Abstract
Objective
The aim of this study was to conduct a pilot study testing whether single-dose, immediate-release dexmethylphenidate (dMPH) can facilitate tic suppression in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) and Tourette's disorder (TD) or chronic tic disorders. The primary hypothesis is that dMPH will improve behaviorally reinforced tic suppression in a standard tic suppression paradigm (TSP).
Methods
Ten children with ADHD and TD were given dMPH on one visit and no medication on another, using a random crossover design. On both days, following a baseline period, subjects were reinforced for suppressing tics using a standard TSP.
Results
Thirteen subjects were enrolled; 10 subjects (mean age 12.7 ± 2.6; 90% male) completed all study procedures. Relative to the no-medication condition, tics were reduced when children were given a single dose of dMPH. Behavioral reinforcement of tic suppression resulted in lower rates of tics compared to baseline, but dMPH did not enhance this suppression.
Conclusion
Preliminary results indicate replication of prior studies of behavioral tic suppression in youths with TD and without ADHD. In addition, our findings indicate tic reduction (and not tic exacerbation) with acute dMPH challenge in children and adolescents with ADHD and TD.
Introduction
Bidirectional overlap of attention-deficit/hyperactivity disorder (ADHD) and tic disorders, including Tourette's disorder (TD), has been frequently described in youths (Spencer et al. 1999a; Spencer et al. 1999b). Co-morbid ADHD is observed in about half of clinically referred children and adolescents with TD, and tic disorders are reported in up to a third of clinically referred children with ADHD (Spencer et al 1999a; Coffey et al. 2000). Both disorders are conceptualized as disorders of central nervous system (CNS) disinhibition; neurobiological substrates for the overlapping motor, attentional, organizational, and planning dysfunction include basal ganglia and premotor and prefrontal pathways (Robertson 2000; Jankovic 2001; Swerdlow and Young 2001).
Treatment of children and adolescents with co-morbid ADHD and TD is challenging, and includes both pharmacological and behavioral interventions. Two problems are often encountered in treatment. First, there remains a general belief that use of stimulants to treat ADHD symptoms in children with co-morbid tic disorders is contraindicated because of concerns about possible tic exacerbation. However, studies over the past decade have provided converging evidence that stimulants are beneficial for ADHD symptoms in children with ADHD and tic disorders, and tics do not increase significantly (Spencer et al. 1999a; Gadow et al. 2007). In fact, in one controlled four-arm study comparing clonidine, methylphenidate (MPH), and the combination to placebo in children and adolescents with ADHD and chronic tics, tics reduced significantly in those treated with MPH alone (The Tourette's Syndrome Study Group SSG 2002).
Second, there is concern that ADHD symptoms may diminish efficacy of behavioral treatment in children with chronic tic disorders. There is growing evidence for the therapeutic benefit of habit reversal therapy (HRT), more recently described as comprehensive behavioral intervention for tics (CBIT) in patients with TD (e.g., Wilhelm et al. 2003; Woods et al. 2003; Deckersbach et al. 2006; Piacentini et al. 2010). However, preliminary results suggest that the therapeutic benefit of HRT may be moderated by the patient's attentional competence. Converging lines of evidence suggest moderating effects of attention and response inhibition on reinforced tic suppression (Peterson et al. 1998; Deckersbach et al. 2006; Himle and Woods 2006; Woods et al. 2008). Such evidence arises from two primary sources.
The first line of evidence comes from laboratory experiments using experimental paradigms to study tic suppression. Using the tic suppression paradigm (TSP) developed by Woods and Himle (2004), Woods and colleagues (2008) examined the ability of children to voluntarily suppress their tics for different time durations (5 minutes, 25 minutes, and 40 minutes) while being reinforced with a monetary reward for 10-second tic-free intervals. This study also examined rebound effects on tic frequency and neuropsychological predictors of tic suppression. Thirteen children, ranging in age from 10 to 17 years, completed the study at two separate sites. Results indicated a nearly 80% reduction in tics with behavioral reinforcement during the active tic suppression phase, regardless of the duration (i.e., 5, 25, or 40 minutes). Results also revealed no rebound (beyond return to baseline) in tic frequency following periods of suppression.
