Abstract
Purpose
The objective of this study was to investigate the role of sodium-dependent multiple vitamin transporter (SMVT) on Biotin-Ganciclovir (biotin-GCV) uptake on both human retinal pigmented epithelium cell line (ARPE-19) and rabbit retina. Study also aims to delineate the vitreal pharmacokinetics of biotin-GCV.
Method
ARPE-19 was employed to study the in vitro uptake experiments. New Zealand white albino rabbits were used to study in vivo retinal uptake and vitreal pharmacokinetics following intravitreal administration of biotin-GCV. In vitro uptake kinetics of [3H] biotin was determined at various initial concentrations. Competitive inhibition studies were conducted in the presence of unlabelled biotin, desthiobiotin, pantothenic acid, and lipoic acid. Various other uptake studies were performed to functionally characterize the transporter. To provide the molecular evidence of this transporter, Reverse Transcription-Polymerase Chain Reaction (RT-PCR) studies were also conducted. In vivo retinal/choroidal uptake studies were carried out with New Zealand albino rabbits. Unconscious animal ocular microdialysis studies were performed in order to evaluate intravitreal pharmacokinetics of GCV and Biotin-GCV.
Results
Uptake of [3H] biotin into ARPE-19 was linear over 7min, and found to be saturable with Km of 138.25 μM and Vmax of 38.85 pmol/min/mg protein. Both pantothenic acid and lipoic acid decreased significantly in uptake of biotin in the concentration-dependent manner. Uptake of biotin into ARPE-19 was found to be temperature, energy, and Na+ dependent but Cl− independent. Further, RT-PCR studies identified a band exhibiting presence of hSMVT on ARPE-19. Biotin-GCV is recognized by SMVT system present on the ARPE-19 and rabbit retina. Vitreal Pharmacokinetics profile reveals that most of the parameters were not significantly different for GCV and Biotin-GCV. However, use of Biotin-GCV may result in sustain levels of regenerated GCV in vitreous.
Conclusions
SMVT was identified and functionally characterized on ARPE-19 cells. Further, Biotin-GCV shares this transport system. Vitreal pharmacokinetics of the conjugate was determined in unconscious rabbit model.
Introduction
Human cytomegalovirus (HCMV) belongs to the β subgroup of herpes viruses. HCMV retinitis is the most common sight-threatening infection in acquired immune deficiency syndrome (AIDS) patients.1 In cytomegalovirus (CMV) retinitis, the inner layer of the retinal blood vessels are first known to get infected and which eventually spread outwards to the other layers including retinal pigmented epithelium (RPE).2 The exact mechanism of viral spread in the retina is not well understood, however it has been suggested that CMV first penetrates the retinal blood vessels following which it spreads into the cells of various layers of retina.3,4 The main reason in the progression of CMV retinitis is the reproduction of virus in the retinal cells, which could occur in the endothelial cells of retinal blood vessels, leading to the breakdown of inner blood–retinal barrier. Breakdown can eventually lead to the migration of virus toward the RPE, nonpigmented epithelium and retinal glial cells.5,6 RPE may become disorganized in some cases that could result in the breakdown of outer BRB. Such processes could be followed by the accumulation of fluid in the subretinal space, leading to retinal hemorrhage and detachment.5–8 In recent years, although the application of highly active antiretroviral therapy (HAART) has induced a dramatic decrease in the incidence of HCMV retinitis, CMV retinitis remains a threat to vision and quality of life in AIDS patients who fail HAART or do not qualify for HAART.9,10 Ganciclovir (GCV), a 2′-deoxyguanosine analog, was the first drug approved by FDA for HCMV infections. Owing to its poor oral bioavailability and virustatic properties, daily intravenous infusion of GCV has been primarily indicated in the maintenance therapy for HCMV retinitis.11 The delivery of therapeutic doses of drugs to the tissues in the posterior segment of the eye is hampered due to ocular barriers, that is, the blood–aqueous barrier and the blood–retinal barrier. Consequently, intravenous infusion of GCV results in poor ocular drug permeation and high systemic toxicity.12 Therefore, GCV is mainly administered intravitreally for the treatment of HCMV retinitis. However, even after the intravitreal administration the accumulation of GCV in to the retinal cells (the sanctuary site where viruses resides) is limited due to its hydrophilic nature. In our laboratory lipophilic prodrug strategies have been attempted to various hydrophilic therapeutic agents to improve their ocular bioavailability,13–15 including GCV. Lipophilic prodrugs of GCV16 have enhanced permeability into ocular cells thereby raising intracellular concentrations. These prodrugs also revert to the parent drug in the vitreous humor at a slow rate resulting in sustained GCV maintenance levels above minimal inhibitory concentration (MIC). Major limitations associated with lipophilic modifications are decreased water solubility of the prodrugs. A balance is needed to be struck in order to have better permeability along with suitable water solubility. Another approach would be to develop transporter-targeted prodrug strategy. Compounds such as prodrugs or analogs that can target membrane transporters, can significantly enhance the absorption of poorly permeating parent drug. Desired membrane permeability can be achieved by proper selection of the promoiety. These prodrugs are recognized by the membrane transporters as substrates and are translocated across the epithelia. Subsequently, the prodrugs are enzymatically cleaved to release the parent drug and the ligand which in most cases is a nutrient and nontoxic. Therefore, transporter-targeted drug delivery has become a promising approach to treat HCMV retinitis. In our laboratory, various nutrient transporters have been investigated on the retina and even some have been utilized for drug delivery purposes. Various transporters are reported to be present on RPE cells that is, monocarboxylic acids, taurine, peptide/histidine, glucose, amino acid, and folic acid. Such membrane transporters are vital for supplying essential nutrients across the cell membrane.
Sodium-dependent multiple vitamin transporter (SMVT) was found to possess excellent capacity (Km) for utilization in drug delivery. SMVT has broad substrate specificity. It can translocate biotin (vitamin H), pantothenic acid (vitamin B 5) and also a cofactor, lipoic acid. These substrates share some degree of structural similarity. Carrier-mediated uptake of biotin is either regulated by specific low-capacity high-affinity biotin transporter or through SMVT. The former transports only biotin and is not inhibited by lipoic and pantothenic acid17 whereas the later co-transports pantothenic and lipoic acid, which is a low-affinity high-capacity transporter.18–24 Biotin prodrugs may be able to utilize SMVT to increase the permeability of conjugated drugs. A protease inhibitor saquinavir conjugated with biotin was taken up by this transport system.25 In another study permeability of a nonapeptide (Retro-Inverso Tat Nonapeptide i.e., R.I.-K-Tat9) linked to biotin (R.I.-K(biotin)-Tat9) was significantly enhanced by 3.2-fold compared to the control across Caco-2 cells. Transport of such biotin conjugates was also found to be concentration dependent and saturable. Biotin conjugates of PEG and CPT-PEG were also demonstrated to be recognized by this transporter which enhanced their permeabilities. No information regarding the presence of SMVT on rabbit retina or ARPE-19 cells is available. Thus identification of biotin transporter on retinal cells may prove to be beneficial for targeted drug delivery. This study provides the first evidence for the presence of SMVT on the RPE.
SMVT is an important transport system that carries vitamins and cofactors essential to the ocular tissue. A report described that in a developing retina an adequate biotin content along with a precise regulation of retinal cell death are required for the correct ocular morphogenesis. Thus it would be of great interest to explore the potential of this transport system to deliver drug conjugates. The purpose of the present study was to identify the presence of functionally characterize SMVT on human RPE cells (i.e., ARPE-19). In this study, we have also demonstrated the presence of SMVT on the rabbit retina/RPE and its possible role in the pharmacokinetics of biotin-ganciclovir (biotin-GCV).
