after the 5 years of treatment are shown in Table 4. All retrobulbar velocities, except PSV in the OA, increased during treatment with DT (Table 4). BT only significantly increased EDV in the CRA at month 6, P < 0.001, and EDV in the SPCA at months 6 and 12, P < 0.001, respectively.
These results were confirmed in the per protocol population (DT, N = 50; BT, N = 35). The per protocol analyses had a statistical power of the 95% to detect a difference of 0.05 units in mean RI in the OA, SPCA, and CRA increase between the two treatment groups, at a significance level of 0.05 (Table 5).
Table 5.
Overview of the Values of Color Doppler Imaging Measurements, Ocular Perfusion Pressure, and Intraocular Pressure and Their Changes to Baseline for Study Treatment Groups (per Protocol Analyses)
| |
Dorzolamide/timolol treatment |
Brinzolamide/timolol treatment |
Difference between treatment groups |
|||
|---|---|---|---|---|---|---|
| Variable | Mean (95% CI) difference from timolol baseline | P value | Mean (95% CI) difference from timolol baseline | P value | Mean (95% CI) | P value |
| Ophthalmic artery | ||||||
| PSV | −0.10 (−1.31 to 1.13) | 0.890 | −0.09 (−1.40 to 1.20) | 0.892 | −0.96 (−2.25 to 0.32) | 0.140 |
| EDV | 1.33 (0.97 to 1.68) | <0.001 | −0.06 (−0.41 to 0.29) | 0.728 | 1.08 (0.70 to 1.46) | <0.001 |
| RI | −0.04 (−0.05 to −0.03) | <0.001 | 0.00 (−0.01 to 0.01) | 0.532 | −0.04 (−0.05 to −0.03) | <0.001 |
| Short posterior ciliary arteries | ||||||
| PSV | 1.15 (0.70 to 1.60) | <0.001 | 0.38 (−0.33 to 1.08) | 0.292 | 1.29 (0.72 to 1.87) | <0.001 |
| EDV | 0.61 (0.42 to 0.81) | <0.001 | −0.02 (−0.28 to 0.24) | 0.896 | 0.61 (0.39 to 0.85) | <0.001 |
| RI | −0.04 (−0.05 to −0.03) | <0.001 | 0.01 (−0.01 to 0.03) | 0.097 | −0.05 (−0.06 to −0.04) | <0.001 |
| Central retinal artery | ||||||
| PSV | 1.17 (0.55 to 1.78) | <0.001 | 0.11 (−0.66 to 0.87) | 0.783 | 1.14 (0.42 to 1.85) | 0.002 |
| EDV | 0.60 (0.31 to 0.90) | <0.001 | −0.01 (−0.35 to 0.34) | 0.968 | 0.48 (0.15 to 0.81) | 0.005 |
| RI | −0.04 (−0.06 to −0.02) | 0.001 | 0.00 (−0.01 to 0.02) | 0.619 | −0.04 (−0.06 to −0.03) | 0.008 |
| OPP | 4.2 (2.4 to 6.1) | <0.001 | 4.2 (2.4 to 6.0) | <0.001 | 0.1 (−0.8 to 1.4) | 0.842 |
| IOP | −4.4 (−4.8 to −3.9) | <0.001 | −4.3 (−4.9 to −3.8) | <0.001 | 0.0 (−0.28 to 0.30) | 0.753 |
Abbreviations: PSV, peak systolic velocity (cm/s); EDV, end diastolic velocity (cm/s); RI, resistance index; OPP, ocular perfusion pressure (mmHg); IOP, intraocular pressure (mmHg).
P values are calculated comparing the parameters at baseline and at the end of the study (two-way ANOVA test); P values were considered statistically significant if lower than 0.005 (Bonferroni correction).