Results also indicated specific predictors of impaired ability to suppress tics with a behavioral reward. Specifically, the number of errors of omission on the Conners' Continuous Performance Test (CPT) (a measure of deficient attentional functioning) was significantly negatively correlated with magnitude of tic suppression both in the 25- and 40-minute suppression conditions. Such results are consistent with earlier findings by Himle and Woods (2005), who demonstrated that tic suppression ability was significantly negatively correlated with scores on the Attention Problems subscale of the Child Behavior Check List (CBCL) (Achenbach 2001), and a study by Peterson and colleagues (1998) suggesting that tic suppression recruits a number of brain regions involved in attentional competence.
The second line of evidence comes from a clinical trial (Deckersbach et al. 2006) in which 30 adults with TD were randomly assigned to either HRT or a supportive psychotherapy control condition. Results replicated earlier findings demonstrating the efficacy of behavior therapy over the control condition (e.g., Azrin and Peterson 1990; Wilhelm et al. 2003). The investigators also showed that poor response on a “go-no go” task (a task measuring attentional competence) was a significant predictor of poor response to HRT. Taken together, laboratory-based studies and the clinical trial suggest that successful tic suppression using behavioral procedures may be significantly impaired by compromised attentional functioning.
On the basis of these findings, a logical next step would be to determine if pharmacological agents designed to enhance attentional/inhibitory processes would improve the efficacy of behavioral procedures designed to facilitate tic suppression. Psychostimulant medication, well established in the treatment of ADHD, may be a useful agent in this regard (DeVito et al. 2009). Reinforced tic suppression can be assessed via a TSP developed by Woods and Himle (Woods and Himle 2004; Himle and Woods 2005).
We describe a pilot challenge study using a short-acting psychostimulant known to enhance attention—dexmethylphenidate (dMPH)—to evaluate its effect on reinforced tic suppression in children with ADHD and co-morbid TD or chronic tic disorder. On the basis of previous work in both ADHD and TD, we seek to test three primary hypotheses. First, we predict that dMPH will produce a decrease in tic rate relative to a no-medication control condition. Second, consistent with prior work on the effects of reinforcement on establishing tic suppression (Woods and Himle 2004; Himle and Woods 2006; Woods et al. 2008), we predict that tic rates will be lower when children are reinforced for successful suppression when compared to a no-suppression baseline condition, and that successful suppression will not result in a tic rate rebound exceeding baseline levels. Finally, given that psychostimulants have been useful in ameliorating deficits in attention and response inhibition, and these processes have been linked to successful tic suppression, we predict that behaviorally reinforced tic suppression will be more successful in the dMPH condition as compared to the no-drug condition.
Methods
Subjects
Subjects were recruited between November, 2008, and October, 2009, through the Tics and Tourette's Clinical and Research Program, referrals from local professionals, the Tourette Syndrome Association, and by telephone screening of interested parties solicited by advertising. All subjects were evaluated with a comprehensive psychiatric assessment by the first or senior author, both with expertise in the diagnosis and treatment of tic disorders. Written informed consent of parents and assent of child were obtained. All study procedures were approved by the institutional review boards (IRB) at University of Wisconsin–Milwaukee and New York University Langone Medical Center.
Approximately 51 subjects were screened for this study and 13 were enrolled. Subjects were eligible for inclusion if they met the following criteria: (1) Age 10–17 years (inclusive) when informed consent was obtained; (2) Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) diagnostic criteria for TD or chronic motor/vocal tic disorder (CTD) confirmed by the Computerized Diagnostic Interview Schedule for Children–4th edition (CDISC4) (Shaffer et al. 2003); (3) DSM-IV-TR diagnostic criteria for co-morbid ADHD, any subtype, confirmed by the CDISC4; (4) Yale Global Tic Severity Scale (YGTSS) (Leckman et al. 1989) Total Tic Score ≥14 for TD or ≥10 for CTD; (5) exhibited one or more motor and/or vocal tics at a rate of at least 1 tic per minute averaged across a 10-minute videotaped observation; (6) intellectual functioning was at least in the low-average range or above as indicated by a score of greater than 75 on the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999); (7) no history of behavioral treatment for tics (greater than 3 weeks in duration) or other treatment in which suppression strategies were a primary component of the intervention; (8) current tic medication (α-adrenergic agonists, typical or atypical neuroleptics) at the time of the study was allowed but had to remain stable at the same dose throughout the study period; (9) previous treatment with stimulants was allowed if the subject had not received stimulants for at least 48 hours prior to testing procedures; and (10) ADHD symptoms must have been associated with impairment in at least one domain (home, school).