ARPE-19 cell line is of human origin and has been extensively employed for in vitro representation of the human RPE. Several studies have shown that this cell line exhibits characteristic epithelial morphology and represents the RPE with respect to polarization and expression of various ion channels, transporters, and RPE-specific markers. The primary objective of this study was to use ARPE-19 as an in vitro model for screening the presence of SMVT and to delineate the uptake mechanism of biotin. The cell line is utilized to study the uptake of ganciclovir and its biotin-conjugated prodrug (i.e., biotin-GCV). Moreover in vivo experimentations to delineate vitreal pharmacokinetic profiles and retinal concentration were performed on the anesthetized New Zealand albino rabbits following intravitreal injections of ganciclovir and biotin-GCV.
Materials
[3H] Biotin (specific activity 44.4 Ci/mmol; radiochemical purity > 98.6%) was obtained from Amersham Biosciences. Unlabeled biotin, desthiobiotin, pantothenic acid, lipoic acid, amiloride, 2, 4 DNP, ouabain, sodium azide, biotin methyl ester, biocytin, biotin-dextran and all other chemicals were purchased from Sigma Chemicals Co (St. Louis, MO). Biotin-fluorescein was procured from Pierce Biotechnology (Rockford, IL). GCV was a generous gift from Hoffman La Roche (Nutley, NJ). Biotin conjugate of GCV (Biotin-GCV) was synthesized in our laboratory. All chemicals were of special reagent grade and were used as received without further purification. Culture flasks (75 cm2 growth area) and 12-well plates (3.8 cm2 growth area per well) were procured from MidSci (St. Louis, MO). Primers were customized and obtained from Invitrogen Life Technologies. Concentric probes (CMA/20, 0.5 × 10 mm polycarbonate membrane and 14-mm shaft) employed for sampling the vitreous chamber. Microinjection pump (CMA/100) for perfusing the isotonic buffer saline was obtained from CMA/Microdialysis (Acton, MA). Ketamine hydrochloride was purchased from Fort Dodge Animal Health (Fort Dodge, IA) and Xylazine was obtained from Bayer Animal Health. Topical wells were custom-made by Hansen Ophthalmic Development Corporation (Iowa City, IA), according to special instructions.
Animals
Adult male New Zealand albino rabbits weighing between 2 and 2.5 kg were obtained from Myrtle's Rabbitry (Thompson Station, TN). This research was conducted under aseptic conditions strictly under the regulation of ARVO statement for the use of animals in ophthalmic and vision research. Protocol for performing all the surgical procedure was also approved by Institutional Animal Care and Use Committee (IACUC) of the University of Missouri–Kansas City.
Methods
Synthesis
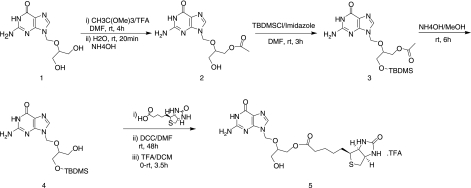
Compounds 2, 3, and 4 were synthesized according to the following published procedure.26
Synthesis of Biotin-GCV-OH(5)
Commercially available biotin (991 mg, 4.06 mmol) was taken in a reaction vessel. Dry DMF (20 mL) was added to dissolve and the solution was cooled down to 0°C using on ice bath. DCC (838 mg, 4.06 mmol) was added and the mixture stirred for 1 h at the same temperature. In a separate reaction flask OTBDMS-GCV-OH(4) (1 g, 2.71 mmol) was dissolved in DMF. DMAP (413 mg, 3.38 mmol) was added with continued stirring for 10 min at room temperature under inert atmosphere to activate the hydroxyl group of OTBDMS-GCV-OH. To the reaction mixture it was added through a syringe. The mixture was brought to the room temperature with continued stirring for 48 h. A small portion of the reaction mixture was taken out and injected in to LC/MS to ensure complete conversion of the starting material to product. The reaction mixture was filtered and solvent evaporated at room temperature under reduced pressure to obtain the crude product. The product OTBDMS-GCV-Biotin was purified by silica gel column chromatography with 10% MeOH/DCM as an eluent with 52% yield. OTBDMS-GCV-Biotin was treated with 80% TFA/CH2Cl2 at 0°C for 3.5 h and the solvent was evaporated under reduced pressure to constant weight. The crude product was purified by recrystallization from cold diethyl ether to obtain the final product5 as a TFA salt with an excellent yield (92%). The prodrug was allowed to dry under vacuum for 10 h.
NMR data of Biotin-GCV-OH(5)
White solid; LC/MS(M/z): 481.3; 1HNMR(DMSO-D6): δ 1.27 – 1.34 (m, 2H, COCH2CH2CH2), 1.40 – 1.52 (m, 2H, COCH2CH2CH2CH2), 1.55 – 1.62 (m, 2H, COCH2CH2), 2.21 – 2.25 (t, J = 8Hz, 2H, SCH2), 2.56 (m, 1H, COCH2), 2.80 – 2.84 (m, 1H, COCH2), 3.05 – 3.10 (m, 1H, SCH), 3.31 – 3.35 (m, 2H, HOCH2), 3.36 – 3.37 (m, 1H, NHCH), 3.65 – 3.68 (m, 1H, NHCH), 4.07 – 4.10 (m, 2H, -OCH2) 4.12 – 4.15 (m, 1H, OCH), 4.29 – 4.33 (m, 1H, NH), 5.34 (s, 2H, NCH2O), 6.38 (brs, 1H, NHCONH), 6.44 (brs, 1H, NHCONH), 6.56 (brs, 2H, NH2), 7.82 (s, 1H, NCH), 10.69 (brs, 1H, OH); 13C NMR(DMSO-D6): 24.44, 27.96, 33.14, 55.38, 59.20, 59.90, 61.05, 62.62, 66.59, 70.38, 71.83, 72.03, 116.48, 137.73, 151.45, 153.95, 156.82, 162.75, 172.78
Cell culture
ARPE-19 cells (passages 18–25) were cultured in D-MEM/F-12 containing 10% heat-inactivated fetal bovine serum, 15 mM hydroxyl ethyl piperazine ethane sulfonic acid (HEPES), 29 mM sodium biocarbonate, penicillin (100 units/mL), streptomycin (100 μg/mL), and were maintained at 37°C, in a humidified atmosphere of 5% CO2 and 90% relative humidity. The medium was replaced every other day. For uptake studies, cells were plated at a density of 250,000 cells per well on 12-well culture plates. The uptake studies were carried out on 21-day old culture.
Uptake experiments
Uptake studies were conducted based on our earlier published method with slight modifications.27 Briefly, at 21 days postseeding, medium was removed and cells were washed twice, 15 min each with 2 mL Dulbecco's phosphate-buffered saline (DPBS), pH 7.4, containing 130 mM NaCl, 2.5 mM KCl, 7.5 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgSO4, 5 mM glucose, and 20 mM HEPES (unless otherwise specified). Then 1 mL of solutions with 0.5 μCi/mL of [3H]biotin in the presence and absence of various competing substrates were added into the wells. After the incubation period, the solution was aspirated off and cells were rinsed three times with 2 mL of ice-cold stop solution (200 mM KCl and 2 mM HEPES). Then the cells were lysed by adding 1 mL of 0.3 N NaOH containing 0.1% Triton-X 100 solution to each well and then leaving overnight at room temperature. Aliquots (500 μL) from each well were transferred to scintillation vials containing 5 mL of scintillation cocktail (Fisher Scientific, Fair Lawn, NJ). Cellular radioactivity was quantitated with a scintillation counter (Model LS-6500; Beckman Counter, Fullerton, CA). The amount of protein was measured by the method of Bradford with BioRad protein estimation kit (BioRad, Hercules, CA, USA).