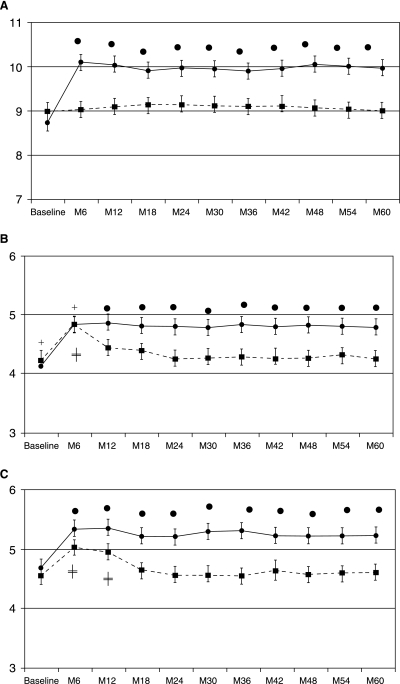
Figure 1 shows mean end-diastolic velocities at baseline and follow-up for the study treatment groups in the OA (A), CRA (B), and SPCA (C).
FIG. 1.
Mean end-diastolic velocities at baseline and follow-up for the study treatment groups. The solid line with solid rhombus corresponds to the dorzolamide/timolol treatment group. The dotted line with solid squares corresponds to the brinzolamide/timolol treatment group. Each point represents the mean value of the indicated hemodynamic parameter. Error bars show 95% confidence interval (CI). (A) Ophthalmic artery. (B) Central retinal artery. (C) Short posterior ciliary artery. • Indicates significant effects of dorzolamide/timolol compared with baseline and brinzolamide/timolol (P < 0.001). ‡ Indicates significant effects of brinzolamide/timolol compared with baseline (P < 0.001). + Indicates no significant differences between dorzolamide/timolol and brinzolamide/timolol treatment groups.
The mean end-diastolic velocities, in the DT-treated group, increased from 8.8 (0.9), 4.7 (0.5), and 4.1 (0.7) to 10.1 (1.3), 5.2 (0.5), and 4.6 (0.8) in the OA, SPCA, and CRA, respectively, P < 0.0001. In the BT group, there was not statistically significant difference in terms of hemodynamic parameters at the end of the study. Nevertheless, the EDVs, in the CRA and in the SPCA, were statistically significantly greater at month 6 and at months 6 and 12 as compared with baseline, respectively (P < 0.001).
The RI in the OA, CRA, and SPCA were significantly reduced during treatment with DT, P < 0.001, but did not change during treatment with BT (Table 4).
OPP significantly increased in both study groups after the 60 months of treatment, P < 0.001 (Table 4).
The amount of general medication changes such as calcium channel blockers, nonsteroidal anti-inflammatory drugs, or diuretics was low and did not differ between the two treatment groups.
No serious adverse events were detected in the study. The incidence and types of adverse events were similar in both treatments (Table 6).
Table 6.
Systemic and Ocular Adverse Events
| Event | DT (70) | BT (76) | P value |
|---|---|---|---|
| Hyperemia | 4 | 5 | 0.906 |
| Tearing | 12 | 8 | 0.358 |
| Foreign body sensation | 17 | 14 | 0.513 |
| Itching | 25 | 22 | 0.478 |
| Burning/stinging | 18 | 7 | 0.014 |
| Blurred vision | 5 | 20 | 0.002 |
| Punctate keratitis | 3 | 3 | 0.743 |
| Taste perversion | 15 | 18 | 0.894 |
| Flu | 8 | 11 | 0.757 |
| Headache | 7 | 5 | 0.656 |
| Back pain | 13 | 15 | 0.967 |
Abbreviations: DT, dorzolamide/timolol; BT, brinzolamide/timolol.
P values were calculated comparing the adverse events at month 60 (Fisher exact test); P values were considered statistically significant if lower than 0.05.
The most frequently reported adverse event was itching, which occurred in 35.7% (25/70) of patients of the DT treatment group and in 28.9% (22/76) of the patients in the BT treatment group (P = 0.478).
A significantly greater proportion of patients in the DT group (25.7%; 18/70) reported burning/stinging as compared with the BT group (9.2%; 7/76), P = 0.014. However, a significantly greater proportion of patients in the BT group reported blurred vision (26.3%; 20/76) as compared with the DT group (7.1%; 5/70), P = 0.002.
