Mps1 regulates its own turnover at kinetochores to ensure mitotic checkpoint silencing in metaphase.
Abstract
Mps1 kinase activity is required for proper chromosome segregation during mitosis through its involvements in microtubule–chromosome attachment error correction and the mitotic checkpoint. Mps1 dynamically exchanges on unattached kinetochores but is largely removed from kinetochores in metaphase. Here we show that Mps1 promotes its own turnover at kinetochores and that removal of Mps1 upon chromosome biorientation is a prerequisite for mitotic checkpoint silencing. Inhibition of Mps1 activity increases its half-time of recovery at unattached kinetochores and causes accumulation of Mps1 protein at these sites. Strikingly, preventing dissociation of active Mps1 from kinetochores delays anaphase onset despite normal chromosome attachment and alignment, and high interkinetochore tension. This delay is marked by continued recruitment of Mad1 and Mad2 to bioriented chromosomes and is attenuated by Mad2 depletion, indicating chronic engagement of the mitotic checkpoint in metaphase. We propose that release of Mps1 from kinetochores is essential for mitotic checkpoint silencing and a fast metaphase-to-anaphase transition.
Introduction
To prevent chromosome missegregations, the onset of anaphase is inhibited by coordinated actions of the error correction and mitotic checkpoint machineries until all chromosomes have stably bioriented. The mitotic checkpoint directs formation of a mitotic checkpoint complex, which is catalyzed on unattached kinetochores and inhibits the anaphase-promoting complex/cyclosome (APC/C; for review see Musacchio and Salmon, 2007). As soon as all kinetochores have attached to microtubules in a stable fashion, the mitotic checkpoint is silenced and inhibition of APC/C is released, ultimately causing anaphase initiation and mitotic exit (for review see Musacchio and Salmon, 2007). Checkpoint silencing in human cells requires dynein-mediated removal of Spindly–RZZ–Mad1/Mad2 from attached kinetochores (Howell et al., 2001; Barisic et al., 2010; Gassmann et al., 2010), p31comet-mediated inhibition of Mad2 conformational activation (Xia et al., 2004; Mapelli et al., 2006), and APC/C-assisted disassembly of the inhibitory complex (Reddy et al., 2007).
The kinase Mps1 is an important player in prevention of chromosomal instability (Jelluma et al., 2008b; Tighe et al., 2008), as its activity is crucial for chromosome biorientation by promoting attachment error correction as well as for APC/C inhibition by the mitotic checkpoint. In human cells, Mps1 regulates error correction (Jelluma et al., 2008b; Santaguida et al., 2010; Sliedrecht et al., 2010) by enhancing Aurora B activity through direct phosphorylation of Borealin (Jelluma et al., 2008b; Bourhis et al., 2009; Kwiatkowski et al., 2010; Sliedrecht et al., 2010), and may in addition use other mechanisms (Espeut et al., 2008; Maciejowski et al., 2010; Santaguida et al., 2010). Mitotic checkpoint regulation by Mps1 has been observed in many model systems (Hardwick et al., 1996; Weiss and Winey, 1996; He et al., 1998; Abrieu et al., 2001; Fisk and Winey, 2001; Stucke et al., 2002; Liu et al., 2003; Fischer et al., 2004; Jelluma et al., 2008b), and its enzymatic activity, at least in humans, directs a number of checkpoint proteins including Mad1 to unattached kinetochores (see Lan and Cleveland, 2010 for a recent summary), allows Mad2 conformational activation (Hewitt et al., 2010), and stabilizes the cytoplasmic APC/C inhibitory complex(es) (Maciejowski et al., 2010).
Mps1 activity rises during mitosis (Stucke et al., 2002), at which time Mps1 dynamically localizes to kinetochores (Howell et al., 2004), dimerizes (Hewitt et al., 2010), and auto-activates by cross-phosphorylation of its activation loop (Kang et al., 2007; Mattison et al., 2007; Jelluma et al., 2008a). The underlying mechanisms of Mps1 kinetochore recruitment and dynamics, however, remain elusive. Mps1 requires the Hec1 component of the microtubule-binding NDC80 complex to reach kinetochores (Martin-Lluesma et al., 2002; Meraldi et al., 2004), likely through a localization signal intrinsic to its N-terminal 300 amino acids that are also required for mitotic checkpoint function (Liu et al., 2003). Interestingly, a mutant lacking the N-terminal 100 amino acids also doesn’t reach kinetochores but still supports a mitotic checkpoint in cells that also express full-length, inactive Mps1 (Maciejowski et al., 2010). GFP-Mps1 only transiently associates with prometaphase kinetochores in PtK2 cells, and this association decreases as chromosomes establish attachments, reaching its lowest levels after chromosomes have aligned on the metaphase plate (Howell et al., 2004). We here address the regulation of Mps1 levels at kinetochores and investigate the reason for its fast turnover at these sites.