Subjects were excluded from the study if they met any of the following exclusion criteria: (1) subjects with pervasive developmental disorder, schizophrenia, major depressive disorder, bipolar disorder, or substance abuse disorder; (2) subjects currently receiving stimulant medication who could not temporarily discontinue it for study procedures; (3) subjects with any medical condition that would contraindicate use of a stimulant, such as seizure disorder, previous hypersensitivity to MPH, glaucoma, or a significant cardiac history, including fainting or dizziness, seizures, rheumatic fever, chest pain or shortness of breath with exercise, unexplained change in exercise tolerance, palpitations, increased heart rate, hypertension, heart murmur other than benign functional murmur, or current viral illness with chest pains or palpitations; (4) subjects with a family history of sudden or unexplained death in someone less than 35 years of age, sudden death during exercise, cardiac arrhythmias, cardiomyopathy including hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy or right ventricular cardiomyopathy, long QT syndrome, short QT syndrome or Brugada syndrome, Wolf–Parkinson–White or abnormal cardiac rhythyms, event requiring resuscitation in family members less than age 35, including syncope requiring resuscitation, or Marfan syndrome; (5) subjects with abnormal electrocardiogram (ECG) at baseline, including prolongation of the QTc interval greater than 450 msec for males and 470 msec for females; (6) subjects who meet full criteria for obsessive-compulsive disorder or another anxiety disorder requiring pharmacological or behavioral treatment.
Two enrolled subjects dropped out prior to completion of the study procedures, one for lack of interest in the study after consent was obtained and the other for failure to meet intellectual functioning criteria. One subject was excluded after completing study procedures for not meeting the inclusion criterion of exhibiting one or more motor and/or vocal tics at a rate of at least 1 tic per minute averaged across a 10-minute videotaped observation. One subject was 8 years old, but was allowed to enroll because he met all other inclusion and exclusion criteria and was able to understand and comply with study procedures.
The final study sample included 10 children and adolescents, ages 8–16 years, with a DSM-IV-TR diagnosis of TD and co-morbid ADHD, any subtype.
Design
Overview of Study Procedures
The study occurred over 3 days. Following initial baseline evaluation on day 1, one experimental session occurred each day on days 2 and 3. On day 1, each subject received an initial evaluation, which included an assessment battery designed to provide sociodemographic information, ensure that inclusion/exclusion criteria were met, and confirm a diagnosis of TD or CTD and ADHD. Demographic information and treatment history (pharmacological and behavioral) were collected for each subject using a demographics form and clinical interview. Current medication status and medical and psychiatric history were also obtained. Subjects were overtly videotaped for 10 minutes for observation to determine whether or not the subject met inclusion criteria of at least 1 tic per minute and to ascertain a list of subject-specific tics. The YGTSS was administered as a semistructured interview to parent and subject by one of the investigators (G.L. or B.C.) at baseline. All subjects received an ECG. Subjects with abnormal ECG readings were referred for a full cardiology evaluation; written clearance was obtained from a pediatric cardiologist prior to participating in study experimental procedures.
On days 2 and 3, the subject received no medication for 1 day and a single, immediate release dose of dMPH (0.15 mg/kg) on the other. The dose administered was designed to fall in the typical therapeutic range for treatment of ADHD. The order of medication versus no medication was randomly assigned. One hour post dMPH dose, or immediately after arrival on the day no medication was given, attention was evaluated with a CPT, followed 15 minutes later by tic suppression evaluation with TSP (as described below). Days 2 and 3 were held at approximately the same time of day to avoid any circadian rhythm effects in attention or tics.
All experimental sessions took place in a 10-foot × 15-foot observation room equipped with a one-way mirror and video recording equipment to allow for covert observation and recording. All conditions in the observation room were identical for each experimental session; placement of the equipment, including the token dispenser and camera were the same for each experimental session. All sessions were video recorded, with videotapes sent to the University of Wisconsin–Milwaukee for data coding. Video raters were blind to participants' medication status. Subjects were unaware of being observed or videotaped during the TSP and were debriefed at the end of the study.
Vital signs (pulse, blood pressure) were obtained on day 1 and before and after TSP procedures on days 2 and 3. The Safety Monitoring Uniform Report Form (SMURF) (Greenhill et al. 2004) was administered by one of the investigators on day 1, and after TSP procedures on days 2 and 3.