At least one adverse event was reported by 47/70 (67.1%) patients receiving DT and by 49/76 (64.5%) of those treated with BT, P = 0.862.
Discussion
The results of this 60-month study indicated that the association of dorzolamide 2% and timolol 0.5% given twice-daily significantly increased retrobulbar blood flow in POAG patients.
In contrast, the association of brinzolamide 1% and timolol 0.5% had a limited effect upon retrobulbar hemodynamics. The vascular effect of brinzolamide did not last longer than 6 months in the CRA and 12 months in the SPCA. In addition, brinzolamide did not exert a statistically significant effect upon the blood flow parameters in the OA.
Our findings of increased retrobulbar blood flow during dorzolamide/timolol treatment agree with previous studies reported by our group.15,18–20
We found that dorzolamide/timolol, in association15,20 or in fixed combination,18,19 not only significantly increased EDV but also significantly decreased RI in the OA, CRA, and in the PCA in patients with OAG.
In agreement with our results, Galassi and colleagues17 found a significant decrease in RI in the temporal posterior ciliary artery after 4 weeks of dorzolamide 2% in patients with newly diagnosed POAG.
However, the results of our study are in contradiction to the findings reported by Bergstrand and colleagues23 in previously untreated glaucoma patients. These authors did not find dorzolamide to exert significant hemodynamic effects.
This difference in circulatory results after dorzolamide treatment in patients with glaucoma may be explained by the existence of variable study designs, treatment periods, and ethnic populations.
The end-diastolic velocities and RI, in all retrobulbar vessels, reported by Bergstrand and colleagues23 seemed to be significantly lower than those reported by our group. These differences in the retrobulbar hemodynamics between the two study patients may be clinically relevant.
Relatively few studies have evaluated the effect of brinzolamide upon OBF. Our findings partially disagree with those published by Kaup and colleagues25 who reported that retrobulbar hemodynamics remained unaltered in healthy subjects treated with brinzolamide 1% BID.
It is very difficult to compare our results with those published by Kaup and colleagues25 because the subjects included in our study were patients with glaucoma. We found that brinzolamide significantly increased EDV in the CRA and SPCA. However, the effect of brinzolamide did not last longer than 6 months in the CRA, and 12 months in the SPCA.
Siesky and colleagues26 have found no significant effects upon retrobulbar hemodynamics after 3 months of treatment with brinzolamide 1%, three times daily, in OAG patients.
Our results partially dissents from those reported by Siesky and colleagues.26 The results of our study suggested that the effect of brinzolamide, on the retrobulbar hemodynamics, remained 6 months in the CRA and 12 months in the SPCA.
It seems that the vascular effects of dorzolamide/timolol found in our study are unrelated to mechanical changes with the drug, and are more related to the pharmacological effects of dorzolamide in the posterior segment. The fact that both treatments had a similar IOP-lowering effect, though with different vascular effects, provides further evidence in support of a local vasoactive effect as opposed to an oculartension mechanism.
The mechanism underlying the vasodilator effect of CAIs in retrobulbar vessels has not been fully established. There is evidence that CAIs may cause extracellular acidosis.35 It is worth noting that an increase in arterial pCO2—a stimulus that produces extracellular acidosis—also strongly increases choroidal blood flow.36–38 Nevertheless, experiments in isolated precontracted bovine retinal arteries indicate that the vasodilator effects of dorzolamide are independent from changes in pH, because dorzolamide-induced vasodilatation is also seen when the pH is kept.39
The different vascular effects of dorzolamide and brinzolamide found in this study may be explained by their different ability to inhibit CA isoenzyme IV. In the eye, CA-IV is located in the endothelial cells of the choriocapillaris.40 Additionally, Sender and colleagues41 found CA isoenzyme IV activity in muscle capillaries.