Results and discussion
Mps1 auto-regulates its dissociation from kinetochores
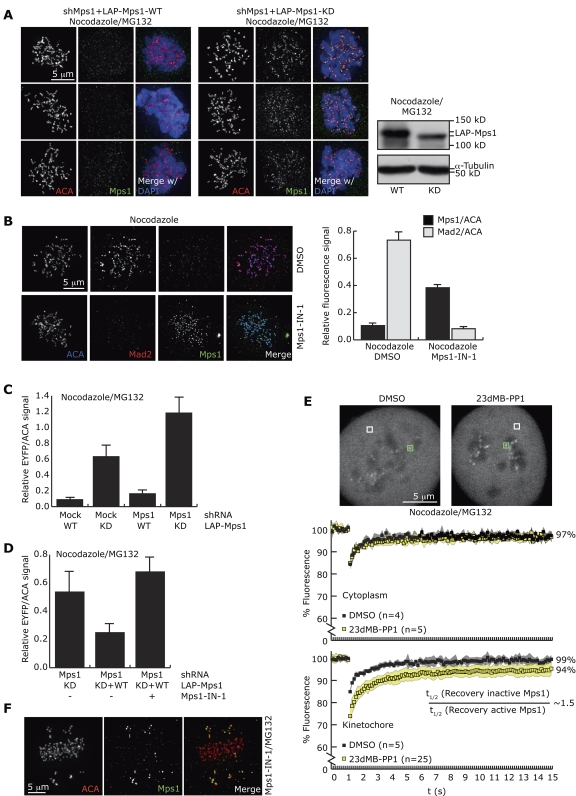
Mps1 exchanges on kinetochores during mitosis in PtK2 cells, showing monophasic recovery of 99% with a half-life of 9 s (Howell et al., 2004). To investigate the role of Mps1 kinase activity in recruitment and release of Mps1 at kinetochores, kinetochore levels of active and inactive Mps1 were examined by immunofluorescence. As noted by others (Hewitt et al., 2010), exogenous kinase-dead (KD, D664A) Mps1 (LAP-Mps1-KD) was found at much higher levels on unattached kinetochores of cells depleted of endogenous Mps1 than its active, wild-type (WT) counterpart (LAP-Mps1-WT; Fig. 1 A, Fig. S1 A). Short-term chemical inhibition of endogenous Mps1 or LAP-Mps1 with the specific inhibitor Mps1-IN-1 (Kwiatkowski et al., 2010) in HeLa cells, or of analogue-sensitive Mps1 (Mps1-as) with 23dMB-PP1 in U2OS-derived cells (Sliedrecht et al., 2010) corroborated this, causing a two- to tenfold increase in kinetochore-bound Mps1 (Fig. 1 B; Fig. S1, B and C). This is in excellent agreement with a recent study using another Mps1 inhibitor, AZ3146 (Hewitt et al., 2010). Together, these results show that the levels of Mps1 at kinetochores in prometaphase increase when Mps1 kinase activity is impaired.
Figure 1.
Mps1 accumulates on kinetochores when inhibited. (A) Immunolocalization of LAP-Mps1 in U2OS cells cotransfected with Mps1 shRNA and LAP-Mps1-WT or LAP-Mps1-KD and treated with nocodazole and MG132. Immunoblot shows expression of LAP-Mps1-WT and -KD in whole-cell lysates. (B) Immunolocalization of Mps1 and Mad2 in HeLa cells treated as indicated. Graph represents quantitation of fluorescence intensities (±SEM, 5 cells per condition, 22 kinetochores/cell). (C and D) Quantitation of fluorescence intensities at kinetochores of U2OS cells transfected and treated as indicated (±SEM, 8 cells per condition, 22 kinetochores/cell). (E) UTRM-LAP-Mps1M602G cells were treated as indicated, and 0.81-µm2 areas around single kinetochores (green squares in top panel) or in the cytoplasm (white squares) were bleached at t = 1 s. Graphs show average fluorescence intensities, shaded areas indicate SDs, and percentages indicate average recovery between 10 and 12 s. (F) Mps1 localization on bioriented and mono-oriented kinetochores in a HeLa cell treated as indicated.
We next addressed what substrates of Mps1 could affect Mps1 localization to kinetochores. Mps1 modifies itself in trans and in cis by autophosphorylation (Kang et al., 2007; Mattison et al., 2007; Jelluma et al., 2008a; Hewitt et al., 2010), phosphorylates the inner-centromere protein Borealin (Jelluma et al., 2008b; Bourhis et al., 2009; Sliedrecht et al., 2010), and likely phosphorylates many kinetochore-localized proteins (Espeut et al., 2008; and see Lan and Cleveland, 2010 for review of kinetochore proteins affected by Mps1 activity). Borealin phosphorylation and subsequent increase in Aurora B activity was not involved, as addition of the Aurora B inhibitor ZM447439 (Ditchfield et al., 2003) did not cause mislocalization of active Mps1 (Fig. S1, D and E). Interestingly, however, LAP-Mps1-KD levels at kinetochores were increased twofold upon depletion of endogenous Mps1 by RNAi compared with control (Fig. 1 C). This increase could be reduced by coexpression of LAP-Mps1-WT, which was, in turn, prevented by Mps1-IN-1 (Fig. 1 D). These results indicate that LAP-Mps1-KD could be displaced from kinetochores by the action of endogenous or exogenous active Mps1. A similar influence of Mps1 activity on its localization was observed when Mps1 was artificially targeted to peroxisomes via a C-terminal peroxisomal targeting sequence (PTS1; Gould et al., 1989). Only inactive Mps1-PTS1 was found in peroxisomes of interphase cells but its localization was prevented by coexpression of active Mps1-PTS1 (Fig. S1 F). These data thus show that Mps1 activity could also regulate its own localization when artificially targeted to a different location in a different phase of the cell cycle. Although the mechanism of auto-regulation at peroxisomes might be different from what happens on kinetochores, structural rearrangement of Mps1 by in trans autophosphorylation could account for delocalization from both sites.
Inhibition of Mps1 increases its residence time at kinetochores
Because short-term inhibition of Mps1 had no overt effects on its total cellular protein levels (Fig. S1 A), we examined if a decreased exchange rate at unattached kinetochores upon inactivation was causing Mps1 to accumulate at these sites by measuring FRAP. After photobleaching, LAP-tagged Mps1-as (Sliedrecht et al., 2010) recovered rapidly to 99% (Fig. 1 E). Addition of 23dMB-PP1 caused Mps1 half-life at kinetochores to increase ∼1.5-fold (95% confidence intervals: uninhibited 0.88–1.36; inhibited 1.38–1.67 s) and recovery was reduced to 94% (Fig. 1 E; see Materials and methods for more details). Similar exchange and recovery kinetics of inhibited Mps1 was observed on kinetochores of PtK2 cells (Fig. S1 G). Although these analyses show a shorter Mps1 half-time of recovery at kinetochores than previously reported (Howell et al., 2004), technical limitations (see Materials and methods) prohibited us from drawing conclusions on the absolute recovery times of LAP-Mps1 on kinetochores, but did allow comparison of kinetics between uninhibited and inhibited Mps1. The small but significant change in half-life and in the size of stable, nonexchanging pool of kinetochore-bound Mps1 may underlie the higher protein levels detected by immunofluorescence (Fig. 1, A and B; Fig. S1, B and C). Nevertheless, inactive Mps1 still had a high exchange rate at the kinetochore, indicating that the exchange of Mps1, although influenced by it, is not fully dependent on its kinase activity.