Sodium and chloride dependence of uptake
To determine the sodium dependence of uptake process, sodium chloride (NaCl) and sodium phosphate dibasic (Na2HPO4) in DPBS were replaced by equimolar quantities on the choline chloride, and the effects of chloride on uptake of biotin was studied by using sodium phosphate monobasic (NaH2PO4), potassium phosphate monobasic (KH2PO4), and calcium acetate to substrate sodium chloride (NaCl), potassium chloride (KCl), and calcium chloride (CaCl2), respectively, in equimolar quantities.
Concentration dependence studies
Various concentrations (5–250 μM) of unlabeled biotin solutions were prepared with DPBS. Then [3H]biotin was added to each biotin solution to prepare donor solutions for determining concentration-dependent uptake of biotin. The data was fitted to a modified Michaelis-Menten equation and then the apparent affinity constant (Km) and maximum uptake velocity (Vmax) were determined.
Energy dependence
These studies were performed in the presence of 1-mM concentrations of either ouabain (inhibitor of Na+ K+ -adenosine triphosphatase [ATPase]) or sodium azide and 2,4 DNP (inhibitors of oxidative phosphorylation).
Substrate specificity
To delineate the structural requirements for interaction with this vitamin carrier system, uptake experiments were performed with competitive inhibitors such as biotin, pantothenic acid, lipoic acid, a structural analog of biotin (desthiobiotin) at 10 and 200 μM concentrations. Cells were incubated simultaneously with [3H] biotin (0.5 μCi/mL) and two different concentrations of unlabeled compounds as inhibitors, and uptake experiments were conducted as described earlier. Similar uptake study was also conducted in the presence of biotin methyl ester and biocytin. Both biotin methyl ester and biocytin do not carry a free carboxylic acid group in their structures.
Temperature dependent studies
Effect of temperature on the uptake of biotin by ARPE-19 cell line was studied at four different temperature viz., 37°C, 25°C, 10°C, and 4 °C. Uptake rate (ln(V)) versus 1/T was plotted (Fig. 1), and activation energy (Ea) was calculated using Arrhenius equation.
FIG. 1.
Schematic representation of the synthesis of Biotin-GCV.
Gene expression using reverse transcription–polymerase chain reaction (RT-PCR)
The presence of SMVT on ARPE-19 was determined with Reverse Transcription–Polymerase Chain Reaction (RT-PCR). Total RNA was extracted from ARPE-19 with a standard protocol. Briefly, cells were transferred to 800 μL of Tri-reagent LS (Molecular Research Center, Inc., Cincinnati, OH), homogenized, and transferred to eppendorf tubes (Fremont, CA). RNA was extracted by phenol-CHCl3-isopropanol method, purified, and resuspended in 20 μL of RNase-DNase-free water. RT-PCR was performed according to a reported method of Sugawara and colleagues,28 with slight modification, using 1 μg of total RNA isolated from ARPE-19 cells. Primers used for the amplification of human SMVT were: forward 5′-CGATTCAATAAAACTGTGCGAGT-3′ and reverse 5′-GGACAGCCACAGATCAAAGC-3′. These primers were adopted from a published human SMVT cDNA sequences.29 The conditions for reverse transcription were as follows: denaturation of the template RNA for 10 min at 70°C; reverse transcription for 60 min at 42°C. The condition of PCR amplification were as follows: denaturation for 45 s at 94°C; annealing for 1 min at 58°C and extension for 45 s at 72°C; 32 cycles; final extension for 10 min at 72°C. PCR product was analyzed by gel electrophoresis on 0.8% agarose in TAE buffer and visualized under UV.
In vivo retinal uptake studies
These studies were performed according to a previously published method.30 For in vivo retinal uptake studies, 100-μL Biotin-GCV prodrug solution (dose equivalent to 58.04 μg GCV or 0.227 μmol) in the presence or absence of biotin (250 mg or 0.98 μmol) was administered by intravitreal injections to anesthetized New Zealand Albino Rabbits into the mid-vitreous region, using a specially prepared canula fitted with a 30-gauge needle. Vitreal and retinal-choroid GCV concentrations were determined following intravitreal administration using published method.30 At the end of an experiment (4 h), animals were euthanized with an overdose of sodium pentobarbital injection through the marginal ear vein. Briefly, eyes were enucleated and carefully vitreous humor was collected, then retina/choroid/sclera part was removed and washed with ice-cold HEPES stop solution. Following washing, the retina/choroid was then carefully extracted and lysed overnight (24 h) in 1 mL of 1 N NaOH at 37°C. Lysed samples were subsequently homogenized and centrifuged at 12,000 rpm for 15 min at 4°C. The supernatant was analyzed for the protein content using Bradford with BioRad protein estimation kit. To the 100 μL of the supernatant a 200 μL of ice-cold trichloroacetic acid was added (10% wt/vol). The mixture was subsequently vortexed and centrifuged at 12,000 rpm for 15 min at 4°C. To the 100 μL of the supernatant 400 μL water was added. Final samples were analyzed for total GCV content. Uptake data was normalized to the protein content. Vitreous humor samples were simply diluted 10 times in water and total GCV content determined by HPLC.
Cell proliferation assay
The assay was carried out to determine any cytotoxicity of Biotin-GCV on the ARPE-19 cells in comparison to dimethyl sulfoxide (DMSO). For cell proliferation assay CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay Kit (Promega, Madison, WI) was used. The assay determines the number of cells in proliferation by utilizing colorimetric method. Briefly, cells (ARPE-19) were plated in 96-well plates. Solutions of GCV and Biotin-GCV (1.5 mM) were made in the culture medium and appropriate volumes were added to make up the final volume to 100 μL in each well. Cells were then incubated at 37°C in a humidified 5% CO2 atmosphere. Effect of drug and prodrug on the cell proliferation was observed by comparing the intensity of absorbance. Also the DMSO was used as a positive control to examine its cellular toxicity.
Posterior chamber microdialysis
Posterior chamber microdialysis was utilized to study the pharmacokinetic profile of both GCV and Biotin-GCV. Here samples are collected as a dialysate from the vitreous humor.
Probe implantation in the vitreous required a 22-gauge needle which was carefully inserted in the posterior part of the eye, that is, about 3 mm below the corneal-scleral limbus through the pars plana. The needle was retracted and immediately the concentric probe was placed through the hole created by the needle. The probe was then adjusted so that it was positioned approximately at the center of vitreous. The probe outlet was fixed in order to prevent any disturbance during the collection of sample. Similar to anterior chamber microdialysis the probe was perfused by isotonic phosphate-buffered saline (IPBS) (pH 7.4) at a flow rate of 2 μL/min using the microdialysis pump. Animals implanted with the probes were left for recovery period of 2 h. Following recovery, intravitreal solutions of drug or prodrug were administered with a 30-gauge needle fitted to specially designed canula. Samples were collected every 20 min for a duration of 10 h. The animals were kept under anesthesia (administered and maintained similarly as mentioned earlier) throughout the experiment right before the implantation of probe and including recovery period.
Following the end of experiments animals were euthanized by administering the overdose of sodium pentobarbital through the marginal ear vein.
Data analysis
Uptake data from the concentration-dependent studies were fitted to Michaelis-Menten equation (Eq. 1).
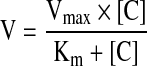 |
(Eq. 1) |
V is the total uptake rate, Vmax is the maximum uptake rate for carrier-mediated process, Km (Michaelis-Menten constant) is the concentration at half the maximum velocity and [C] is the substrate concentration. Data analysis was performed according to Equation 1 with a nonlinear least square regression analysis program (Kaleida Graph Version 3.09, Synergy Software, PA).