The vasorelaxation observed in vitro or after systemic administration of CAIs can therefore be assumed to be due to an effect on the vascular smooth muscle cells alone or on these cells in interplay with other cellular elements in the surrounding tissue. However, this response depended on the type of the CAI. The vasorelaxing effect of dorzolamide was significantly reduced in isolated arterioles, as evidenced by both a reduction in the maximum relaxation and an increase in EC50.42 It is therefore highly likely that the vasorelaxing effect of these drugs depends on carbonic anhydrases in the perivascular tissue. This effect may have involved different membrane-bound isoenzyme IV and XIV located in retinal astrocytes and Müller cells.43–45
Dorzolamide is a potent sulfonamide inhibitor of CA-IV, with an IC50 of 6.9 nM.46 IC50 for brinzolamide against CA-IV is 45.3 nM.47 In other words, the inhibitory activity of dorzolamide against CA-IV is 6.5 times greater than that of brinzolamide.
Regarding the ocular side effects, the symptoms reported in this study have been observed in previous studies involving dorzolamide48,49 or brinzolamide,50,51 used alone or as adjunctive therapy to timolol.52
There are limitations of our study that need to be taken into account on interpreting the data obtained. The first limitation results from the use of CDI. This ultrasound technique has been used in several studies with the aim of assessing changes in retrobulbar circulation.9,15,53,54 As it is impossible to measure the diameter of retrobulbar vessels in vivo, CDI does not reflect blood volume, and only blood velocity can be estimated.55 Nevertheless, at constant perfusion pressure and blood viscosity, increased flow velocities are associated with increased blood flow (in volume per time unit).56
Our second limitation results from the use of a singular ocular imaging technology to study ocular blood perfusion, without providing data on the retina or other vascular beds.
The third limitation is the single-center nature of the study, with a limited number of patients. Nevertheless, the sample size was calculated prior the study.
Another limitation of the study is the fact that CCT was measured when the study was already ongoing. Dorzolamide and brinzolamide are potent selective inhibitors of the carbonic anhydrase isoenzyme II.46,47 The prolonged presence of dorzolamide or brinzolamide in the cornea could increase corneal thickness. In two clinical studies57,58 it was reported that dorzolamide significantly increased CCT in patients with cornea guttata. In addition, Ornek and colleagues59 suggested that brinzolamide may cause a short-term increase in the human CCT, particularly on the first day. Nevertheless, a 1-year clinical study of 298 patients with elevated IOP and normal corneas at baseline found that dorzolamide was equivalent to timolol and betaxolol in terms of the change in central endothelial cell density and thickness.60
Despite these limitations, the results of our study suggest that dorzolamide added to timolol significantly increased the retrobulbar blood flow parameters in POAG patients after 60 months of treatment.
In addition, dorzolamide and brinzolamide each added to timolol not only significantly reduced IOP but also increased mean OPP in patients with POAG, after a 5-year therapy period.
The importance of these findings is critically dependent on whether an increase of the blood flow parameters contributes to visual field preservation in glaucoma.
Further clinical studies, particularly multicenter randomized clinical trials, are needed to establish the vascular effects of these treatments.
Acknowledgments
The authors wish to express their gratitude to Celia Rodriguez-Suarez for her collaboration with the Doppler imaging.
The authors wish to express their gratitude to Cristina Fernandez for her statistical advice.
Financial support: This study was funded in part by research grant no. C-00-13 from the Red Tematica en Oftalmología (Ophthalmology Thematic Network) and by research grant no. XUGA IN825B2005/4-0 from the Galician Authorities.
Meeting presentation: This study was partially presented as a poster at the 8th European Glaucoma Society meeting in Berlin, Germany, June 1–6, 2008.
Author Disclosure Statement
Conflict of interest: Antonio Martínez: Received honoraria for lectures from MSD.
Antonio Martinez: Received reimbursement for attending symposia from MSD.
Antonio Martinez: Received payments for research performed for Alcon.