To examine in what circumstances Mps1 is recruited to kinetochores, cells were treated with Mps1-IN-1 but without spindle drugs, allowing accumulation of Mps1 on kinetochores of aligned and misaligned chromosomes that are nevertheless attached (Jelluma et al., 2008b). Under this condition, Mps1 was present at kinetochores of misaligned chromosomes, but its levels were strongly reduced on bioriented chromosomes (Fig. 1 F). Inhibiting Aurora B prevented the accumulation of inhibited Mps1 on non-bioriented chromosomes (Fig. S1 H; Santaguida et al., 2010). Thus, recruitment of Mps1 to kinetochores is strongly diminished as soon as chromosomes have bioriented. In agreement with this, Mps1 levels diminish on attached prometaphase kinetochores of PtK2 cells compared with unattached prometaphase kinetochores, and diminish further on late metaphase kinetochores (Howell et al., 2004).
Combined, the data support a model in which Mps1 is recruited to non-bioriented chromosomes where it is rapidly released through a mechanism that involves Mps1-dependent phosphorylation. Upon biorientation Mps1 is no longer recruited, causing its depletion from kinetochores in metaphase cells.
Preventing dissociation of Mps1 from kinetochores prolongs metaphase
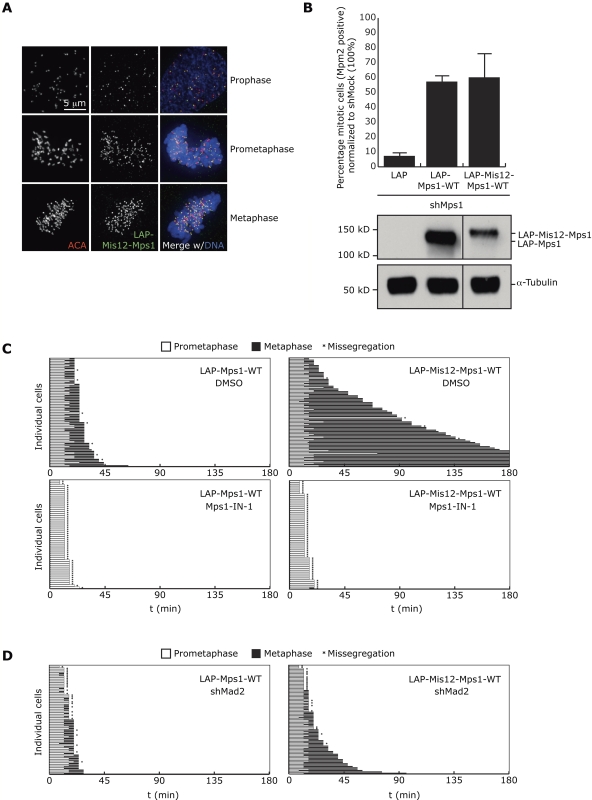
To address the functional relevance of rapid release of Mps1 in prometaphase and its depletion in metaphase, a pool of Mps1 was prevented from leaving the kinetochore by fusion to the Mis12 protein. Mis12 is a constitutive kinetochore protein in mitosis (Cheeseman et al., 2004; Obuse et al., 2004) and fusion to INCENP was previously shown to efficiently recruit INCENP/Aurora B to metaphase kinetochores (Liu et al., 2009). LAP-tagged Mis12-Mps1 (LAP-Mis12-Mps1-WT) was readily visible on kinetochores in prophase, prometaphase, and metaphase (Fig. 2 A) at a similar location as wild-type Mps1 (nocodazole-treated; Fig. S2 A). Mis12-Mps1 fully supported mitotic checkpoint activity in Mps1-depleted cells, showing the fusion did not prevent Mps1 functioning (Fig. 2 B). Please note that ∼60% of Mis12-Mps1 associated transiently with kinetochores (Fig. S2, B and C), indicating that this approach could not tether all cellular Mps1 to kinetochores and thus maintained essential cytoplasmic pools of Mps1 (Maciejowski et al., 2010). We speculate that Mps1 dimerization (Hewitt et al., 2010) and/or targeting via the Mps1-intrinsic kinetochore localization domain may have been responsible for recruitment and release of this exchanging pool of Mis12-Mps1. Nevertheless, a significant fraction (∼40%) of Mis12-Mps1 was stably associated with kinetochores (Fig. S2 B), allowing evaluation of the effect of sustained presence of Mps1 at kinetochores on chromosome segregation.
Figure 2.
Tethering Mps1 to kinetochores extends metaphase. (A) Immunolocalization of LAP-Mis12-Mps1(-WT) in U2OS during indicated phases of cell cycle. (B) Percentage of mitotic (Mpm2 positive) U2OS cells that were transfected and treated as indicated, as determined by flow cytometry. Immunoblot shows levels of LAP-Mps1 and LAP-Mis12-Mps1 in total cell lysate. (C and D) Time spent in prometaphase and metaphase of transiently transfected U2OS cells with indicated plasmids and treated as indicated. Each horizontal bar represents a single cell.
Strikingly, expression of Mis12-Mps1-WT but not -KD or LAP-Mps1-WT in U2OS cells caused pronounced extension of metaphase in more than 70% of cells, even up to 10 h in some cases (Fig. 2 C; Fig. S2, D and E). Treatment with Mps1-IN-1 showed that these prolonged metaphases were due to sustained Mps1 activity on kinetochores (Fig. 2 C). Removal of Mad2 by expression of Mad2 shRNA effectively prevented the delays in anaphase onset in cells expressing Mis12-Mps1, showing Mad2 and thus mitotic checkpoint activity was responsible for the delays (Fig. 2 D).