In vitro probe calibration
In vitro probe calibration or recovery was performed by placing the semipermeable membrane portion of the probes (linear or concentric) in the known concentration of compound (solution made in IPBS, pH7.4) under study. The probes were then perfused at a flow rate of 2 μL/min with IPBS and dialysate was collected every 20 min. Recovery of drug or prodrug was calculated with equation 2.
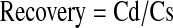 |
(Eq. 2) |
Where Cd is the dialysate concentration and Cs is the known concentration of compound dissolved in IPBS.
Concentrations of respective drug or prodrug in aqueous or vitreous humor (in a pharmacokinetic experiment) can be calculated by dividing the dialysate sample concentration with the in vitro recovery value obtained by equation 2.
Statistical analysis
All uptake studies were performed in atleast quadruplicate and results are expressed as mean ± SD. Unpaired student's t-test was applied to calculate statistical significance. A difference between the means was considered significant at P ≤ 0.05.
Results
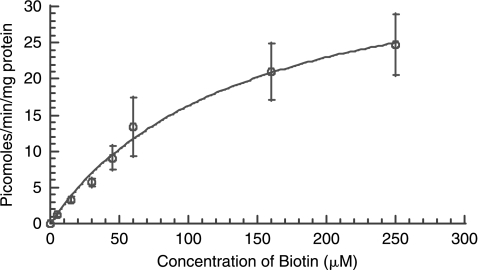
Uptake of [3H] biotin in ARPE-19 cells was linear over 7 min at 37°C and reached equilibrium after 10 min. Therefore, an uptake time of 7 min was selected as standard for all subsequent uptake experiments. In order to study saturation kinetics, cells were incubated at various concentrations of unlabeled biotin for 7 min at 34°C. [3H] Biotin uptake by ARPE-19 was shown to be concentration dependent and saturable with a Km and Vmax of 138.25 μM and 38.85 picomoles/mg of protein/min, respectively [r2 = 0.99] (Fig. 2).
FIG. 2.
Uptake of [3H] biotin by ARPE-19 as a function of substrate concentration at 37°C, pH 7.4. Each data point represents the mean ± S.D. of 4–6 separate uptake determinations.
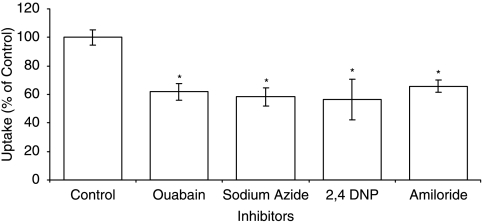
To delineate the role of sodium on biotin uptake, experiments were conducted in sodium-free buffer. As depicted in Fig. 3, a marked decrease in biotin uptake is evident in the absence of sodium ions. In contrast, the uptake of biotin was unaltered in chloride-free buffer (Fig. 3). Energy requirement for the uptake process in to ARPE-19 was determined with metabolic inhibitors. The results indicate ouabain, sodium azide and 2,4 DNP caused a significant reduction in biotin uptake (Fig. 4).
FIG. 3.
Uptake of [3H] biotin by ARPE-19 in the presence and absence of sodium and chloride ion. Each data point represents the mean ± SD of 4–6 separate uptake determinations. Asterisk (*) represents significant difference from the control (P < 0.05).
FIG. 4.
Uptake of [3H] biotin by ARPE-19 cells in the presence of ouabain (1 mM), sodium azide (1 mM), 2,4 DNP, and amiloride. Each data point represents the mean ± SD of 4–6 separate uptake determinations. Asterisk (*) represents significant difference from the control (P < 0.05).
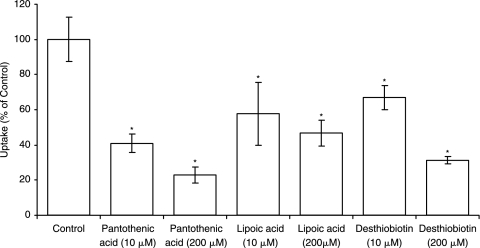
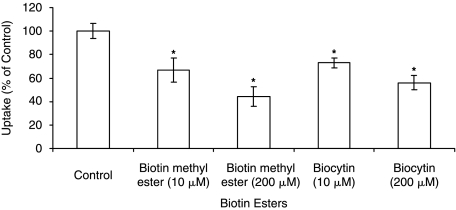
Substrate specificity at two different concentrations (10 and 200 μM) was determined by performing uptake experiments in the presence of unlabeled biotin analog desthiobiotin. Similar uptake experiments were performed in the presence of pantothenic acid and lipoic acid which are known substrates of SMVT. In all cases, [3H] biotin uptake by ARPE-19 was significantly inhibited in a concentration-dependent manner (Fig. 5). Since biotin has a functional carboxylic group, we further studied the involvement of such a functional group in the carrier-mediated process. Thus uptake was performed in the presence of biocytin and biotin methyl ester which are devoid of a free carboxylic acid group. Both biocytin and biotin methyl ester caused significant concentration-dependent lowering in uptake (Fig. 6).
FIG. 5.
Uptake of [3H] biotin by ARPE-19 in the presence of pantothenic acid (10 μM and 200 μM), lipoic acid (10 μM and 200 μM), and desthiobiotin (10 μM and 200 μM). Each data point represents the mean ± SD of 4–6 separate uptake determinations. Asterisk (*) represents significant difference from the control (P < 0.05).
FIG. 6.
Uptake of [3H] biotin by ARPE-19 cells in the presence of biotin esters such as biotin methyl ester (10 μM and 200 μM) and biocytin (10 μM and 200 μM). Each data point represents the mean ± SD of 4–6 separate uptake determinations. Asterisk (*) represents significant difference from the control (P < 0.05).
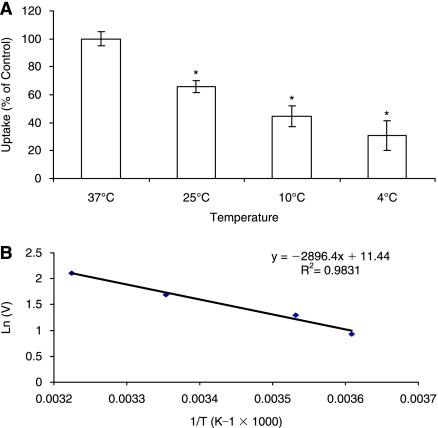
Effect of temperature on the uptake of biotin by ARPE-19 was studied. Initial uptake rates of biotin were 8.23 ± 0.428, 5.41 ± 0.357, 3.67 ± 0.604, and 2.53 ± 0.862 fmol min−1 (mg of protein)−1 at 37°C, 10°C, 25°C, and 4°C, respectively. Uptake significantly diminished as incubation temperature was lowered, suggesting that the process is sensitive to temperature. Uptake rate (ln(V))vs 1/T was plotted (Fig. 7), and activation energy (Ea) was obtained as 5.75 kcal/mol.
FIG. 7.
(A) Uptake of [3H] biotin by ARPE-19 cells as a function of temperature (37°C, 25°C, 10°C, and 4°C). Each data point represents the mean ± SD of 4–6 separate uptake determinations. Asterisk (*) represents significant difference from the control (P < 0.05). (B) Arrhenius plot of the effect of temperature on uptake of [3H] biotin by ARPE-19 cells.
