Abstract
Knowing one's HIV status is particularly important in the setting of recent tuberculosis (TB) exposure. Blood tests for assessment of tuberculosis infection, such as the QuantiFERON Gold in-tube test (QFT; Cellestis Limited, Carnegie, Victoria, Australia), offer the possibility of simultaneous screening for TB and HIV with a single blood draw. We performed a cross-sectional analysis of all contacts to a highly infectious TB case in a large meatpacking factory. Twenty-two percent were foreign-born and 73% were black. Contacts were tested with both tuberculin skin testing (TST) and QFT. HIV testing was offered on an opt-out basis. Persons with TST ≥10 mm, positive QFT, and/or positive HIV test were offered latent TB treatment. Three hundred twenty-six contacts were screened: TST results were available for 266 people and an additional 24 reported a prior positive TST for a total of 290 persons with any TST result (89.0%). Adequate QFT specimens were obtained for 312 (95.7%) of persons. Thirty-two persons had QFT results but did not return for TST reading. Twenty-two percent met the criteria for latent TB infection. Eighty–eight percent accepted HIV testing. Two (0.7%) were HIV seropositive; both individuals were already aware of their HIV status, but one had stopped care a year previously. None of the HIV-seropositive persons had latent TB, but all were offered latent TB treatment per standard guidelines. This demonstrates that opt-out HIV testing combined with QFT in a large TB contact investigation was feasible and useful. HIV testing was also widely accepted. Pairing QFT with opt-out HIV testing should be strongly considered when possible.
Introduction
Tuberculosis (TB) and HIV often afflict the same hosts, with devastating consequences. Persons infected with HIV have significantly higher risk of progression to TB disease after TB infection than persons without HIV, with an annual risk of progression to TB disease of 5–10%, compared with a lifetime risk of 5–10% for HIV-uninfected, otherwise immunocompetent persons.1,2 Furthermore, HIV infection reduces the sensitivity of skin testing for TB infection making diagnosis difficult.3,4
This synergistic interaction of HIV and TB has implications for TB contact investigations. Per Centers for Disease Control and Prevention (CDC) guidelines, persons who are close contacts to a case of infectious TB and who have HIV infection are advised to undergo treatment for latent TB infection regardless of skin test result.3 With 25% of HIV-infected persons unaware of their infection, there are a significant number of persons in a contact investigation who may not receive the appropriate intervention as outlined by the guidelines. Obtaining HIV status during a TB contact investigation can be logistically challenging, but new technologies may overcome some of these difficulties. Blood-based interferon gamma release assays (IGRA) such as the QuantiFERON Gold in-tube test (QFT; Cellestis Limited, Carnegie, Victoria, Australia) offer the possibility of simultaneous screening for TB and HIV with a single blood draw by measuring interferon gamma levels in vitro following stimulation by antigens unique to Mycobacterium tuberculosis. The results of interferon gamma release assays correlate well with the extent of exposure to the source case,4,5 and may reduce the rate of false-positive testing due to prior nontuberculous mycobacterial exposure or Bacille Calmette-Guérin (BCG) vaccination.6 These assays also obviate the need for a second visit (as required for reading the tuberculin skin test), so may reduce health department labor time and increase the proportion of contacts with valid test results. We assessed the feasibility of an approach using opt-out HIV testing paired with QFT after a mass exposure to a highly infectious index TB case.
Materials and Methods
We conducted a retrospective, cross-sectional analysis of persons exposed to a highly infectious TB case in a large workplace. The source case had been ill with pulmonary TB for at least a year prior to diagnosis. The workplace was large and well ventilated, and initial contact investigation of coworkers who had worked most closely with the source patient did not reveal an unexpectedly large number of positive tuberculin skin tests. However, several months after the initial contact investigation, four coworkers were diagnosed with tuberculosis. As only one of these had been identified and tested during the initial contact investigation, screening was expanded to include all workers in the same building as the source case. The screening strategy consisted of symptom screening, limited risk factor assessment, and QFT. As this was the first time that QFT had been clinically used in North Carolina, concurrent tuberculin skin testing was performed (in case of laboratory error or other problems). Persons with a history of a prior positive tuberculin skin test (TST) did not have repeat tuberculin skin testing, but QFT and HIV testing were performed for these persons. Testing was conducted at the workplace and confidentiality was a concern, so no questions about HIV status or risk factors were asked during the interview; HIV testing (antibody followed by pooled viral load) was provided on an opt-out basis.