Sustained Mps1 activity at kinetochores prevents mitotic checkpoint silencing
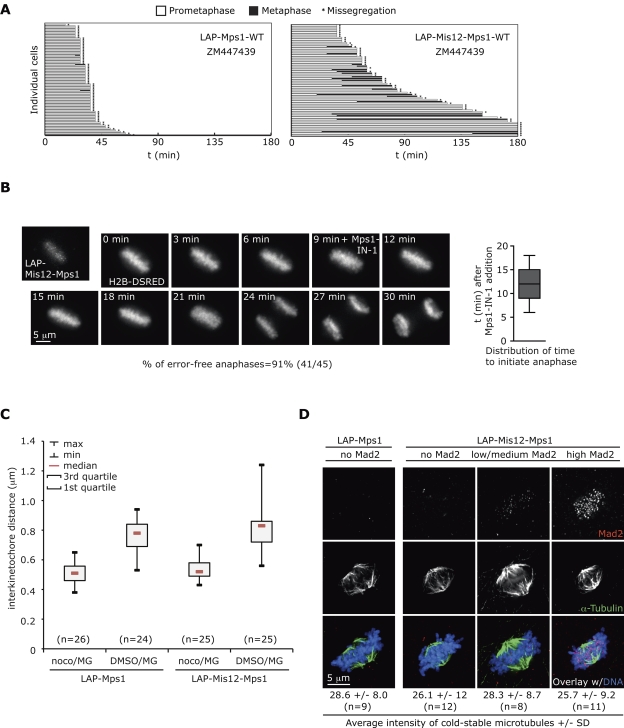
The previous data suggest that removal of Mps1 is required for efficient checkpoint silencing, or, alternatively, that persistent kinetochore Mps1 might have caused unstable attachments that engaged the mitotic checkpoint, for instance by increasing local Aurora B activity or otherwise affecting kinetochore function. Five lines of evidence strongly argued against the presence of unstable attachments. First, if Aurora B–mediated destabilization of kinetochore microtubules promoted checkpoint activity in Mis12-Mps1–expressing cells, stabilization of these attachments by inhibition of Aurora B is predicted to revert the extension of metaphase in these cells, much like in the case of monastrol- or taxol-treated cells (Hauf et al., 2003; Yang et al., 2009). However, addition of ZM447439 to cells expressing Mis12-Mps1 did not prevent the mitotic extension (Fig. 3 A). Second, attachment defects that can engage the checkpoint for hours are expected to have at least some effect on ability of cells to quickly biorient all chromosomes (see Hanisch et al., 2006; Gaitanos et al., 2009; Liu et al., 2009; Raaijmakers et al., 2009; and Fig. S2, F and G for examples). Mis12-Mps1–expressing cells did not show any significant increase in prometaphase time (Fig. S2 H). Third, forced anaphase initiation in Mis12-Mps1–expressing, metaphase-arrested cells via addition of Mps1-IN-1 showed no increase in segregation errors compared with control cells (∼10%; Jelluma et al., 2008a,b; Videos 1 and 2; Fig. 3 B). Such increase would be expected if metaphase cells with (minor) attachment defects are induced to undergo anaphase. Fourth, interkinetochore distances of chromosomes in Mis12-Mps1 cells were similar to control cells, with no sisters that were under less tension than any of the sister pairs in control cells (Fig. 3 C). Fifth, overall microtubule density and appearance of cold-stable kinetochore fibers upon Mis12-Mps1 expression was indistinguishable from that of normal cells (Fig. 3 D).
Figure 3.
Sustained Mps1 activity at kinetochores does not affect microtubule attachment or chromosome biorientation. (A) Time spent in prometaphase and metaphase of U2OS cells transfected with indicated plasmids and treated as indicated. Each horizontal bar represents a single cell. (B) Stills of Video 1 showing a LAP-Mis12-Mps1-WT–expressing U2OS cell after Mps1-IN-1 addition during metaphase. Graph shows distribution of time after Mps1-IN-1 addition for cells to initiate anaphase. Average time is 12.4 min ± 3.0 min (SD), n = 45 cells. (C) Distribution of interkinetochore distances in U2OS cells transfected and treated as indicated. For LAP-Mis12-Mps1–expressing cells, only the cells showing clear Mad2 staining on kinetochores were analyzed. (D) Cold-stable microtubules in U2OS cells expressing the indicated constructs. Examples are shown for metaphases that show no, low/medium, or high Mad2 at kinetochores. Averages of absolute total spindle intensities per cell are indicated (±SD).
Kinetochore-tethered Mps1 maintains Mad1 and Mad2 on attached, bioriented kinetochores
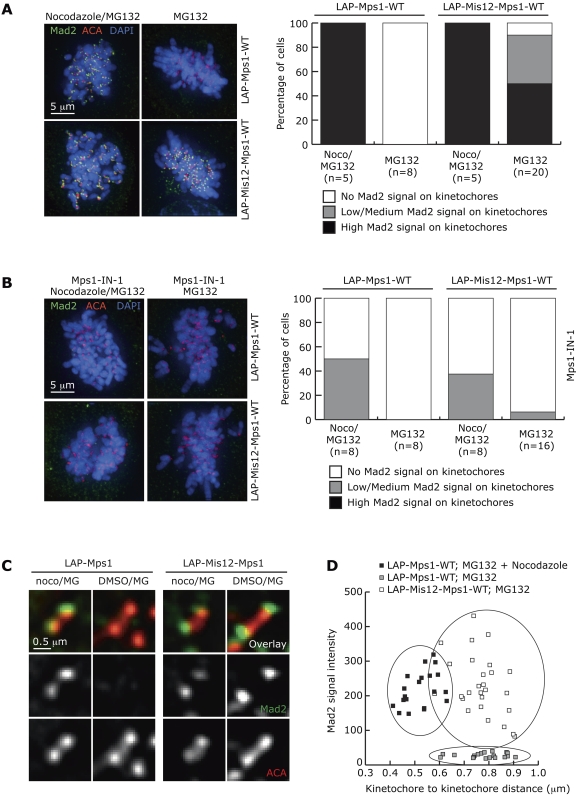
Despite normal attachment and alignment, Mad1 and Mad2 were localized to kinetochores of bioriented chromosomes in metaphase cells expressing Mis12-Mps1, but not LAP-Mps1 (Fig. 4 A and Fig. S3), and this depended on Mps1 kinase activity (Fig. 4 B). Strikingly, high levels of Mad2, similar to those in nocodazole-treated cells, were apparent on kinetochore pairs that were under full tension (Fig. 4, C and D) and that had normal, cold-stable k-fibers (Fig. 3 D). Importantly, Mis12-Mps1–expressing metaphase cells with kinetochore-bound Mad2 contained Mad2 on all kinetochores (Fig. 4 A). In contrast, metaphase figures of cells with slight destabilization of k-fibers or other attachment defects that allow alignment but significantly prolong prometaphase and metaphase had no or very few kinetochores with detectable Mad1 or Mad2 (Fig. S2 G; Hanisch et al., 2006; Daum et al., 2009; Liu et al., 2009). The results strongly suggest that preventing Mps1 from leaving kinetochores is sufficient to cause persistent mitotic checkpoint-mediated APC/C inhibition, independent of the attachment status of kinetochores, and argue that Mps1 removal is a prerequisite for mitotic checkpoint silencing. Interestingly, prolonged metaphases with high levels of Mad1 and Mad2 on bioriented chromosomes were also observed when Spindly–Dynein interaction was inhibited but not when Spindly was depleted (Barisic et al., 2010; Gassmann et al., 2010), showing that Spindly removal, like Mps1 removal, is a prerequisite for checkpoint silencing. It may be of interest to examine whether Mps1 influences Spindly function or vice versa.