References
- 1.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal tension glaucoma and patients with therapeutically reduced intraocular pressures. Am. J. Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 2.AGIS investigators. The Advance Glaucoma Intervention Study (AGIS):7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 3.Heijl A. Leske M.C. Bengtsson B., et al. Reduction of intraocular pressure and glaucoma progression: results from the Early manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 4.Carter C.J. Brooks D.E. Doyle D.L., et al. Investigation into a vascular aetiology for low tension glaucoma. Ophthalmology. 1990;97:49–55. doi: 10.1016/s0161-6420(90)32627-1. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh S.S. Progress in the understanding of the vascular aetiology of glaucoma. Curr. Opin. Ophthalmol. 1994;5:26–35. [Google Scholar]
- 6.Flammer J. Orgül S. Costa V.P., et al. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 7.Galassi F. Sodi A. Ucci F., et al. Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch Ophthalmol. 2003;121:1711–1715. doi: 10.1001/archopht.121.12.1711. [DOI] [PubMed] [Google Scholar]
- 8.Satilmis M. Orgul S. Doubler B., et al. Rate of progression of glaucoma correlates with retrobulbar circulation and intraocular pressure. Am. J. Ophthalmol. 2003;135:664–669. doi: 10.1016/s0002-9394(02)02156-6. [DOI] [PubMed] [Google Scholar]
- 9.Martinez A. Sanchez M. Predictive value of color Doppler imaging in a prospective study of visual field progression in primary open-angle glaucoma. Acta Ophthalmol. Scand. 2005;83:716–723. doi: 10.1111/j.1600-0420.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 10.Becker B. Decrease in intraocular pressure in a man by a carbonic anhydrase inhibitor, Diamox. Am. J. Ophthalmol. 1954;37:13. doi: 10.1016/0002-9394(54)92027-9. [DOI] [PubMed] [Google Scholar]
- 11.Nishimori I. Vullo D. Innocenti A., et al. Carbonic anhydrase inhibitors: inhibition of the transmembrane isoenzyme XIV with sulfonamides. Bioorg. Med. Chem. Lett. 2005;15:3828–3833. doi: 10.1016/j.bmcl.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 12.Harris A. Arend O. Arend S., et al. Effects of topical dorzolamide on retinal and retrobulbar hemodynamics. Acta Ophthalmol. Scand. 1996;74:569–572. doi: 10.1111/j.1600-0420.1996.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 13.Harris A. Arend O. Kagemann L., et al. Dorzolamide, visual function and ocular hemodynamics in normal-tension glaucoma. J. Ocul. Pharmacol. Ther. 1999;15:189–197. doi: 10.1089/jop.1999.15.189. [DOI] [PubMed] [Google Scholar]
- 14.Fuchsjäger-Mayrl G. Wally B. Rainer G., et al. Effect of dorzolamide and timolol on ocular blood flow in patients with primary open angle glaucoma and ocular hypertension. Br. J. Ophthalmol. 2005;89:1293–1297. doi: 10.1136/bjo.2005.067637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez A. Gonzalez F. Capeans C., et al. Dorzolamide effect on the ocular blood flow. Invest. Ophthalmol. Vis. Sci. 1999;40:1270–1275. [PubMed] [Google Scholar]
- 16.Avunduk A.M. Sari A. Akyol N., et al. The one-month effects of topical betaxolol, dorzolamide and apraclonidine on ocular blood flow velocities in patients with newly diagnosed primary open-angle glaucoma. Ophthalmologica. 2001;215:361–365. doi: 10.1159/000050886. [DOI] [PubMed] [Google Scholar]
- 17.Galassi F. Sodi A. Renieri G., et al. Effects of timolol and dorzolamide on retrobulbar hemodynamics in patients with newly diagnosed primary open-angle glaucoma. Ophthalmologica. 2002;216:123–128. doi: 10.1159/000048311. [DOI] [PubMed] [Google Scholar]