Tic suppression paradigm
The TSP involved exposing the subjects to three different tic suppression conditions: (1) a 10-minute baseline condition (BL) during which subjects were told not to suppress their tics; (2) a 10-minute suppression condition (SUP) during which subjects were reinforced for suppressing their tics using a standard tic suppression protocol; and (3) a 5-minute rest condition (REST) during which subjects were instructed not to suppress their tics. After the initial three conditions were presented, the suppression and rest conditions were repeated once. On each day, each subject received the following sequence of components for a total of 40 minutes: BL, SUP, REST, SUP, REST.
Baseline
During the 10-minute BL condition, the subject was seated in the observation room by him/herself with a token dispenser (a 12-inch × 12-inch × 24-inch box with an attached, nonfunctioning web camera and a clear plastic receptacle attached to the front) placed in front of him/her. Following the protocol established by Woods and Himle (2004), the subject was told by a research assistant that the machine is a “tic detector” which would monitor and count the subject's tics. The device was actually a token dispenser, which was manually operated by study personnel from behind the one-way mirror. The subject was asked to sit in front of the machine for 10 minutes and was told to tic freely as much or as little as needed while remaining in his/her seat with his/her arms on the arm rest of the chair or in his/her lap. The subject was then asked to repeat the instructions to ensure that he/she understood the task. No instructions to suppress were delivered and no tokens were delivered during the BL condition.
Reinforced suppression
During the 10-minute SUP conditions, the subject remained seated with the token dispenser directly in front of him/her. The subject was told again that the device is a “tic detector,” which would monitor and count his/her tics. To produce reinforced tic suppression, the subject was told that he/she could earn a token for each 10-second interval during which he/she had no tics. The subject was also told that each token would be exchangeable for a small monetary reward upon completion of the study. Next, the research assistant provided a detailed overview of the subject-specific tics the subject was to suppress. Subject-specific tics were ascertained from tics reported on the YGTSS and from tics observed and identified during the 10-minute video observation at visit 1. The subject was then asked to repeat the instructions to ensure that he/she understood the task.
During the suppression component, the “tic detector” was controlled by a study investigator and research assistant observing from behind the one-way mirror. For each consecutive 10-second interval during which the subject did not have a tic, a token was delivered into a receptacle visible to the subject on the front of the token dispenser. Whenever a tic was observed, the 10-second interval was reset without token delivery. Each subject received the same compensation at the end of the study, regardless of the number of tokens earned.
Rest
During the 5-minute REST component, the subject remained seated with the token dispenser directly in front of him/her. The subject was reminded by the research assistant that the device was a “tic detector,” which monitored and counted his/her tics. The subject was specifically instructed not to attempt tic suppression during the REST condition. He or she was told to tic if necessary. The subject was then asked to repeat the instructions to ensure that he/she understood the task.
Measurements and analytic methods
Reinforced tic suppression was measured using videotaped tic counts during the TSP. ADHD symptoms were measured using the ADHD Rating Scale (ADHD-RS; Conners 1997). Attention was measured by the CPT II (Conners 2000). Tic severity was measured using the YGTSS (Leckman et al. 1989). Adverse events were collected using the SMURF after each experimental trial (Greenhill et al. 2004). Adverse events were defined as any undesirable change from the subject's baseline condition, including any intercurrent illness that occurs during the study, whether considered related to the investigational procedures or not.
Descriptive measures, including sociodemographic and diagnostic data, were tabulated with means, standard deviations, and proportions where appropriate. A 2 (drug vs. no drug) × 3 (baseline vs. suppression vs. rest) within-subjects analysis of variance (ANOVA) was conducted to compare tics per minute observed under each condition.
Results
Sociodemographic data
Sociodemographic data are described in Table 1. The sample included 9 males (90%) and 1 female (10%) with a mean age of 12.7 (standard deviation [SD] = 2.6). All 10 subjects (100%) met DSM-IV-TR criteria for TD and co-morbid ADHD. Five subjects (50%) met criteria for ADHD Combined type and 5 subjects (50%) met criteria for ADHD Inattentive type. Seven subjects (70%) self-identified as white non-Hispanic and 3 subjects (30%) self-identified as Hispanic. Subjects had a mean intelligence quotient (IQ) of 104 (SD = 13.3). Seven subjects (70%) had normal ECG readings; 3 (30%) subjects were referred for cardiology consultation for abnormal readings, and were cleared by a cardiologist prior to participation in the experimental procedures.
Table 1.