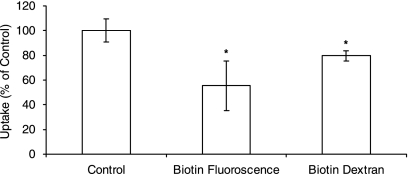
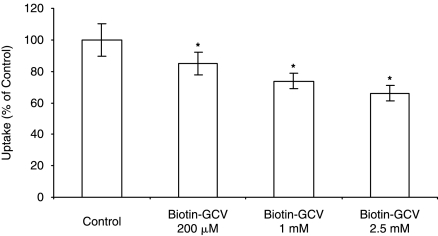
Studies were also performed to establish whether biotin conjugates are able to interact with the SMVT. Uptake of radiolabeled biotin were performed in the presence of biotin-fluorescein (mol wt: 732.80 Da) and biotin-dextran (mol wt ∼ 10,000 Da). Uptake rate of labeled biotin significantly diminished in the presence of biotin-fluorescein and biotindextran (Fig. 8). Further uptake studies with Biotin-GCV conjugate has shown that higher concentrations of Biotin-GCV have decreased the uptake rate significantly (Fig. 9).
FIG. 8.
Uptake of [3H] biotin by ARPE-19 cells in the presence of Biotin-Fluorescein (200 μM) and Biotin-dextran (200 μM). Each data point represents the mean ± SD of 4–6 separate uptake determinations. Asterisk (*) represents significant difference from the control (P < 0.05).
FIG. 9.
Uptake of [3H] biotin by ARPE-19 cells in the presence of Biotin-GCV at 200 μM, 1 mM, and 2.5 mM concentrations. Each data point represents the mean ± SD of 4–6 separate uptake determinations. Asterisk (*) represents significant difference from the control (P < 0.05).
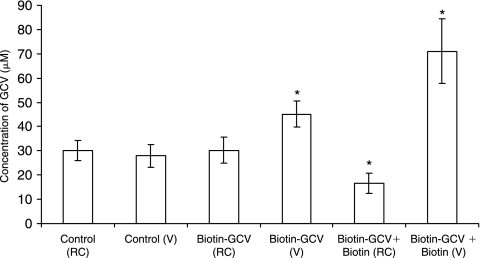
In vivo retinal uptake studies were performed, to evaluate the total retinal accumulation of GCV and GCV remaining in vitreous. Four hours after intravitreal concentrations of total GCV were obtained following administration of GCV, Biotin-GCV and Biotin-GCV along with biotin (biotin+ Biotin-GCV). Such studies revealed that total GCV accumulated in retina-choroid (RC) following intravitreal administration of GCV and Biotin-GCV was similar. However it was significantly lower in case of biotin+Biotin-GCV. But vitreal (V) levels of GCV in case of Biotin-GCV and biotin+ Biotin-GCV was significantly elevated relative to GCV alone (Fig. 10).
FIG. 10.
Retina-choroid (RC) and vitreal (V) ganciclovir (GCV) concentrations 4 h after administration to rabbits of GCV (control) or the prodrugs alone and in the presence of biotin. Each data point represents the mean ± SD of 4 separate retinal uptake and vitreal GCV concentration determinations. Asterisk (*) represents significant difference from the control (P < 0.05).
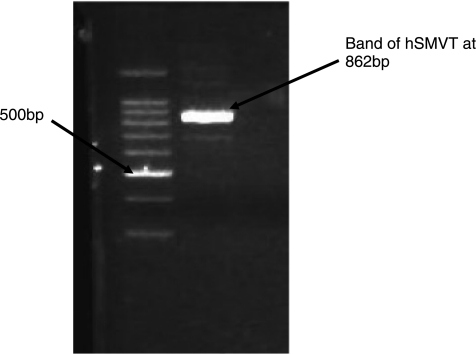
Molecular identification of SMVT was conducted with RT-PCR. PCR products were analyzed by gel electrophoresis on 0.8% agarose. cDNA generated from total RNA isolated from ARPE-19 was PCR amplified with the primers specific for human SMVT sequence. The band at 862 bp during the gel electrophoresis confirms the presence of hSMVT according to the previously published data.31 A band between 800 and 900 bp corresponding to hSMVT can be observed in Figure 11 (lane 2).
FIG. 11.
hSMVT cDNA was generated by RT-PCR amplification of total RNA from ARPE-19 cells (lane 2). Aliquots of PCR products were analyzed by gel electrophoresis on 0.8% agarose. Ethidium bromide staining of the gel showed a ∼862 bp band corresponding to the amplified rabbit SMVT cDNA (lane 3). Lane 1: 1-kbp DNA ladder.
Cell proliferation assay
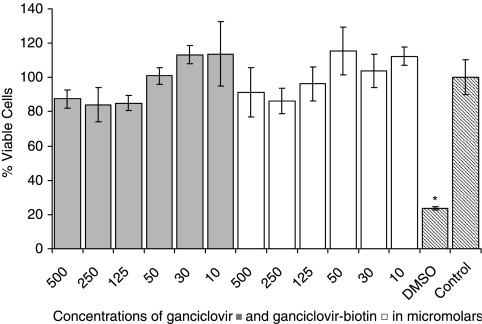
Results indicate that both GCV and Biotin-GCV are not cytotoxic even at 500 μM in either cell types. However, DMSO which acts as a positive control showed considerably low % viable cells in both the cases. Culture medium was used as a control for both types of cells and the % viability for control were kept to 100% and rest of the data was normalized to the control (Fig. 12).
FIG. 12.
ARPE-19 cell-proliferation assay showing the percentage viable cells in presence of various concentrations of GCV and Biotin-GCV. Each data point represents the mean ± SD of 4–6 separate determinations. Asterisk (*) represents significant difference from the control (P < 0.05).
Vitreal pharmacokinetics of GCV and Biotin-GCV following intravitreal administration
The vitreal concentration profile of GCV is depicted in Figure 13. Intravitreal dose of GCV consisted of 0.227 μmol (58.04 μg GCV) in a volume of 50 μL sterile IPBS, pH 7.4. The results generated from the non compartmental analysis of concentration-time profile of GCV are tabulated in the Table 1. Vitreal elimination half-life of GCV was 270 ± 15.7 min. Clearance (Cl) and area under the vitreous time concentration curve (AUC) values of GCV were 4.39 ± 0.603 μL/min and 10.6 ± 1.27 mg*min.mL−1, respectively.
FIG. 13.
Vitreous concentration-time profile of ganciclovir (GCV). Mean values are represented (n = 4).
Table 1.
Vitreous Pharmacokinetic Parameters of GCV and Biotin-GCV Following Intravitreal Administration
| |
|
(Biotin-GCV) |
|
|---|---|---|---|
| Parameters | GCV | Biotin-GCV | Regenerated GCV |
| AUC (mg.min.mL−1) | 10.6 ± 1.27 | 17.5 ± 1.38* | 1.85 ± 0.744 |
| λz (×10−3 min−1) | 2.58 ± 0.124 | 3.19 ± 0.536 | |
| T1/2 (min) | 270 ± 15.7 | 222 ± 40.5 | |
| Vss (mL) | 1.56 ± 0.100 | 1.47 ± 0.106 | |
| Cl (μL.min−1) | 4.39 ± 0.603 | 5.45 ± 0.673 | |
| MRT last (min) | 197 ± 22.2 | 175 ± 17.6 | 264 ± 9.26 |
| Clast (μg/mL) | 7.06 ± 1.38 | 8.28 ± 2.27 | |
| Cmax = 5.37 ± 0.435 μg/mL | |||
| Tmax = 66.7 ± 23.1 min | |||
Each data point represents the mean ± SD of four separate determinations. Asterisk (*) represents significant difference from the control (P < 0.05).