- 18.Martinez A. Sanchez M. A comparison of the effects of 0.005% latanoprost and fixed combination dorzolamide/timolol on retrobulbar haemodynamics in previously untreated glaucoma patients. Curr. Med. Res. Opin. 2006;22:67–73. doi: 10.1185/030079906X80215. [DOI] [PubMed] [Google Scholar]
- 19.Martinez A. Sanchez M. Retrobulbar haemodynamic effects of the latanoprost/timolol and the dorzolamide/timolol fixed combinations in newly diagnosed glaucoma patients. Int. J. Clin. Pract. 2007;61:815–821. doi: 10.1111/j.1742-1241.2006.01126.x. [DOI] [PubMed] [Google Scholar]
- 20.Martinez A. Sanchez M. Effects of dorzolamide 2% added to timolol maleate 0.5% on intraocular pressure, retrobulbar blood flow, and progression of visual field damage in patients with primary open-angle glaucoma: a single-centre,4-year, open-label study. Clin. Ther. 2008;30:1120–1134. doi: 10.1016/j.clinthera.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Grunwald J.E. Mathur S. DuPont J. Effects of dorzolamide hydrochloride 2% on the retinal circulation. Acta Ophtahlmol. 1997;75:236–238. doi: 10.1111/j.1600-0420.1997.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 22.Pillunat L.E. Bohm A.G. Koller A.U., et al. Effect of topical dorzolamide on optic nerve head blood flow. Graefes Arch. Clin. Exp. Ophthalmol. 1999;237:495–500. doi: 10.1007/s004170050268. [DOI] [PubMed] [Google Scholar]
- 23.Bergstrand I.C. Heijl A. Harris A. Dorzolamide and ocular blood flow in previously untreated glaucoma patients: a controlled double-masked study. Acta Ophthalmol. Scand. 2002;80:176–182. doi: 10.1034/j.1600-0420.2002.800211.x. [DOI] [PubMed] [Google Scholar]
- 24.Barnes G.E. Li B. Dean T., et al. Increased optic nerve head blood flow after 1 week of twice daily topical brinzolamide treatment in Dutch-belted rabbits. Surv. Ophthalmol. 2000;44:S131–S140. doi: 10.1016/s0039-6257(99)00124-1. [DOI] [PubMed] [Google Scholar]
- 25.Kaup M. Plange N. Niegel M., et al. Effects of brinzolamide on ocular haemodynamics in healthy volunteers. Br. J. Ophthalmol. 2004;88:257–262. doi: 10.1136/bjo.2003.021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siesky B. Harris A. Cantor L.B., et al. A comparative study of the effects of brinzolamide and dorzolamide on retinal oxygen saturation and ocular microcirculation in patients with primary open-angle glaucoma. Br. J. Ophthalmol. 2008;92:500–504. doi: 10.1136/bjo.2007.125187. [DOI] [PubMed] [Google Scholar]
- 27.Ferris F.L., 3rd Kassoff A. Bresnick G.H., et al. New visual acuity charts for clinical research. Am. J. Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 28.Asman P. Heijl A. Glaucoma hemifield test. Automated visual field evaluation. Arch. Ophthalmol. 1992;110:812–819. doi: 10.1001/archopht.1992.01080180084033. [DOI] [PubMed] [Google Scholar]
- 29.Hodapp E. Parrish R. Anderson D. Clinical Decisions in Glaucoma. St. Louis: Mosby Year Book, Inc.; 1993. [Google Scholar]
- 30.Dong J. Chihara E. Slope analysis of the optic disc in eyes with ocular hypertension and early normal tension glaucoma by confocal scanning laser ophthalmoscope. Br. J. Ophthalmol. 2001;85:56–62. doi: 10.1136/bjo.85.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieb W.E. Cohen S.M. Merton D.A., et al. Color Doppler imaging of the eye and orbit: technique and normal vascular anatomy. Arch Ophthalmol. 1991;109:527–531. doi: 10.1001/archopht.1991.01080040095036. [DOI] [PubMed] [Google Scholar]
- 32.Martinez A. Sanchez M. Ocular haemodynamics in pseudoexfoliative and primary open-angle glaucoma. Eye. 2008;22:515–520. doi: 10.1038/sj.eye.6702676. [DOI] [PubMed] [Google Scholar]