Figure 4.
Persistent presence of Mps1 maintains Mad1 and Mad2 on attached, bioriented kinetochores. (A and B) Mad2 localization at kinetochores of U2OS cells transiently transfected with LAP-Mps1-WT or LAP-Mis12-Mps1-WT, treated as indicated. Graph shows distribution of cells with high, low/medium, or no Mad2 signal on kinetochores. (C) Close-up of Mad2 localization at individual kinetochore pairs of U2OS cells treated and transfected as in A. (D) Mad2 intensity related to interkinetochore distance of kinetochore pairs in cells transfected and treated as in A.
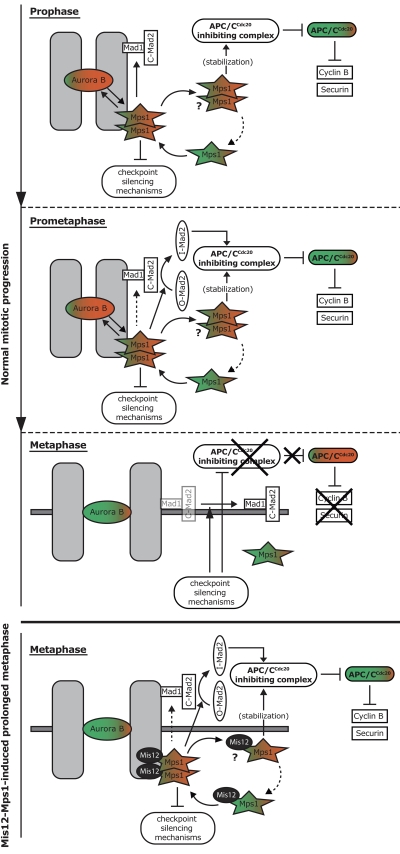
A model for mitotic checkpoint regulation by Mps1
Combining the present and recent studies (Howell et al., 2001, 2004; Jelluma et al., 2008a,b; Tighe et al., 2008; Hewitt et al., 2010; Maciejowski et al., 2010; Santaguida et al., 2010; Sliedrecht et al., 2010), we propose the following model for the roles of Mps1 in the control of the metaphase-to-anaphase transition (Fig. 5): in prophase and prometaphase, Mps1 is recruited to unattached and/or tension-less kinetochores where its activity, possibly aided by dimerization, promotes error correction and ensures kinetochore-dependent catalysis of APC/CCdc20 inhibitory complexes via establishing kinetochore binding of Mad1, via conformational activation of Mad2, and via inhibition of checkpoint silencing mechanisms. Once activated, Mps1 contributes to its own release from kinetochores to ensure cytoplasmic stabilization of APC/C–Cdc20 inhibitory complexes and to allow its removal from kinetochores for efficient checkpoint silencing once stable, bioriented attachment has been achieved. Deep understanding of how Mps1 promotes these various processes will require the identification of the direct Mps1 substrates and elucidation of the recruitment mechanisms of Mps1 and the checkpoint machinery.
Figure 5.
A model for mitotic checkpoint regulation by Mps1. Prophase: Mps1 is recruited in an Aurora B–dependent manner to unattached and/or tensionless kinetochores, where its activity is stimulated (red), possibly through dimerization. At kinetochores, Mps1 promotes error correction by enhancing Aurora B activity, ensures kinetochore binding of Mad1, inhibits checkpoint silencing mechanisms, and replenishes an interphasic pool of cytoplasmic Mps1 that stabilizes APC/CCdc20 inhibitory complexes. Prometaphase: After establishing Mad1 localization, Mps1 promotes kinetochore-dependent catalysis of APC/CCdc20 inhibitory complexes via conformational activation of Mad2, and contributes to its own removal from kinetochores. Dotted arrow indicates possibility that Mps1 contributes to maintenance of Mad1 at unattached kinetochores. The unknown details of what pools of Mps1 are dimers is represented by the question mark. Metaphase: The fast turnover of Mps1 at kinetochores allows its removal from kinetochores after stable, bioriented attachment, causing checkpoint silencing and ultimately APC/CCdc20 activity toward Cyclin B and Securin. Mis12-Mps1–induced prolonged metaphase: When Mps1 is not removed from kinetochores after biorientation, checkpoint silencing cannot occur. Question mark indicates the likely contribution of a cycling, and thus also cytoplasmic, pool of Mis12-Mps1 (∼60%) with the uncertainty of whether this fusion protein functions as a dimer.