AUC: Area under the vitreous time concentration curve; λz: Elimination rate constant; T1/2: Vitreal elimination half-life; Vss: Volume of distribution at steady; Cl: Clearance state; MRT: Mean residence time.
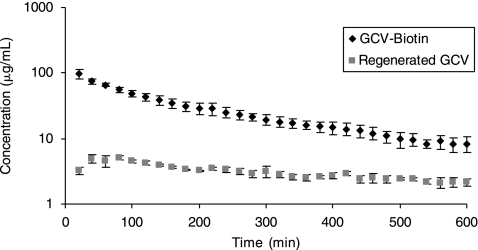
Vitreous concentration-time profiles of the Biotin-GCV and the regenerated GCV from the intravitreal dose of Biotin-GCV are depicted in Figure 14. The vitreal elimination half-life of Biotin-GCV was 222 ± 40.5 min and was not found to be statistically different than the GCV. However, AUC of Biotin-GCV, that is, 17.5 ± 1.38 mg*min.mL−1, was significantly higher than GCV. Other parameters such as elimination rate constants, volume of distribution at steady state, clearance, mean residence time and even Clast were not significantly different for Biotin-GCV relative to GCV. The results generated from the non compartmental analysis of concentration time profile of Biotin-GCV and regenerated GCV are summarized in the Table 1.
FIG. 14.
Vitreous concentration-time profiles of Biotin-GCV and regenerated GCV. Mean values are represented (n = 4).
Figure 14 also reveals that sustained level of GCV is generated following intravitreal administration of Biotin-GCV. Although the level of GCV obtained following intravitreal injection of GCV is higher compared to the level of regenerated GCV achieved due to administration of Biotin-GCV, but it is considerably higher than the minimum inhibitory concentrations (0.25–1.22 μg/mL) required.32
Discussion
Biotin is a water soluble vitamin required for normal cellular function, growth, and development. This vitamin is not synthesized in the body and must be supplemented. Therefore, specific transport system may be involved in cellular translocation. One such system (SMVT) belongs to the class of multiple vitamin transporters in human placenta that co-transports biotin, pantothenate, and lipoate.33,34 SMVT has also been identified in other human cell membranes. It belongs to the family of sodium-dependent glucose transporter and is known to share significant amino acid sequence homology with the members of the family.35
Our results demonstrate the biotin uptake in ARPE-19 to be saturable at micromolar concentration range, with Km of 138.25 μM and Vmax of 38.85 pmoles/mg of protein /min. These results are also consistent with previous reports of saturable biotin uptake in other cell types.19,23,34,36–38 Previously, it has been shown that the apparent Km for SMVT is in micromolar range.18,19,21–24 Further, uptake of biotin was inhibited by pantothenic and lipoic acid indicating that this transporter is not specific for biotin. Similar results had been reported in previous studies in various cell lines, and in all cases SMVT was suggested as the transporter involved.23,37,39
Uphill transport of SMVT substrates is energized by a transmembrane sodium gradient as well as the membrane potential.37 In this study, biotin transport via SMVT is found to be coupled with the electrochemical gradient of Na+ but not H+. Reduced uptake in the presence of amiloride, a sodium ion transport inhibitor, further strengthens the result that sodium is required for the functioning of SMVT. Even though other studies have shown that some active transport system require specific coupling of a downhill Cl−gradient into the cells, but rate of biotin uptake remained unaltered in the absence of chloride ion in the buffer.
Uptake of biotin in ARPE-19 diminished in the presence of ouabain, suggesting that this process is dependent on the normal function of Na+K+-ATPase. Further, sodium azide and 2,4 DNP that block oxidative phosphorylation also caused diminished uptake. These results confirm that biotin uptake into ARPE-19 is an energy (ATP) dependent process.
Substrate specificity studies revealed that the transporter is specific for biotin (and certain biotin analogs), pantothenic acid and lipoic acid. Interestingly, uptake process was significantly inhibited by biocytin and biotin methyl ester which was not found in our previous studies with rPCECs.27 These results along with previous reports suggest that a free valeric carboxylic group of biotin may not always be necessary for specific binding to the transporter.37 Although results from this study are contradictory to what were observed previously, such contradictions do exist in literature.40 Still the molecular evidence using RT-PCR studies confirm the presence of SMVT in the retinal pigmented epithelium (ARPE-19 cells).
Results from the uptake of biotin in presence of biotin-conjugated compound such as biotin-fluorescein (mol wt: 732.80 Da) and biotin-dextran (mol wt ∼ 10,000 Da) have revealed that uptake rate of labeled biotin was significantly lower in the presence of biotin-fluorescein and biotin-dextran. One aim of this study was to deliver GCV into targeted cells that is, retina and therefore the molecular weight of biotin conjugated with GCV via an ester bond would fall in the range which is even lower than biotin-fluorescein. Further studies were thus performed to synthesize Biotin-GCV conjugate. Such a conjugate generates higher concentrations of GCV in target cells/tissues relative to parent drug.
Another set of studies were performed which involves use of animals to test whether such transport system present on rabbit retina would recognize the prodrug (Biotin-GCV) and transport it across through a carrier mediated process. In vivo retinal uptake experiment was conducted to establish how much of the total GCV (may be in the form of drug and prodrug) accumulates in the retina/choroid versus in the vitreous humor, after 4 h of administration following an intravitreal injection of equimolar concentration of GCV or Biotin-GCV. Similar experiments were performed when Biotin-GCV was administered with excess dose of unconjugated biotin. Results clearly suggest that accumulation of GCV in retina/choroid following intravitreal dose of GCV or Biotin-GCV is similar, while the vitreous levels of total GCV left were more in case of Biotin-GCV. It is possible that either the elimination of Biotin-GCV is slower or some amount of GCV is regenerated in the vitreous. However, a comparison equimolar doses of drug and prodrug clearly suggest that Biotin-GCV permeability into the retina-choroid is significantly higher and total elimination of GCV (i.e., GCV and Biotin-GCV) from the vitreous is slower. Finally the data also reveal that Biotin-GCV accumulation in retina-choroid diminishes while concentrations left in vitreous increases in the presence of excess dose of biotin. This observation clearly suggests that Biotin-GCV does interact with the transporter present on the retina and biotin competitively inhibits its uptake in the retina.
Principle of diffusion governs the sampling using the techniques called microdialysis. The technique involves implantation of special microdialysis probes either in vitreous or aqueous or both ocular chambers. A concentration gradient results in the diffusion through the semipermeable membrane which is placed in the tissue or fluids under study and probes are continuously perfused with a physiological solution (isotonic phosphate-buffered saline or IPBS). The technique allows dynamic sampling of aqueous and vitreous humor without euthanizing the animal. Microdialysis has served as an important tool in sampling of ocular fluids both by reducing the number of animals and providing statistically powered data.41–47
The main objective of this study was to perform the ocular pharmacokinetics of GCV and its prodrug (Biotin-GCV). The pharmacokinetic studies were done to conclude whether the prodrug had better or if not comparable pharmacokinetic profiles to parent drug. Results could be extrapolated for their pharmacological efficacy in the treatment of CMV retinitis. Prodrugs by themselves are inactive and needs to be converted to the parent compound for their efficacy. However, if the role of prodrug is to increase the permeability of parent drug into a cell or tissue, such as observed in this study, then higher drug concentrations can be generated in retina/choroid resulting in higher virustatic activity.
The prodrug proposed in the current study has shown to interact with the vitamin transporter, SMVT, and to compete with biotin in the transcellular passage through SMVT. Thus, Biotin-GCV permeates the cell membrane of corneal epithelium and retinal pigment epithelium by a carrier-mediated process. Such processes can altogether alter the pharmacokinetic profile of a parent drug.