For the purposes of this investigation, persons who met any of the following criteria were referred to the health department for further evaluation: (1) TST ≥10 mm of induration, (2) positive QFT test, (3) positive HIV test (antibody or pooled viral load testing), or (4) symptoms concerning for TB disease. Persons with a 10 mm or greater tuberculin skin test were considered to have latent TB infection in the absence of a positive QFT as a compromise between sensitivity and specificity in the setting of apparent widespread infection. Previous reports have suggested that moving the cutoff from 5 to 10 mm might reduce the number of positive tests by over 50% without missing persons who would subsequently progress to active TB.5 Persons referred to the health department for any of the above criteria had a more detailed medical history and a postero-anterior chest radiograph. If there was no evidence of active TB, these persons were offered 4 months of rifampin for latent TB treatment, given better tolerability and completion rates when compared to isoniazid.7,8
Statistical Analysis
A dataset was provided to the investigators by Wake County Human Services with all identifiers removed. Use of the dataset for analysis was approved by the Duke University Institutional Review Board. SAS version 9.1 (SAS Institute, Cary, NC) was used for data analysis. Categorical variables were analyzed using Fisher's exact test or the χ2 test, as appropriate, with statistical significance defined by a p value of <0.05.
Results
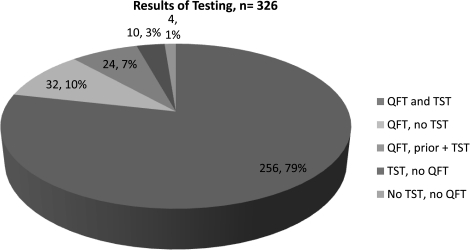
Three hundred twenty-six people were screened onsite (Table 1). Of those, the mean age was 44 (range, 21–67) with 56.8% being female. Twenty-two percent were foreign born and 11.4% reported prior BCG vaccination. TST results were available for 266 people, and an additional 24 reported a prior positive TST and were not retested, for a total of 290 (89.0%) persons with any TST result. Adequate QFT specimens were successfully obtained for 312 (95.7%) of persons. Thirty-two persons had adequate QFT specimens obtained but did not return for TST reading, and 10 persons had a TST placed and read, but did not have an adequate QFT specimen (either due to phlebotomy failure or refusing to have blood drawn). Four persons had neither a TST nor a QFT result (Fig. 1).
Table 1.
Subject Characteristics (n = 326)
| Characteristic | n (%) |
|---|---|
| Female gender | 185 (56.8%) |
| Mean age | 44 (range, 21–67) |
| White | 36 (11.0%) |
| Black | 239 (73.3%) |
| Hispanic | 16 (4.9%) |
| Asian | 27 (8.3%) |
| Other/unknown race/ethnicity | 8 (2.5%) |
| Foreign born | 73 (22.4%) |
| Prior reported BCG | 37 (11.4%) |
| Known diabetes | 20 (6.2%) |
BCG, Bacille Calmette-Guérin.
FIG. 1.
Number of subjects who had any available result for tuberculin skin testing (TST), QuantiFERON Gold in-tube test (QFT; Cellestis Limited, Carnegie, Victoria, Australia), or both. A subject could have no QFT result because of phlebotomy failure, refusal, or test failure. A subject could have no TST result because of refusal or failure to return for reading 48–72 hours later. Labels include raw number and percentage.
Overall, 54 of 266 (20.3%) of persons tested had a positive TST and 41 of 312 (13.1%) had a positive QFT. Among the foreign born 53.7% had a positive TST and 26.1% had a positive QFT. Among the U.S. born, 11.7% had a positive TST and 9.4% had a positive QFT. Twenty-two percent of all persons tested met criteria for latent TB infection. There were 6 individuals with a positive QFT but a negative TST and 26 with a positive TST and negative QFT (Table 2).
Table 2.
PPD and QFT Results
| PPD ≥10 mm | No result | Negative | Positive | Indeterminate |
|---|---|---|---|---|
| Negative | 4 | 201 | 6 | 1 |
| Positive | 5 | 26 | 23 | 0 |
| No result | 4 | 44 | 12 | 0 |
QFT, QuantiFERON Gold in-tube test (Cellestis Limited, Carnigie, Victoria, Australia).