- 33.Martinez A. Sanchez M. Retrobulbar hemodynamic parameters in pseudoexfoliation syndrome and pseudoexfoliative glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2008;246:1341–1349. doi: 10.1007/s00417-008-0841-4. [DOI] [PubMed] [Google Scholar]
- 34.Pourcelot L. Indications de l'ultrasonographie Doppler dans l'etude des vaisseaux peripheriques. Rev. Prat. 1975;25:4671–4680. [PubMed] [Google Scholar]
- 35.Friberg L. Kastrup J. Rizzi D., et al. Cerebral blood flow and end-tidal pCO2 during prolonged acetazolamide treatment in humans. Am. J. Physiol. 1990;258:H954–H959. doi: 10.1152/ajpheart.1990.258.4.H954. [DOI] [PubMed] [Google Scholar]
- 36.Friedman E. Chandra S.R. Choroidal blood flow: effects of oxygen and carbon dioxide. Arch. Ophthamol. 1972;87:70–71. doi: 10.1001/archopht.1972.01000020072015. [DOI] [PubMed] [Google Scholar]
- 37.Riva C.E. Petrig B.L. Choroidal blood flow by laser Doppler flowmetry. Opt. Eng. 1995;34:746–752. [Google Scholar]
- 38.Schmetterer L. Lexer F. Graselli U., et al. The effcet of different mixtures of O2 and CO2 on ocular fundus pulsations. Exp. Eye Res. 1996;63:351–355. doi: 10.1006/exer.1996.0125. [DOI] [PubMed] [Google Scholar]
- 39.Josefsson A. Sigurdsson S.B. Bang K., et al. Dorzolamide induces vasodilatation in isolated pre-contracted bovine retinal arteries. Exp. Eye Res. 2004;78:215–221. doi: 10.1016/j.exer.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Hageman G.S. Zhu X.L. Waheed A., et al. Localization of carbonic anhydrase IV in a specific capillary bed of the human eye. Proc. Natl Acad. Sci. USA. 1991;88:2716–2720. doi: 10.1073/pnas.88.7.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sender S. Gros G. Waheed A., et al. Immunohistochemical localization of carbonic anhydrase IV in Capillaries of rat and human skeletal muscle. J. Histochem. Cytochem. 1994;42:1229–1236. doi: 10.1177/42.9.8064130. [DOI] [PubMed] [Google Scholar]
- 42.Kehler A.K. Holmgaard K. Hessellund A., et al. Variable involvement of the perivascular retinal tissue in carbonic anhydrase inhibitor-induced relaxation of porcine retinal arterioles in vitro. Invest. Ophthalmol. Vis. Sci. 2007;48:4688–4693. doi: 10.1167/iovs.07-0048. [DOI] [PubMed] [Google Scholar]
- 43.Wistrand P.J. Carbonic anhydrase inhibition in ophthalmology: carbonic anhydrases in cornea, lens, retina and lacrimal gland. EXS. 2000;90:413–424. doi: 10.1007/978-3-0348-8446-4_20. [DOI] [PubMed] [Google Scholar]
- 44.Inoue J. Oka M. Aoyama Y., et al. Effects of dorzolamide hydrochloride on ocular tissues. J. Ocul. Pharmacol. Ther. 2004;20:1–13. doi: 10.1089/108076804772745419. [DOI] [PubMed] [Google Scholar]
- 45.Nagelhus E.A. Mathiisen T.M. Bateman A.C., et al. Carbonic anhydrase XIV is rich in specific membrane domains of retinal pigment epithelium, Müller cells, and astrocytes. Proc. Natl Acad. Sci. USA. 2005;102:8030–8035. doi: 10.1073/pnas.0503021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugrue M.F. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog. Retin. Eye Res. 2000;19:87–112. doi: 10.1016/s1350-9462(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 47.Stams T. Chen Y. Boriack-Sjodin P.A., et al. Structures of murine carbonic anhydrase IV and human carbonic anhydrase II complexed with brinzolamide: molecular basis of isoenzyme-drug discrimination. Protein Sci. 1998;7:556–563. doi: 10.1002/pro.5560070303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strahlman E. Tipping R. Vogel R. for the International Dorzolamide Study Group. A double-masked, randomized 1-year study comparing dorzolamide (Trusopt), timolol, and betaxolol. Arch. Ophthalmol. 1995;113:1009–1016. doi: 10.1001/archopht.1995.01100080061030. [DOI] [PubMed] [Google Scholar]