Sociodemographic Data (n = 10)
| Mean ± SD | Range | |
|---|---|---|
| Age | 12.7 ± 2.6 | 8–16 |
| IQ | 104 ± 13.3 | 85–118 |
| dMPH dose (mg) | 7.5 ± 3.1 | 2.5–12.5 |
| N | % | |
|---|---|---|
| Male | 9 | 90% |
| Hispanic | 3 | 30% |
| White non-Hispanic | 7 | 70% |
| Tourette's disorder diagnosis | 10 | 100% |
| ADHD diagnosis | 10 | 100% |
| Combined type | 5 | 50% |
| Inattentive type | 5 | 50% |
| ADHD-RS | 25.3 ± 10.8 | 9–43 |
| Concomitant medications | 7 | 70% |
Abbreviations: SD = standard deviation; IQ = intelligence quotient; dMPH = dexmethylphenidate; ADHD = attention-deficit/hyperactivity disorder; ADHD-RS = ADHD Rating Scale.
Mean baseline YGTSS motor tic score was 13.2 (SD = 3.5) (mild-to-moderate severity), vocal tic score was 10.6 (SD = 5.0) (mild severity), and total tic score was 23.8 (SD = 7.5) (mild-to-moderate severity). Mean baseline YGTSS impairment scores were 18.0 (SD = 8.9) (minimal-to-mild severity) and YGTSS global severity scores of 41.8 (SD = 13.4) (mild-to-moderate severity) (see Table 2). Mean ADHD-RS score at baseline was 25.3 (SD = 10.8) (mild to moderate).
Table 2.
Yale Global Tic Severity Scale Subscale Scores by Study Condition
| |
Baseline |
Nonmedication |
Medication |
|||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| Motor tic | 13.2 ± 3.5 | 8–18 | 13.6 ± 2.9 | 9–19 | 12.1 ± 2.0 | 10–15 |
| Vocal tic | 10.6 ± 5.0 | 0–17 | 8.6 ± 4.8 | 0–15 | 4.9 ± 6.9 | 0–16 |
| Total tic | 23.8 ± 7.5 | 10–35 | 22.1 ± 7.6 | 9–34 | 17.0 ± 8.4 | 10–31 |
| Impairment score | 18.0 ± 8.9 | 10–40 | 22.8 ± 7.6 | 20–40 | 19.4 ± 9.4 | 10–40 |
| Global severity | 41.8 ± 13.4 | 20–64 | 45 ± 10.6 | 29–62 | 36.4 ± 15.2 | 20–63 |
Abbreviations: SD = standard deviation.
Mean weight of the sample was 58.4 kg (SD = 21.8) and mean height was 157 cm (SD = 15) at baseline. Subjects received mean dose of 7.5 mg (SD = 3.1) of dMPH during the medication condition. Mean systolic (SBP) and diastolic blood pressure (DBP) at baseline were 114.7 (SD = 9.1) and 62.8 (SD = 3.6), respectively. Mean sitting pulse reading at baseline was 74.0 (SD = 19.4). Blood pressure and sitting pulse readings were recorded before and after TSP during each study condition. No significant differences between SBP and DBP or pulse rates were found between readings before or after TSP, regardless of medication status.
Seven (70%) subjects were taking concomitant medications during this study. These medications included clonidine, aripiprazole, guanfacine, lamotrigine, and benztropine for TD, citalopram for obsessive-compulsive disorder (OCD), budesonide and albuterol for asthma, and fluoxetine and sertraline for anxiety.