HIV testing was well received in this context, with 288 (88.3%) agreeing to opt-out testing (Table 3). Two (0.7%) were enzyme-linked immunosorbent assay (ELISA) positive, with confirmation by Western blot. Both individuals previously knew their HIV status; one was on antiretroviral therapy, but the second patient had stopped care 1 year prior. Both persons had negative TST and QFT results. Persons of black race were less likely to accept HIV testing than persons in other racial/ethnic groups (85.4% versus 96.6%, p = 0.0053); age and gender were not significantly associated with acceptance of HIV testing.
Table 3.
Proportion of Persons Accepting Opt-Out HIV Testing, by Demographic Group
| Characteristic | n (%) |
|---|---|
| Male | 122/141 (86.5%) |
| Female | 166/185 (89.7%) |
| White | 34/36 (94.4%) |
| Black | 204/239 (85.4%)a |
| Hispanic | 16/16 (100%) |
| Asian | 26/27 (96.3%) |
| Other/unknown race/ethnicity | 8/8 (100%) |
| U.S.-born | 213/244 (87.3%) |
| Foreign born | 67/73 (91.8%) |
| Unknown birthplace | 8/9 (88.9%) |
p = 0.005 compared to other racial/ethnic groups.
Interestingly, of persons who did not accept HIV testing on site, 1 of 38 (2.6%) was later found to be HIV-seropositive in health department records.
Of persons screened during this contact investigation, one was found to have active pulmonary TB. This individual had a 7 mm TST and a positive QFT. A second person had a positive TST at 19 mm and a positive QFT; she was asymptomatic at the time of screening and was offered rifampin, but did not initiate treatment. She was subsequently hospitalized and found to have disseminated TB infection affecting multiple bony sites, with an isolate matching the outbreak strain. A third work contact had a 0 mm TST and indeterminate QFT (with a negative HIV test and no other known immunosuppressive condition), so was not offered rifampin, but developed culture-confirmed sacral TB approximately two years after the outbreak investigation, with an isolate that matched the outbreak strain. Of 72 persons determined to have latent TB infection, all were offered rifampin. Thirty-five (48.6%) initiated rifampin, and 23 of the 35 (65.7%) completed a 4-month course. Three were presribed INH due to potential drug–drug interations; 2 completed a full 9-month course.
Discussion
Opt-out HIV testing paired with QFT was a feasible and efficient screening strategy in this large contact investigation. Of the 326 people screened onsite, 95.7% had QFT results obtained. HIV-testing using an opt-out strategy was well accepted, with 88.3% undergoing testing. Two individuals who were seropositive for HIV were offered LTBI therapy in accordance with CDC guidelines (regardless of TST or QFT result); this would not have occurred without concurrent HIV testing. The use of QFT in this contact investigation had some logistical advantages. Despite the fact that the contact investigation was conducted at the workplace where all of the contacts were currently employed, 32 (9.8%) persons did not return for TST reading, but a QFT result was available for all of these persons. Conversely, a smaller number of persons (10, 3.1%) had an available TST result but no QFT result, usually because phlebotomy was refused. This demonstrates that the use of QFT or any other interferon gamma release assay has the potential to improve both the efficiency and completeness of TB contact investigations. Although QFT is a more expensive test than TST, overall costs may be lower with QFT because of reduced TB control staff time (one visit instead of two), reduced patient costs (no need to take time from work for a second visit), and greater test specificity of the QFT compared with TST, particularly in BCG-vaccinated individuals.9 Formal cost-effectiveness analyses have arrived at different conclusions regarding optimum use of QFT with TST,10–14 but the data presented here demonstrate that rates of failure to present for TST reading and phlebotomy/laboratory failures are key parameters to include in such analyses.
We were surprised by the fact that QFT did not enable us to greatly reduce the number of persons offered LTBI treatment. One study of 601 close contacts to TB cases found that 40.4% had a positive TST if a 5-mm cutoff was used and 18.3% were positive if a 10-mm cutoff was used; admittedly these contacts had a high rate of BCG vaccination.5 In our contacts, only one individual had a TST between 5–9 mm (and a positive QFT), and this person was found to have active pulmonary TB. We did find that the prevalence of a positive TST with a negative QFT was disproportionately higher in foreign-born than U.S.-born contacts. This may be a reflection of prior BCG vaccination or remote infection; some data suggest that QFT is less sensitive in the setting of remote infection given decreased interferon gamma secretion to M. tuberculosis-specific antigens over time or following treatment.15–17
Our study is limited by the fact that we were unable to link information about prior BCG vaccination to specific individuals, so the proportion of those whose positive TST may have been due to BCG vaccination is unknown.