Results from both in vitro and in vivo uptake studies on ARPE-19 (Fig. 9) and retina/choroid (Fig. 10) respectively suggest that Biotin-GCV has affinity toward SMVT. An in vivo retinal uptake study confirms that Biotin-GCV transport into the retina-choroid was decreased in the presence biotin. Such data clearly suggest that Biotin-GCV uptake into retina-choroid is mediated by a carrier that also recognizes biotin. Such transport system needs to have a broad substrate specificity and capacity to allow the accumulation of Biotin-GCV into RPE cells.
Vitreal Pharmacokinetic data of drug (GCV) and prodrug (Biotin-GCV) seems to differ only in the AUC values. Vitreal concentration-time profile following administration of Biotin-GCV reveals the regeneration of GCV. The concentrations of regenerated GCV during the entire set of study were above the minimum inhibitory concentrations (0.25–1.22 μg/mL) required for GCV.32 This result along with the MRT values of regenerated GCV suggest that not just the concentrations were above MIC but also were sustained compared to the simple administration of GCV. AUC of regenerated GCV was almost 1/6th of the AUC of GCV. Thus Biotin-GCV administration increases the total residence of GCV and thereby would be more effective in controlling viral replication.
Retinal uptake studies were performed by administration of equimolar concentrations of GCV and Biotin-GCV and evaluating the concentration of cumulative GCV attained in the retina-choroid and the vitreous humor. Results from such studies reveal that even though the cumulative amount of GCV achieved in the retina-choroid is same in case of both drug and prodrug, higher cumulative concentrations of GCV were found in vitreous humor. Higher permeation of Biotin-GCV along with it slower elimination causes such high GCV concentrations. Similar PK profiles of Biotin-GCV and GCV prolongs the residence time of GCV and thus provides nearly similar concentrations in retina-choroid, for a longer period.
Conclusions
In conclusion, this study demonstrates molecular and functional evidence of a sodium dependent multivitamin carrier system, SMVT, in the human retinal pigment epithelium ARPE-19. GCV prodrug (Biotin-GCV) is recognized by this transport system on both ARPE-19 cell line and rabbit retina. Moreover, Biotin-GCV was found to have better and more therapeutically desirable pharmacological profile in the vitreous fluid which may be therapeutically more advantageous than the drug itself.
Acknowledgments
This study was supported by National Institutes of Health grants R01 EY 09171-14 and R01 EY 10659-12.
References
- 1.Freeman W.R. Lerner C.W. Mines J.A., et al. A prospective study of the ophthalmologic findings in the acquired immune deficiency syndrome. Am. J. Ophthalmol. 1984;97:133–142. doi: 10.1016/s0002-9394(14)76082-9. [DOI] [PubMed] [Google Scholar]
- 2.Bodaghi B. Slobbe-van Drunen M.E. Topilko A., et al. Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Invest. Ophthalmol. Vis. Sci. 1999;40:2598–2607. [PubMed] [Google Scholar]
- 3.Bodaghiand B. Michelson S. Cytomegalovirus: Virological facts for clinicians. Ocul. Immunol. Inflamm. 1999;7:133–137. doi: 10.1076/ocii.7.3.133.3998. [DOI] [PubMed] [Google Scholar]
- 4.Burd E.M. Pulido J.S. Puro D.G., et al. Replication of human cytomegalovirus in human retinal glial cells. Invest. Ophthalmol. Vis. Sci. 1996;37:1957–1966. [PubMed] [Google Scholar]
- 5.Pepose J.S. Holland G.N. Nestor M.S., et al. Acquired immune deficiency syndrome. Pathogenic mechanisms of ocular disease. Ophthalmology. 1985;92:472–484. doi: 10.1016/s0161-6420(85)34008-3. [DOI] [PubMed] [Google Scholar]
- 6.Rao N.A. Zhang J. Ishimoto S. Role of retinal vascular endothelial cells in development of CMV retinitis. Trans. Am. Ophthalmol. Soc. 1998;96:111–123. discussion 124–126. [PMC free article] [PubMed] [Google Scholar]
- 7.Magone M.T. Nussenblatt R.B. Whitcup S.M. Elevation of laser flare photometry in patients with cytomegalovirus retinitis and AIDS. Am. J. Ophthalmol. 1997;124:190–198. doi: 10.1016/s0002-9394(14)70783-4. [DOI] [PubMed] [Google Scholar]
- 8.Nussenblattand R.B. Lane H.C. Human immunodeficiency virus disease: Changing patterns of intraocular inflammation. Am. J. Ophthalmol. 1998;125:374–382. doi: 10.1016/s0002-9394(99)80149-4. [DOI] [PubMed] [Google Scholar]
- 9.Jabs D.A. Van Natta M.L. Thorne J.E., et al. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 2. Second eye involvement and retinal detachment. Ophthalmology. 2004;111:2232–2239. doi: 10.1016/j.ophtha.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Jabs D.A. Van Natta M.L. Thorne J.E., et al. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 1. Retinitis progression. Ophthalmology. 2004;111:2224–2231. doi: 10.1016/j.ophtha.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Markhamand A. Faulds D. Ganciclovir. An update of its therapeutic use in cytomegalovirus infection. Drugs. 1994;48:455–484. doi: 10.2165/00003495-199448030-00009. [DOI] [PubMed] [Google Scholar]
- 12.Fauldsand D. Heel R.C. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs. 1990;39:597–638. doi: 10.2165/00003495-199039040-00008. [DOI] [PubMed] [Google Scholar]
- 13.Narurkarand M.M. Mitra A.K. Synthesis, physicochemical properties, and cytotoxicity of a series of 5′-ester prodrugs of 5-iodo-2′-deoxyuridine. Pharm. Res. 1988;5:734–737. doi: 10.1023/a:1015968113838. [DOI] [PubMed] [Google Scholar]
- 14.Narurkarand M.M. Mitra A.K. Prodrugs of 5-iodo-2′-deoxyuridine for enhanced ocular transport. Pharm. Res. 1989;6:887–891. doi: 10.1023/a:1015968724007. [DOI] [PubMed] [Google Scholar]
- 15.Hughes P.M. Krishnamoorthy R. Mitra A.K. Effect of acylation on the ocular disposition of acyclovir. I: Synthesis, physicochemical properties, and antiviral activity of 2′-esters. J. Ocul. Pharmacol. 1993;9:287–297. doi: 10.1089/jop.1993.9.287. [DOI] [PubMed] [Google Scholar]
- 16.Dias C.S. Anand B.S. Mitra A.K. Effect of mono- and di-acylation on the ocular disposition of ganciclovir: physicochemical properties, ocular bioreversion, and antiviral activity of short chain ester prodrugs. J. Pharm. Sci. 2002;91:660–668. doi: 10.1002/jps.10072. [DOI] [PubMed] [Google Scholar]
- 17.Zempleniand J. Mock D.M. Uptake and metabolism of biotin by human peripheral blood mononuclear cells. Am. J. Physiol. 1998;275:C382–C388. doi: 10.1152/ajpcell.1998.275.2.C382. [DOI] [PubMed] [Google Scholar]