Materials and methods
Cell culture, plasmids, and transfections
U2OS and HeLa cells were grown in DME with 8% FBS, supplemented with penicillin/streptomycin. UTRM10-WT cells (Jelluma et al., 2008a) and UTRM-LAP-Mps1M602G cells (Sliedrecht et al., 2010) were grown in DME with 8% FBS, supplemented with penicillin/streptomycin, and 1 mg ml−1 doxycycline (Sigma-Aldrich) for continuous knockdown of the endogenous protein (Jelluma et al., 2008a). PtK2 cells (a gift from Jagesh Shah, Harvard Institutes of Medicine, Boston, MA) were grown in EMEM with 10% FBS, supplemented with glutamine, nonessential amino acids, and penicillin/streptomycin.
pSuper-Mock, pSuper-Mps1, pcDNA-LAPMps1, and pSuper-Mad2 have been described previously (Kops et al., 2004; Jelluma et al., 2008b). pcDNA-LAPMps1-M602G was created by site-directed mutagenesis of pcDNA-LAPMps1. Endogenous Mps1 replacement assays were done as in Jelluma et al. (2008b). To add a PTS1 signal to Mps1, point mutations in pcDNA3-LAP-Mps1 construct were introduced by site-directed mutagenesis to alter the last three C-terminal amino acids to Serine-Lysine-Leucine or to Alanine-Lysine-Leucine (Gould et al., 1989). pcDNA-LAP-Mis12-Mps1 constructs were created by inserting the full Mis12 sequence in pcDNA-LAP-Mps1. All sequences were verified by automated sequencing. Plasmid transfections in U2OS cells were done with calcium phosphate, and LAP-Mps1-WT was expressed transiently in PtK2 cells via standard electroporation.
Time-lapse live-cell imaging
U2OS cells were grown in 8-well chambered glass-bottom slides (LabTek), and co-transfected with the indicated plasmids and H2B-pEYFP or H2B-pDSRED for visualization of DNA. Cells were blocked in S phase with 2.5 mM thymidine (Sigma-Aldrich) 24 h after transfection for 24 h. After release from thymidine, mitotic progression was followed with live-cell imaging as described below. For prometaphase time measurements in Fig. S2 F, stable H2B-EYFP–expressing HeLa cells were treated with 10 or 50 µM noscapine (Sigma-Aldrich) or with 20 or 660 nM nocodazole (Sigma-Aldrich). See Vasquez et al. (1997) and Zhou et al. (2002) for effects of noscapine and low nocodazole on microtubule dynamics. Live-cell imaging was done on a microscope (model IX-81; Olympus), controlled by Cell-M software (Olympus), in a heated chamber (37°C and 5% CO2) using a 20x/0.5NA UPLFLN objective. Every 3 min fluorescent images of H2B-EYFP were acquired (15-msec exposure) with a camera (ORCA-ER; Hamamatsu Photonics). Images were processed for analysis to maximum intensity projections of all Z-planes and using Cell-M software.
Immunoblotting and immunofluorescence
Immunoblotting was performed according to standard procedures; antibodies used were anti–Mps1-NT (Millipore) and anti–α-tubulin (Sigma-Aldrich).
For immunofluorescence analysis, cells were treated 45 min before fixation with the indicated compounds: 660 nM nocodazole (Sigma-Aldrich), 10 µM MG132 (Sigma-Aldrich), 2 µM ZM447439 (Tocris Bioscience), 1 µM 23dMB-PP1 (a gift from Chao Zhang and Kevan Shokat, UCSF, San Francisco, CA), and 10 µM Mps1-IN-1 (a gift from N. Gray; Kwiatkowski et al., 2010). Cold-shock treatments were done by replacing warm media with ice-cold media for 15 min before fixation. Fixation and immunostaining were done as described in Jelluma et al. (2008b). Anti–Mps1-NT (Millipore), anti-GFP (custom rabbit serum), anti–phospho-(T232)-Aurora B (Rockland), anti-Mad2 (custom rabbit serum), anti-Mad1 (a gift from Andrea Musacchio, IFOM-IEO, Milan, Italy), and anti–α-tubulin (Roche) were used as primary antibodies and detected with Alexa Fluor 488, 568, or 647 (Invitrogen). Images were acquired at room temperature on a DeltaVision RT system (Applied Precision) with a 100x/1.40 NA UPlanSApo objective (Olympus) equipped with a CoolSnap HQ camera and using SoftWorx software. Images are maximum projections of a deconvolved stack and adjusted (identically within experiments) with SoftWorx and Adobe Photoshop CS3. Quantitations were done as described previously (Jelluma et al., 2008b).
FRAP
UTRM-LAP-Mps1M602G cells were grown in glass-bottom dishes (Willco-Wells), released from a 24-h thymidine block in nocodazole for 16 h. The media was replaced with Leibovitz L-15 media (Invitrogen) supplemented with 10% FCS, 2 mM glutamine, and 100 U/ml penicillin/streptomycin, and cells were transferred to an incubator with atmospheric CO2 at 37°C. Cells were treated with 10 µM MG132 (Sigma-Aldrich) ± 1 µM 23dMB-PP1 30 min before imaging. Samples were imaged on a microscope (LSM 510 META; Carl Zeiss, Inc.) equipped with a heated chamber and lens warmer (both set at 37°C), using Zeiss LSM software. The EYFP-based LAP-tag of LAP-Mps1M602G was both excited and bleached using the 514-nm laser line of an Argon laser (max 30 mW) set to 60% (Tube current 5.5 A). Excitation was done using 6% laser power and emission was detected on the META detector set from 529 to 614 nm. Areas of 25 × 25 pixels (0.81 µm2) were bleached at 100% laser power for 10 iterations once the fluorescence signal of LAP-Mps1M602G had become stable for a few seconds (after ∼6 s). Fluorescence intensity in the bleached square was acquired every 125 ms before and after bleaching. For each measurement, the average fluorescence intensity in the 25 × 25-pixel square during the last second before bleaching was set to 100% and the measured signal after bleaching was normalized to this value. Because kinetochores are highly mobile, only those measurements were taken into account in which the kinetochore remained visible within the 25 × 25-pixel square during the entire measurement. FRAP analysis: Log transformation of the FRAP data indicates that in our experiments the recovery follows a double exponential (log transformation was done by plotting −ln((Finf − F(t))/(Finf − F(0))) vs. time where Finf is the fluorescence reached at the plateau, F(t) is the fluorescence at a certain time point, and F(0) is the fluorescence observed immediately after bleaching, as was done in Howell et al. (2004). The double exponential that we observe consists of a fast component that equals the kinetics found for cytoplasmic LAP-Mps1M602G, both in the uninhibited and inhibited states. The slow component in the double exponential represents the LAP-Mps1 kinetics on the kinetochore. Recovery half-times were determined by double-exponential curve fitting using GraphPad Prism software that confirmed the log transformation analysis. FRAP measurements of LAP-Mps1 in PtK2 cells, after inhibition with 10 µM Mps1-IN-1, were done as described above.