Opt-out HIV testing was both feasible and effective in this study. Routine testing of TB contacts for HIV infection is important both as a means for assessing risk of progression to active TB and as an opportunity to extend HIV screening efforts into relatively high-risk populations. The CDC recommends routine screening for HIV infection as part of routine medical care for persons age 13–64 in the United States18 and such screening is cost-effective in populations with HIV prevalence of 0.05% or greater.19 The limited published data on HIV prevalence in TB contacts report varying HIV prevalence. In five areas of the United States examined in 1996, only 19% of TB contacts were HIV tested, and 9% of these were HIV-seropositive.20 Anonymous HIV testing in a London clinic in 1998–1999 reported 5% HIV prevalence among TB contacts presenting for evaluation.21 Of 569 TB contacts offered HIV testing (opt-in) in New York City 2002–2003, only 39% had known HIV status, and 10.7% of these were HIV-seropositive. Finally, a cross-sectional evaluation of TB contacts in Chang Rai, Thailand, in 2000 and 2002 reported that 890 of 1200 (74.2%) accepted HIV testing (opt-in) and 286 (23.8%) were HIV-seropositive (compared to HIV prevalence among pregnant women and blood donors of 4% and 0.06%, respectively, in this region).22 This suggests that contacts to TB cases may have higher rates of HIV prevalence than the general population, supporting the importance of HIV testing in conjunction with contact investigations.
In this investigation a low proportion of individuals refused opt-out HIV testing. In prior studies, the most common reasons given for refusal of HIV testing were lack of perceived risk or having been recently tested.23–25 In a primary care setting, acceptance of opt-out testing was low (35%).25 In another study in our community, however, door-to-door rapid HIV testing among high-risk individuals was very well accepted, suggesting convenience and perceived risk are factors in acceptance of testing.26 Race and age may also play a role; in one study of HIV testing in an area of low seroprevalence (South Carolina) patients over the age of 50 were less likely to accept HIV testing and African Americans were more likely to accept than other racial/ethnic groups.24 In our study, however, African Americans were less likely to accept testing. It is also notable that the point estimate for minimum HIV prevalence among those who refused testing (2.6%) was nearly 4 times the prevalence for those who accepted (0.7%). The high rate of acceptance of opt-out HIV testing in this study may be attributed to its pairing with QFT testing. It has been shown that in the setting of blood testing for another reason individuals are more likely to accept HIV testing.25 Based on our experience, we would advocate for routine opt-out testing among TB contacts, particularly when such testing can be paired with blood testing for TB with an interferon gamma release assay. In addition, HIV testing among other high-risk demographic groups such as prisoners and homeless individuals could be combined with TB testing using an interferon gamma release assay. Combined TB/HIV testing with a single blood draw has the potential to be a potent strategy in many settings.
Acknowledgment
This work was supported by grant No. 5T32 AI007392 (A.K. Person) from the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Selwyn PA. Hartel D. Lewis VA, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 2.Pape JW. Jean SS. Ho JL, et al. Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 3.Jensen PA. Lambert LA. Iademarco MF, et al. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 4.Lee SS. Liu YC. Huang TS, et al. Comparison of the interferon-gamma release assay and the tuberculin skin test for contact investigation of tuberculosis in BCG-vaccinated health care workers. Scand J Infect Dis. 2008;40:373–380. doi: 10.1080/00365540701730743. [DOI] [PubMed] [Google Scholar]
- 5.Diel R. Loddenkemper R. Meywald-Walter K, et al. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2008;177:1164–1170. doi: 10.1164/rccm.200711-1613OC. [DOI] [PubMed] [Google Scholar]
- 6.Harada N. Nakajima Y. Higuchi K, et al. Screening for tuberculosis infection using whole-blood interferon-gamma and Mantoux testing among Japanese healthcare workers. Infect Control Hosp Epidemiol. 2006;27:442–448. doi: 10.1086/504358. [DOI] [PubMed] [Google Scholar]