- 49.Strahlman E. Tipping R. Vogel R. for the Dorzolamide Dose-Response Study Group. A six-week dose-response study of the ocular hypotensive effect of dorzolamide with a one-year extension. Am. J. Ophthalmol. 1996;122:183–194. doi: 10.1016/s0002-9394(14)72009-4. [DOI] [PubMed] [Google Scholar]
- 50.Silver L.H. the brinzolamide primary therapy study group. Clinical efficacy and safety of brinzolamide (Azopt TM), a new topical carbonic anhydrase inhibitor for primary open-angle glaucoma and ocular hypertension. Am. J. Ophthalmol. 1998;126:400–408. doi: 10.1016/s0002-9394(98)00095-6. [DOI] [PubMed] [Google Scholar]
- 51.March W.F. Ochsner K.I. the brinzolamide long-term therapy study group. The long-term safety and efficacy of brinzolamide 1.0% (Azopt) in patients with primary open-angle glaucoma or ocular hypertension. Am. J. Ophthalmol. 2000;129:136–143. doi: 10.1016/s0002-9394(99)00343-8. [DOI] [PubMed] [Google Scholar]
- 52.Laibovitz R. Boyle J. Snyder E., et al. Dorzolamide versus pilocarpine as adjunctive therapies to timolol: a comparison of patient preference and impact on daily life. Clin. Ther. 1996;18:821–832. doi: 10.1016/s0149-2918(96)80042-7. [DOI] [PubMed] [Google Scholar]
- 53.Repo L.P. Suhonen M.T. Teräsvirta M.E., et al. Color Doppler imaging of the ophthalmic artery blood flow spectra of patients who have had a transient ischemic attack. Correlations with generalized iris transluminance and Pseudoexfoliation syndrome. Ophthalmology. 1995;102:1199–1205. doi: 10.1016/s0161-6420(95)30890-1. [DOI] [PubMed] [Google Scholar]
- 54.Detorakis E.T. Achtaropoulos A.K. Drakonaki E.E., et al. Hemodynamic evaluation of the posterior ciliary circulation in exfoliation syndrome and exfoliation glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:516–521. doi: 10.1007/s00417-006-0439-7. [DOI] [PubMed] [Google Scholar]
- 55.Kagemann L. Harris A. Chung H.S., et al. In: Current Concepts on Ocular Blood Flow in Glaucoma. Pillunat L.E, editor; Harris A, editor; Anderson D.R., et al., editors. The Hague, The Netherlands: Kugler Publications; 1999. pp. 103–110. [Google Scholar]
- 56.Sergott R.C. Aburn N.S. Trible J.R., et al. Colour Doppler imaging: methodology and preliminary results in glaucoma. Surv. Ophthalmol. 1994;38:S65–S70. doi: 10.1016/0039-6257(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 57.Wirtitsch M.G. Findl O. Kiss B., et al. Short-term effect of dorzolamide hydrochloride on central corneal thickness in humans with cornea guttata. Arch. Ophthalmol. 2003;21:621–625. doi: 10.1001/archopht.121.5.621. [DOI] [PubMed] [Google Scholar]
- 58.Wirtitsch M.G. Findl O. Heinzl H., et al. Effect of dorzolamide hydrochloride on central corneal thickness in humans with cornea guttata. Arch. Ophthalmol. 2007;125:1345–1350. doi: 10.1001/archopht.125.10.1345. [DOI] [PubMed] [Google Scholar]
- 59.Ornek K. Gullu R. Ogurel T., et al. Short-term effect of topical brinzolamide on human central corneal thickness. Eur. J. Ophthalmol. 2008;18:338–340. doi: 10.1177/112067210801800303. [DOI] [PubMed] [Google Scholar]
- 60.Lass J.H. Khosrof S.A. Laurence J.K., et al. A double-masked, randomized, 1-year study comparing the corneal effects of dorzolamide, timolol, and betaxolol. Dorzolamide Corneal Effects Study Group. Arch. Ophthalmol. 1998;116:1003–1010. doi: 10.1001/archopht.116.8.1003. [DOI] [PubMed] [Google Scholar]