Effects on tics
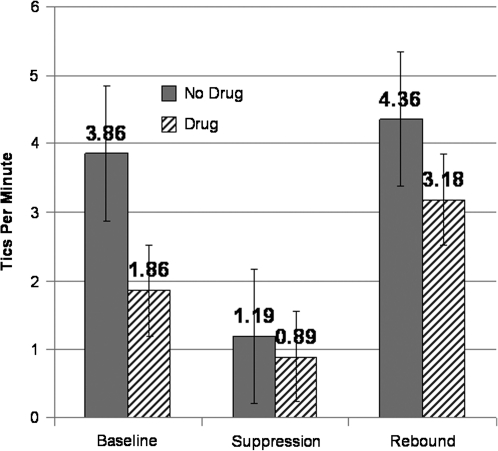
A 2 (drug vs. no drug) × 3 (baseline, suppression, rest) within-subjects ANOVA was conducted to test the three primary hypotheses. Confirming our first hypothesis, results showed a main effect of drug, F(1,9) = 5.41, p < 0.05, η2 = 0.38; tics occurred less frequently when subjects were taking dMPH (M = 1.98 tics per minute, standard error [SE] = 0.40) than when they were medication free (M = 3.14, SE = 0.59). Our second hypothesis was also confirmed; the main effect of suppression condition was statistically significant, F(2,18) = 7.74, p < 0.01, η2 = 0.46. As predicted, when reinforced for tic suppression (M = 1.04 tics per minute, standard error [SE] = 0.22), children exhibited significantly fewer tics compared to baseline (M = 2.86, SE = 0.58; p < 0.01) or rest (M = 3.77, SE = 0.83, p < 0.01) conditions. Consistent with previous reports (Himle and Woods 2006; Woods et al. 2008), there was no evidence of rebound in tics (beyond return to unmedicated baseline) following suppression regardless of being in the drug or no drug condition (p = 0.30). Contrary to expectations, our results did not support our third hypothesis; dMPH did not significantly improve tic suppressibility (see Fig. 1), as the interaction between drug and suppression condition was not significant, F(2,18) = 0.93, p = 0.41, η2 = 0.09.
FIG. 1.
Mean number of tics per minute under the nonmedication and one-time dose of dexmethylphenidate (dMPH) conditions during the TSP.
Effects on CPT measures
To ensure that dMPH significantly improved attentional functioning as measured by the CPT, separate one-way (baseline, drug day, no drug day) within-subjects ANOVAs were conducted for errors of commission and errors of omission. Data from 3 subjects were unusable as a result of computer malfunction. As predicted, errors of commission significantly differed between the three conditions, F(2,12) = 5.49, p < 0.05, η2 = 0.48; fewer errors of commission occurred during the dMPH condition (M = 42.97, SE = 6.2) than during the baseline condition (M = 53.08, SE = 2.89; p < 0.05) or during the no-drug day (M = 49.54, SE = 5.03; p < 0.05). Contrary to our prediction, errors of omission were not differentially effected across conditions, F(2,12) = 0.43, p = 0.66, η2 = 0.07. There were no statistically significant differences between the baseline (M = 49.17, SE = 4.4), dMPH (M = 46.51, SE = 2.1), and the no-drug conditions (M = 49.57, SE = 3.4).
Adverse events
Subjects tolerated the procedures generally well, and any adverse events were minor. Seven (70%) subjects experienced at least one minor adverse event during the study. The most common adverse events possibly related to study drug were drowsiness or sedation (20%) and stomach discomfort (20%).
Discussion
The clinical management of children with TD and co-morbid ADHD is challenging, and our study provides encouraging information for clinicians. Supporting our first hypothesis, our results demonstrate that a one-time dose of short-acting stimulants does not increase tics in children with ADHD and TD, and may, in fact, be beneficial. Second, our results replicate prior research demonstrating that reinforced tic suppression yields significant tic reduction and does not lead to rebound effects. Contrary to our third hypothesis, our results showed that one-time administration of dMPH did not significantly improve tic suppression ability. This was true even though a study indicator of response inhibition (the CPT–Errors of Commission) was improved in the dMPH condition when compared to the no-treatment condition.
Reasons for the failure to support our hypothesis that dMPH would enhance reinforced tic suppression are unclear, but a number of possible explanations exist. First, it is possible that in our sample stimulant-associated tic reduction yielded a floor effect, in that further benefit simply could not be achieved because tics were reduced so significantly by the behavioral suppression task alone. Second, the data suggest that tic suppression abilities were being slightly enhanced by dMPH, and perhaps relatively low power to detect the interaction prevented the detection of the small effect. A third possibility is that it is not the inhibitory deficits in ADHD that are related to poor suppression, but rather deficits in working memory (e.g., ability to retain instructions), attentional selection (e.g., focus on something else in the suppression environment), or interference control (e.g. distraction by other stimuli) that lead to suppression deficits. Last, a possible explanation for our lack of disrupted suppression in the nondrug condition is that perhaps the ADHD symptoms were not severe enough to disrupt suppression. Supporting the role of attentional selection in behavioral tic suppression is our finding that errors of omission did not reduce significantly with the stimulant in these children. Future research will need to be conducted to examine these possibilities.