- 7.Menzies D. Long R. Trajman A, et al. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection: A randomized trial. Ann Intern Med. 2008;149:689–697. doi: 10.7326/0003-4819-149-10-200811180-00003. [DOI] [PubMed] [Google Scholar]
- 8.Horsburgh CR., Jr. Goldberg S. Bethel J, et al. Latent tuberculosis infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137:401–409. doi: 10.1378/chest.09-0394. [DOI] [PubMed] [Google Scholar]
- 9.Pai M. Zwerling A. Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent tuberculosis infection: An update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Perio MA. Tsevat J. Roselle GA, et al. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med. 2009;169:179–187. doi: 10.1001/archinternmed.2008.524. [DOI] [PubMed] [Google Scholar]
- 11.Oxlade O. Schwartzman K. Menzies D. Interferon-gamma release assays and TB screening in high-income countries: A cost-effectiveness analysis. Int J Tuberc Lung Dis. 2007;11:16–26. [PubMed] [Google Scholar]
- 12.Marra F. Marra CA. Sadatsafavi M, et al. Cost-effectiveness of a new interferon-based blood assay, QuantiFERON-TB Gold, in screening tuberculosis contacts. Int J Tuberc Lung Dis. 2008;12:1414–1424. [PubMed] [Google Scholar]
- 13.Diel R. Nienhaus A. Loddenkemper R. Cost-effectiveness of interferon-gamma release assay screening for latent tuberculosis infection treatment in Germany. Chest. 2007;131:1424–1434. doi: 10.1378/chest.06-2728. [DOI] [PubMed] [Google Scholar]
- 14.Diel R. Wrighton-Smith P. Zellweger JP. Cost-effectiveness of interferon-gamma release assay testing for the treatment of latent tuberculosis. Eur Respir J. 2007;30:321–332. doi: 10.1183/09031936.00145906. [DOI] [PubMed] [Google Scholar]
- 15.Pathan AA. Wilkinson KA. Klenerman P, et al. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: Associations with clinical disease state and effect of treatment. J Immunol. 2001;167:5217–5225. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 16.Aiken AM. Hill PC. Fox A, et al. Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect Dis. 2006;6:66. doi: 10.1186/1471-2334-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrara S. Vincenti D. Petrosillo N, et al. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis. 2004;38:754–756. doi: 10.1086/381754. [DOI] [PubMed] [Google Scholar]
- 18.Branson BM. Handsfield HH. Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. ; quiz CE11–14. [PubMed] [Google Scholar]
- 19.Sanders GD. Bayoumi AM. Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 20.Reichler MR. Bur S. Reves R, et al. Results of testing for human immunodeficiency virus infection among recent contacts of infectious tuberculosis cases in the United States. Int J Tuberc Lung Dis. 2003;7:S471–478. [PubMed] [Google Scholar]
- 21.Bowen EF. Rice PS. Cooke NT, et al. HIV seroprevalence by anonymous testing in patients with Mycobacterium tuberculosis and in tuberculosis contacts. Lancet. 2000;356:1488–1489. doi: 10.1016/S0140-6736(00)02876-2. [DOI] [PubMed] [Google Scholar]
- 22.Suggaravetsiri P. Yanai H. Chongsuvivatwong V, et al. Integrated counseling and screening for tuberculosis and HIV among household contacts of tuberculosis patients in an endemic area of HIV infection: Chiang Rai, Thailand. Int J Tuberc Lung Dis. 2003;7:S424–431. [PubMed] [Google Scholar]
- 23.White DA. Scribner AN. Huang JV. A comparison of patient acceptance of fingerstick whole blood and oral fluid rapid HIV screening in an emergency department. J Acquir Immune Defic Syndr. 2009;52:75–78. doi: 10.1097/QAI.0b013e3181afd33d. [DOI] [PubMed] [Google Scholar]
- 24.Weis KE. Liese AD. Hussey J, et al. A routine HIV screening program in a South Carolina community health center in an area of low HIV prevalence. AIDS Patient Care STDs. 2009;23:251–258. doi: 10.1089/apc.2008.0167. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham CO. Doran B. DeLuca J, et al. Routine opt-out HIV testing in an urban community health center. AIDS Patient Care STDs. 2009;23:619–623. doi: 10.1089/apc.2009.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sena AC. Hammer JP. Wilson K, et al. Feasibility and acceptability of door-to-door rapid HIV testing among latino immigrants and their HIV risk factors in North Carolina. AIDS Patient Care STDs. 2010;24:165–173. doi: 10.1089/apc.2009.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]