Flow cytometry analysis
Cells were released from a 24-h thymidine-induced block into nocodazole for 16 h and analyzed by flow cytometry as described previously (Kops et al., 2005). Flow cytometric analysis of transfected cells was based on Spectrin-GFP expression.
Measurement of sister interkinetochore distances
Cells were grown on cover-glasses, transfected, treated, and fixed as described above. Center-to-center distances of the ACA dots of sisters within the same focal plane were measured using SoftWorx software.
Online supplemental material
Figure S1 shows that Mps1 auto-regulates its turnover at kinetochores. Figure S2 shows that a pool of Mis12-Mps1 is tethered to kinetochores and causes prolonged metaphases without detectable prior defects in prometaphase. Figure S3 shows that Mad1 and Mad2 localize to metaphase kinetochores of Mis12-Mps1–expressing cells. Videos 1 and 2 show two examples of Mis12-Mps1–expressing, metaphase-arrested cells that were forced to initiate anaphase via addition of Mps1-IN-1. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201003038/DC1.
Acknowledgments
We thank Jagesh Shah and Yinghua Guan for efforts on Mps1 FRAP and for PtK2 cells; Adrian Saurin, Chao Zhang, Kevan Shokat, Nathanael Gray, Andrea Musacchio, and Susanne Lens for providing reagents; the Kops, Medema, and Lens laboratories for discussions and insights; and Adrian Saurin and René Medema for critically reading the manuscript.
This work was supported by a VIDI grant of the Netherlands Organization for Scientific Research (NWO-91776336), an ERC Starting Independent Researcher Grant (KINSIGN) to G.J.P.L. Kops, and by the Netherlands Genomics Initiative.
Footnotes
Abbreviations used in this paper:
- APC/C
- anaphase-promoting complex/cyclosome
- KD
- kinase dead
- WT
- wild type
References
- Abrieu A., Magnaghi-Jaulin L., Kahana J.A., Peter M., Castro A., Vigneron S., Lorca T., Cleveland D.W., Labbé J.C. 2001. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 106:83–93 10.1016/S0092-8674(01)00410-X [DOI] [PubMed] [Google Scholar]
- Barisic M., Sohm B., Mikolcevic P., Wandke C., Rauch V., Ringer T., Hess M., Bonn G., Geley S. 2010. Spindly/CCDC99 is required for efficient chromosome congression and mitotic checkpoint regulation. Mol. Biol. Cell. 21:1968–1981 10.1091/mbc.E09-04-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourhis E., Lingel A., Phung Q., Fairbrother W.J., Cochran A.G. 2009. Phosphorylation of a borealin dimerization domain is required for proper chromosome segregation. Biochemistry. 48:6783–6793 10.1021/bi900530v [DOI] [PubMed] [Google Scholar]
- Cheeseman I.M., Niessen S., Anderson S., Hyndman F., Yates J.R., III, Oegema K., Desai A. 2004. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 18:2255–2268 10.1101/gad.1234104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum J.R., Wren J.D., Daniel J.J., Sivakumar S., McAvoy J.N., Potapova T.A., Gorbsky G.J. 2009. Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr. Biol. 19:1467–1472 10.1016/j.cub.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield C., Johnson V.L., Tighe A., Ellston R., Haworth C., Johnson T., Mortlock A., Keen N., Taylor S.S. 2003. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161:267–280 10.1083/jcb.200208091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeut J., Gaussen A., Bieling P., Morin V., Prieto S., Fesquet D., Surrey T., Abrieu A. 2008. Phosphorylation relieves autoinhibition of the kinetochore motor Cenp-E. Mol. Cell. 29:637–643 10.1016/j.molcel.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Fischer M.G., Heeger S., Häcker U., Lehner C.F. 2004. The mitotic arrest in response to hypoxia and of polar bodies during early embryogenesis requires Drosophila Mps1. Curr. Biol. 14:2019–2024 10.1016/j.cub.2004.11.008 [DOI] [PubMed] [Google Scholar]
- Fisk H.A., Winey M. 2001. The mouse Mps1p-like kinase regulates centrosome duplication. Cell. 106:95–104 10.1016/S0092-8674(01)00411-1 [DOI] [PubMed] [Google Scholar]
- Gaitanos T.N., Santamaria A., Jeyaprakash A.A., Wang B., Conti E., Nigg E.A. 2009. Stable kinetochore-microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. EMBO J. 28:1442–1452 10.1038/emboj.2009.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R., Holland A.J., Varma D., Wan X., Civril F., Cleveland D.W., Oegema K., Salmon E.D., Desai A. 2010. Removal of Spindly from microtubule-attached kinetochores controls spindle checkpoint silencing in human cells. Genes Dev. 24:957–971 10.1101/gad.1886810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S.J., Keller G.A., Hosken N., Wilkinson J., Subramani S. 1989. A conserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 108:1657–1664 10.1083/jcb.108.5.1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch A., Silljé H.H., Nigg E.A. 2006. Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. EMBO J. 25:5504–5515 10.1038/sj.emboj.7601426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick K.G., Weiss E., Luca F.C., Winey M., Murray A.W. 1996. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 273:953–956 10.1126/science.273.5277.953 [DOI] [PubMed] [Google Scholar]
- Hauf S., Cole R.W., LaTerra S., Zimmer C., Schnapp G., Walter R., Heckel A., van Meel J., Rieder C.L., Peters J.M. 2003. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161:281–294 10.1083/jcb.200208092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Jones M.H., Winey M., Sazer S. 1998. Mph1, a member of the Mps1-like family of dual specificity protein kinases, is required for the spindle checkpoint in S. pombe. J. Cell Sci. 111:1635–1647 [DOI] [PubMed] [Google Scholar]
- Hewitt L., Tighe A., Santaguida S., White A.M., Jones C.D., Musacchio A., Green S., Taylor S.S. 2010. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J. Cell Biol. 190:25–34 10.1083/jcb.201002133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.J., McEwen B.F., Canman J.C., Hoffman D.B., Farrar E.M., Rieder C.L., Salmon E.D. 2001. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 155:1159–1172 10.1083/jcb.200105093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.J., Moree B., Farrar E.M., Stewart S., Fang G., Salmon E.D. 2004. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr. Biol. 14:953–964 10.1016/j.cub.2004.05.053 [DOI] [PubMed] [Google Scholar]