Limitations
Some limitations of the study design need to be taken into account. First, our sample size was small and subjects were recruited from a specialized clinic. Thus, findings may not generalize to nonspecialty settings. Second, 70% of subjects were receiving medication for TD, anxiety, OCD, or asthma, and our results might have differed if the subjects were not receiving concomitant medication. Third, investigators, parents, and subjects were not blind to medication status, because medication was administered openly. Nevertheless, order of receiving medication was randomized, and video raters were blind to study condition. In addition, because we only used single doses of mid-range immediate release dMPH, we were unable to explore a dose range of effects on ADHD and tics. However, our results appear to be valid, in that CPT commission errors reduced on medication compared to no medication, replicating known effects of psychostimulants on CPT (Losier et al. 1996). Clearly, future research is needed to replicate our results with placebo control conditions and a substantially larger sample before drawing firm conclusions.
Conclusion and clinical implications
Preliminary results suggest that dMPH does not appear to enhance tic suppressibility in children with ADHD and TD; however, given the small sample size, and the possibility of a floor effect, firm conclusions cannot be drawn yet. Nevertheless, important findings emerged. First, there was a clear tic-reduction effect, and not exacerbation, with a one-time dose of dMPH compared to no medication in these children. Second, youths with TD and ADHD appear to be able to suppress their tics with a behavioral reward comparable to youths with TD without ADHD. Taken together, these findings justify the need for further studies with larger numbers of subjects and suggest that treatment with dMPH (0.15 mg/kg/dose) should be further explored in treatment of youths with co-morbid ADHD and TD.
Footnotes
This research was supported by generous grants from the Elaine Schlosser Lewis Fund of the American Academy of Child and Adolescent Psychiatry (AACAP) (to G.J.L.) and the Tourette Syndrome Association (to G.J.L., B.C., D.W., X.C.). It was also supported in part by grant 1 UL1 RR029893 from the National Center for Research Resources, National Institutes of Health and by grant M01 RR00096 from the National Center for Research Resources (NCRR).
Disclosures
Barbara Coffey has received research support from Boehringer Ingelheim, Bristol Myers Squibb, National Institute of Mental Health, National Institute of Neurological Diseases and Stroke, Tourette Syndrome Association, American Academy of Child and Adolescent Psychiatry (AACAP), Otsuka Pharmaceuticals, and Shire Pharmaceuticals; was on the advisory boards of Jazz Pharmaceuticals and Novartis; and was on the research/advisory board of Eli Lilly, and is a paid speaker for the Tourette Syndrom Association. Gholson Lyon has received research support from the Tourette Syndrome Association and AACAP. Douglas Woods has received research support from the National Institutes of Health and the Tourette Syndrome Association and is a paid speaker for the Tourette Syndrome Association; and has received royalties from Guilford Press, Oxford University Press, New Harbinger Press, and Springer Press. F. Xavier Castellanos has received research support from the Tourette Syndrome Association and the National Institutes of Health. Stephanie M. Samar, Christine Conelea, Marcel R. Trujillo, Christina M. Lipinski, Christopher C. Bauer, Bryan C. Brandt, Joshua J. Kemp, Zoe E. Lawrence and Jonathan Howard have no financial ties or conflicts of interest to report.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington: University of Vermont Press; 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- Azrin NH. Peterson AL. Treatment of Tourette syndrome by habit reversal: A wait-list control group comparison. Behav Ther. 1990;21:305–318. [Google Scholar]
- Coffey B. Biederman J. Spencer T. Geller D. Faraone S. Bellordre C. Informativeness of structured diagnostic interviews in the identification of Tourette's Disorder in referred youth. J Nerv Ment Dis. 2000;188:583–588. doi: 10.1097/00005053-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners' Rating Scales–Revised. North Tonowanda (New York): Multi-Health Systems; 1997. [Google Scholar]
- Conners CK. Conners' Continuous Performance Test II. Toronto: MHS; 2000. [Google Scholar]