- 18.Ma T.Y. Dyer D.L. Said H.M. Human intestinal cell line Caco-2: A useful model for studying cellular and molecular regulation of biotin uptake. Biochim. Biophys. Acta. 1994;1189:81–88. doi: 10.1016/0005-2736(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 19.Said H.M. Redha R. Nylander W. A carrier-mediated, Na+ gradient-dependent transport for biotin in human intestinal brush-border membrane vesicles. Am. J. Physiol. 1987;253:G631–G636. doi: 10.1152/ajpgi.1987.253.5.G631. [DOI] [PubMed] [Google Scholar]
- 20.Said H.M. Redha R. Nylander W. Biotin transport in basolateral membrane vesicles of human intestine. Gastroenterology. 1988;94:1157–1163. doi: 10.1016/0016-5085(88)90007-8. [DOI] [PubMed] [Google Scholar]
- 21.Said H.M. Hoefs J. Mohammadkhani R., et al. Biotin transport in human liver basolateral membrane vesicles: A carriermediated, Na+ gradient-dependent process. Gastroenterology. 1992;102:2120–2125. doi: 10.1016/0016-5085(92)90341-u. [DOI] [PubMed] [Google Scholar]
- 22.Said H.M. Ma T.Y. Kamanna V.S. Uptake of biotin by human hepatoma cell line, Hep G2: A carrier-mediated process similar to that of normal liver. J. Cell Physiol. 1994;161:483–489. doi: 10.1002/jcp.1041610311. [DOI] [PubMed] [Google Scholar]
- 23.Said H.M. Ortiz A. McCloud E., et al. Biotin uptake by human colonic epithelial NCM460 cells: A carrier-mediated process shared with pantothenic acid. Am. J. Physiol. 1998;275:C1365–C1371. doi: 10.1152/ajpcell.1998.275.5.C1365. [DOI] [PubMed] [Google Scholar]
- 24.Said H.M. Cellular uptake of biotin: Mechanisms and regulation. J. Nutr. 1999;129:490S–493S. doi: 10.1093/jn/129.2.490S. [DOI] [PubMed] [Google Scholar]
- 25.Luo S. Kansara V.S. Zhu X., et al. Functional characterization of sodium-dependent multivitamin transporter in MDCK-MDR1 cells and its utilization as a target for drug delivery. Mol. Pharm. 2006;3:329–339. doi: 10.1021/mp0500768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaoand H. Mitra A.K. Regioselective synthesis of various prodrugs of ganciclovir. Tetrahedron. Lett. 2000;41:1131–1136. [Google Scholar]
- 27.Janoria K.G. Hariharan S. Paturi D., et al. Biotin uptake by rabbit corneal epithelial cells: Role of sodium-dependent multivitamin transporter (SMVT) Curr. Eye Res. 2006;31:797–809. doi: 10.1080/02713680600900206. [DOI] [PubMed] [Google Scholar]
- 28.Sugawara M. Nakanishi T. Fei Y.J., et al. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J. Biol. Chem. 2000;275:16473–16477. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- 29.Balamurugan K. Vaziri N.D. Said H.M. Biotin uptake by human proximal tubular epithelial cells: Cellular and molecular aspects. Am. J. Physiol. Renal. Physiol. 2005;288:F823–F831. doi: 10.1152/ajprenal.00375.2004. [DOI] [PubMed] [Google Scholar]
- 30.Majumdar S. Kansara V. Mitra A.K. Vitreal pharmacokinetics of dipeptide monoester prodrugs of ganciclovir. J. Ocul. Pharmacol. Ther. 2006;22:231–241. doi: 10.1089/jop.2006.22.231. [DOI] [PubMed] [Google Scholar]
- 31.Kansara V. Luo S. Balasubrahmanyam B., et al. Biotin uptake and cellular translocation in human derived retinoblastoma cell line (Y-79): A role of hSMVT system. Int. J. Pharm. 2006;312:43–52. doi: 10.1016/j.ijpharm.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 32.Machaand S. Mitra A.K. Ocular disposition of ganciclovir and its monoester prodrugs following intravitreal administration using microdialysis. Drug Metab. Dispos. 2002;30:670–675. doi: 10.1124/dmd.30.6.670. [DOI] [PubMed] [Google Scholar]
- 33.Grassl S.M. Human placental brush-border membrane Na(+)-pantothenate cotransport. J. Biol. Chem. 1992;267:22902–22906. [PubMed] [Google Scholar]
- 34.Grassl S.M. Human placental brush-border membrane Na(+)-biotin cotransport. J. Biol. Chem. 1992;267:17760–17765. [PubMed] [Google Scholar]
- 35.Prasadand P.D. Ganapathy V. Structure and function of mammalian sodium-dependent multivitamin transporter. Curr. Opin. Clin. Nutr. Metab. Care. 2000;3:263–266. doi: 10.1097/00075197-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Tak R.V. Pal D. Gao H., et al. Transport of acyclovir ester prodrugs through rabbit cornea and SIRC-rabbit corneal epithelial cell line. J. Pharm. Sci. 2001;90:1505–1515. doi: 10.1002/jps.1101. [DOI] [PubMed] [Google Scholar]
- 37.Prasad P.D. Wang H. Huang W., et al. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch. Biochem. Biophys. 1999;366:95–106. doi: 10.1006/abbi.1999.1213. [DOI] [PubMed] [Google Scholar]
- 38.Baurand B. Baumgartner E.R. Na(+)-dependent biotin transport into brush-border membrane vesicles from human kidney cortex. Pflugers Arch. 1993;422:499–505. doi: 10.1007/BF00375078. [DOI] [PubMed] [Google Scholar]
- 39.Wang H. Huang W. Fei Y.J., et al. Human placental Na+-dependent multivitamin transporter. Cloning, functional expression, gene structure, and chromosomal localization. J. Biol. Chem. 1999;274:14875–14883. doi: 10.1074/jbc.274.21.14875. [DOI] [PubMed] [Google Scholar]
- 40.Ramanathan S. Pooyan S. Stein S., et al. Targeting the sodium-dependent multivitamin transporter (SMVT) for improving the oral absorption properties of a retro-inverso Tat nonapeptide. Pharm. Res. 2001;18:950–956. doi: 10.1023/a:1010932126662. [DOI] [PubMed] [Google Scholar]
- 41.Rittenhouse K.D. Pollack G.M. Microdialysis and drug delivery to the eye. Adv. Drug Deliv. Rev. 2000;45:229–241. doi: 10.1016/s0169-409x(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 42.Hughes P.M. Krishnamoorthy R. Mitra A.K. Vitreous disposition of two acycloguanosine antivirals in the albino and pigmented rabbit models: A novel ocular microdialysis technique. J. Ocul. Pharmacol. Ther. 1996;12:209–224. doi: 10.1089/jop.1996.12.209. [DOI] [PubMed] [Google Scholar]
- 43.Machaand S. Mitra A.K. Ocular pharmacokinetics of cephalosporins using microdialysis. J. Ocul. Pharmacol. Ther. 2001;17:485–498. doi: 10.1089/108076801753266866. [DOI] [PubMed] [Google Scholar]
- 44.Waga J. Ohta A. Ehinger B. Intraocular microdialysis with permanently implanted probes in rabbit. Acta Ophthalmol. (Copenh) 1991;69:618–624. doi: 10.1111/j.1755-3768.1991.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 45.Wagaand J. Ehinger B. Intravitreal concentrations of some drugs administered with microdialysis. Acta Ophthalmol. Scand. 1997;75:36–40. doi: 10.1111/j.1600-0420.1997.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 46.Waga J. Nilsson-Ehle I. Ljungberg B., et al. Microdialysis for pharmacokinetic studies of ceftazidime in rabbit vitreous. J. Ocul. Pharmacol. Ther. 1999;15:455–463. doi: 10.1089/jop.1999.15.455. [DOI] [PubMed] [Google Scholar]
- 47.Stempels N. Tassignon M.J. Sarre S. A removable ocular microdialysis system for measuring vitreous biogenic amines. Graefes Arch. Clin. Exp. Ophthalmol. 1993;231:651–655. doi: 10.1007/BF00921960. [DOI] [PubMed] [Google Scholar]