- Jelluma N., Brenkman A.B., McLeod I., Yates J.R., III, Cleveland D.W., Medema R.H., Kops G.J.P.L. 2008a. Chromosomal instability by inefficient Mps1 auto-activation due to a weakened mitotic checkpoint and lagging chromosomes. PLoS One. 3:e2415 10.1371/journal.pone.0002415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelluma N., Brenkman A.B., van den Broek N.J.F., Cruijsen C.W.A., van Osch M.H.J., Lens S.M.A., Medema R.H., Kops G.J.P.L. 2008b. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 132:233–246 10.1016/j.cell.2007.11.046 [DOI] [PubMed] [Google Scholar]
- Kang J., Chen Y., Zhao Y., Yu H. 2007. Autophosphorylation-dependent activation of human Mps1 is required for the spindle checkpoint. Proc. Natl. Acad. Sci. USA. 104:20232–20237 10.1073/pnas.0710519105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops G.J., Foltz D.R., Cleveland D.W. 2004. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc. Natl. Acad. Sci. USA. 101:8699–8704 10.1073/pnas.0401142101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops G.J., Kim Y., Weaver B.A., Mao Y., McLeod I., Yates J.R., III, Tagaya M., Cleveland D.W. 2005. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 169:49–60 10.1083/jcb.200411118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski N., Jelluma N., Filippakopoulos P., Soundararajan M., Manak M.S., Kwon M., Choi H.G., Sim T., Deveraux Q.L., Rottmann S., et al. 2010. Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nat. Chem. Biol. 6:359–368 10.1038/nchembio.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W., Cleveland D.W. 2010. A chemical tool box defines mitotic and interphase roles for Mps1 kinase. J. Cell Biol. 190:21–24 10.1083/jcb.201006080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Vader G., Vromans M.J.M., Lampson M.A., Lens S.M.A. 2009. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 323:1350–1353 10.1126/science.1167000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.T., Chan G.K., Hittle J.C., Fujii G., Lees E., Yen T.J. 2003. Human MPS1 kinase is required for mitotic arrest induced by the loss of CENP-E from kinetochores. Mol. Biol. Cell. 14:1638–1651 10.1091/mbc.02-05-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejowski J., George K.A., Terret M.E., Zhang C., Shokat K.M., Jallepalli P.V. 2010. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J. Cell Biol. 190:89–100 10.1083/jcb.201001050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli M., Filipp F.V., Rancati G., Massimiliano L., Nezi L., Stier G., Hagan R.S., Confalonieri S., Piatti S., Sattler M., Musacchio A. 2006. Determinants of conformational dimerization of Mad2 and its inhibition by p31comet. EMBO J. 25:1273–1284 10.1038/sj.emboj.7601033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Lluesma S., Stucke V.M., Nigg E.A. 2002. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 297:2267–2270 10.1126/science.1075596 [DOI] [PubMed] [Google Scholar]
- Mattison C.P., Old W.M., Steiner E., Huneycutt B.J., Resing K.A., Ahn N.G., Winey M. 2007. Mps1 activation loop autophosphorylation enhances kinase activity. J. Biol. Chem. 282:30553–30561 10.1074/jbc.M707063200 [DOI] [PubMed] [Google Scholar]
- Meraldi P., Draviam V.M., Sorger P.K. 2004. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell. 7:45–60 10.1016/j.devcel.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Musacchio A., Salmon E.D. 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8:379–393 10.1038/nrm2163 [DOI] [PubMed] [Google Scholar]
- Obuse C., Iwasaki O., Kiyomitsu T., Goshima G., Toyoda Y., Yanagida M. 2004. A conserved Mis12 centromere complex is linked to heterochromatic HP1 and outer kinetochore protein Zwint-1. Nat. Cell Biol. 6:1135–1141 10.1038/ncb1187 [DOI] [PubMed] [Google Scholar]
- Raaijmakers J.A., Tanenbaum M.E., Maia A.F., Medema R.H. 2009. RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment. J. Cell Sci. 122:2436–2445 10.1242/jcs.051912 [DOI] [PubMed] [Google Scholar]
- Reddy S.K., Rape M., Margansky W.A., Kirschner M.W. 2007. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 446:921–925 10.1038/nature05734 [DOI] [PubMed] [Google Scholar]
- Santaguida S., Tighe A., D’Alise A.M., Taylor S.S., Musacchio A. 2010. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J. Cell Biol. 190:73–87 10.1083/jcb.201001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliedrecht T., Zhang C., Shokat K.M., Kops G.J. 2010. Chemical genetic inhibition of Mps1 in stable human cell lines reveals novel aspects of Mps1 function in mitosis. PLoS One. 5:e10251 10.1371/journal.pone.0010251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucke V.M., Silljé H.H., Arnaud L., Nigg E.A. 2002. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 21:1723–1732 10.1093/emboj/21.7.1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe A., Staples O., Taylor S. 2008. Mps1 kinase activity restrains anaphase during an unperturbed mitosis and targets Mad2 to kinetochores. J. Cell Biol. 181:893–901 10.1083/jcb.200712028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez R.J., Howell B., Yvon A.M., Wadsworth P., Cassimeris L. 1997. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell. 8:973–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Winey M. 1996. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 132:111–123 10.1083/jcb.132.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G., Luo X., Habu T., Rizo J., Matsumoto T., Yu H. 2004. Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 23:3133–3143 10.1038/sj.emboj.7600322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Kenny A.E., Brito D.A., Rieder C.L. 2009. Cells satisfy the mitotic checkpoint in Taxol, and do so faster in concentrations that stabilize syntelic attachments. J. Cell Biol. 186:675–684 10.1083/jcb.200906150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Gupta K., Yao J., Ye K., Panda D., Giannakakou P., Joshi H.C. 2002. Paclitaxel-resistant human ovarian cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine. J. Biol. Chem. 277:39777–39785 10.1074/jbc.M203927200 [DOI] [PubMed] [Google Scholar]