- Deckersbach T. Rauch S. Buhlmann U. Wilhelm S. Habit reversal versus supportive psychotherapy in Tourette's disorder: A randomized controlled trial and predictors of treatment response. Behav Res Ther. 2006;44:1079–1090. doi: 10.1016/j.brat.2005.08.007. [DOI] [PubMed] [Google Scholar]
- DeVito EE. Bllackwell AD. Clark L. Kent L. Dezsery AM. Turner DC. Aitken MRF. Sahakian BJ. Methylphenidate improves response inhibition but not reflection impulsivity in children with attention deficit hyperactivity disorder (ADHD) Psychopharmacology. 2009;202:531–539. doi: 10.1007/s00213-008-1337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow K. Sverd J. Nolan E. Sprafkin J. Schneider J. Immediate release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:840–848. doi: 10.1097/chi.0b013e31805c0860. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Vitiello B. Fisher P. Levine J. Davies M. Abikoff H. Chrisman AK. Chuang S. Findling RL. March J. Scahill L. Walkup J. Riddle MA. Comparison of increasingly detailed elicitation methods for the assessment of adverse events in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2004;43:1488–1496. doi: 10.1097/01.chi.0000142668.29191.13. [DOI] [PubMed] [Google Scholar]
- Himle MB. Woods DW. An experimental evaluation of tic suppression and the tic rebound effect. Behav Res Ther. 2005;43:1443–1451. doi: 10.1016/j.brat.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Tourette's Syndrome. N Eng J Med. 2001;345:1184–1192. doi: 10.1056/NEJMra010032. [DOI] [PubMed] [Google Scholar]
- Leckman JF. Riddle MA. Hardin MT. Ort SI. Swartz KL. Stevenson J. Cohen DJ. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child and Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Losier BJ. McGrath PJ. Klein RM. Error patterns of the continuous performance test in non-medicated samples of children with and without ADHD: A meta-analytic review. J Child Psychol Psychiatry. 1996;37:971–987. doi: 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Peterson BS. Skudlarski P. Anderson AW. Zhang H. Gatenby JC. Lacadie CM. Leckman JF. Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Piacentini JC. Woods DW. Scahill LD. Wilhelm S. Peterson A. Chang S. Ginsburg G. Deckersbach T. Dzuria J. Levi-Pearl S. Walkup JT. Behavior therapy for children with Tourette syndrome: A randomized controlled trial. JAMA. 2010;303:1929–1937. doi: 10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123:425–462. doi: 10.1093/brain/123.3.425. [DOI] [PubMed] [Google Scholar]
- Spencer T. Biederman J. Coffey BJ. Geller DA. Wilens T. Faraone S. The four-year course of tic disorders in boys with Attention Deficit Hyperactivity Disorder. Arch Gen Psychiatry. 1999a;56:842–847. doi: 10.1001/archpsyc.56.9.842. [DOI] [PubMed] [Google Scholar]
- Spencer T. Biederman J. Harding M. O'Donnell D. Wilens T. Faraone S. Coffey B. Geller D. Disentangling the overlap between Tourette's disorder and ADHD. J Child Psychol Psychiatry. 1999b;39:1037–1044. [PubMed] [Google Scholar]
- Shaffer D. Fischer P. Lucas C. Comer J. Diagnostic Interview Schedule for Children (DISC-IV) New York: Columbia University; 2003. [Google Scholar]
- Swerdlow NR. Young AB. Neuropathology in Tourette Syndrome: An update. Adv Neurol. 2001;85:151–161. [PubMed] [Google Scholar]
- The Tourette Syndrome Study Group (TSSG) Treatment of ADHD in children with tics: A randomized controlled trial. Neurology. 2002;58:527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio (Texas): The Psychological Corporation; 1999. [Google Scholar]
- Wilhelm S. Deckersbach T. Coffey BJ. Bohne A. Peterson AL. Baer L. Habit reversal versus supportive psychotherapy for Tourette's Disorder: a randomized controlled trial. Am J Psychiatry. 2003;160:1175–1177. doi: 10.1176/appi.ajp.160.6.1175. [DOI] [PubMed] [Google Scholar]
- Woods DW. Himle MB. Creating tic suppression: Comparing the effects of verbal instruction to differential reinforcement. J Appl Behav Anal. 2004;37:417–420. doi: 10.1901/jaba.2004.37-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DW. Twohig MP. Flessner CA. Roloff TE. Treatment of vocal tics in children with Tourette Syndrome: investigating the efficacy of habit reversal. J Appl Behav Anal. 2003;36:109–112. doi: 10.1901/jaba.2003.36-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DW. Himle MB. Miltenberger RG. Carr JE. Osmon DC. Karsten AM. Jostad C. Bosch A. Durability, negative impact, and neuropsychological predictors of tic suppression in children with chronic tic disorders. J Abnorm Child Psychol. 2008;36:237–245. doi: 10.1007/s10802-007-9173-9. [DOI] [PubMed] [Google Scholar]