Abstract
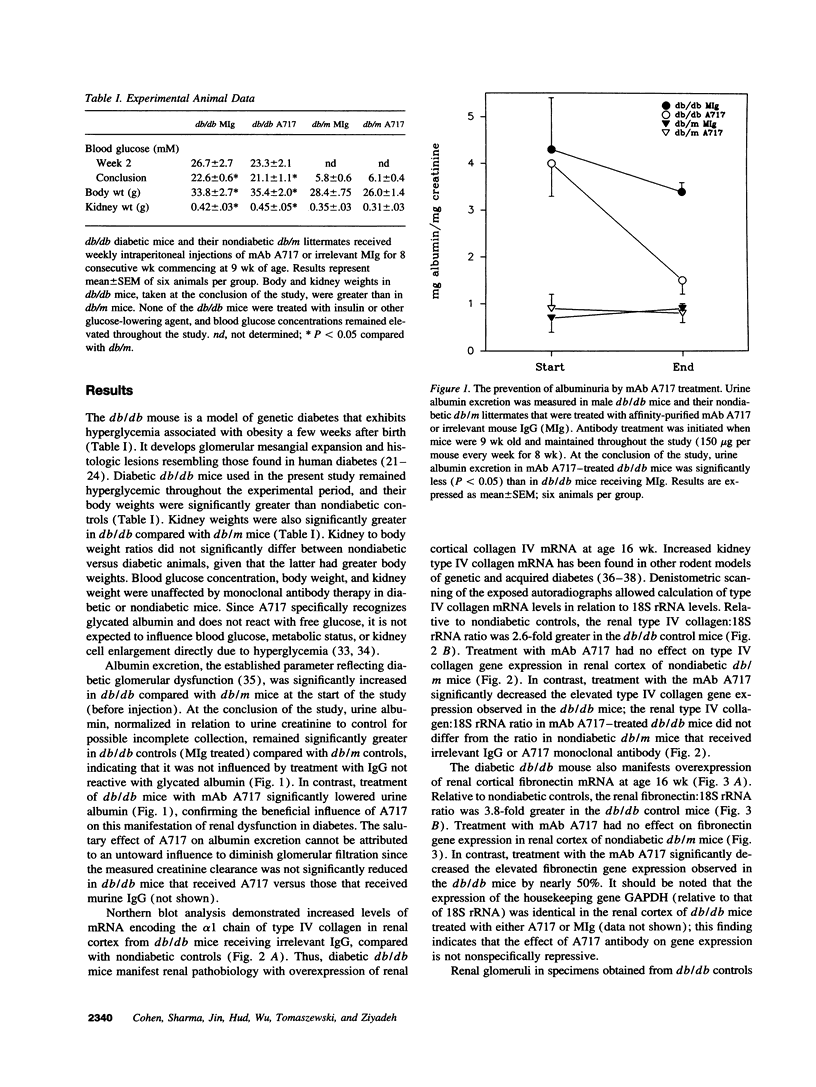
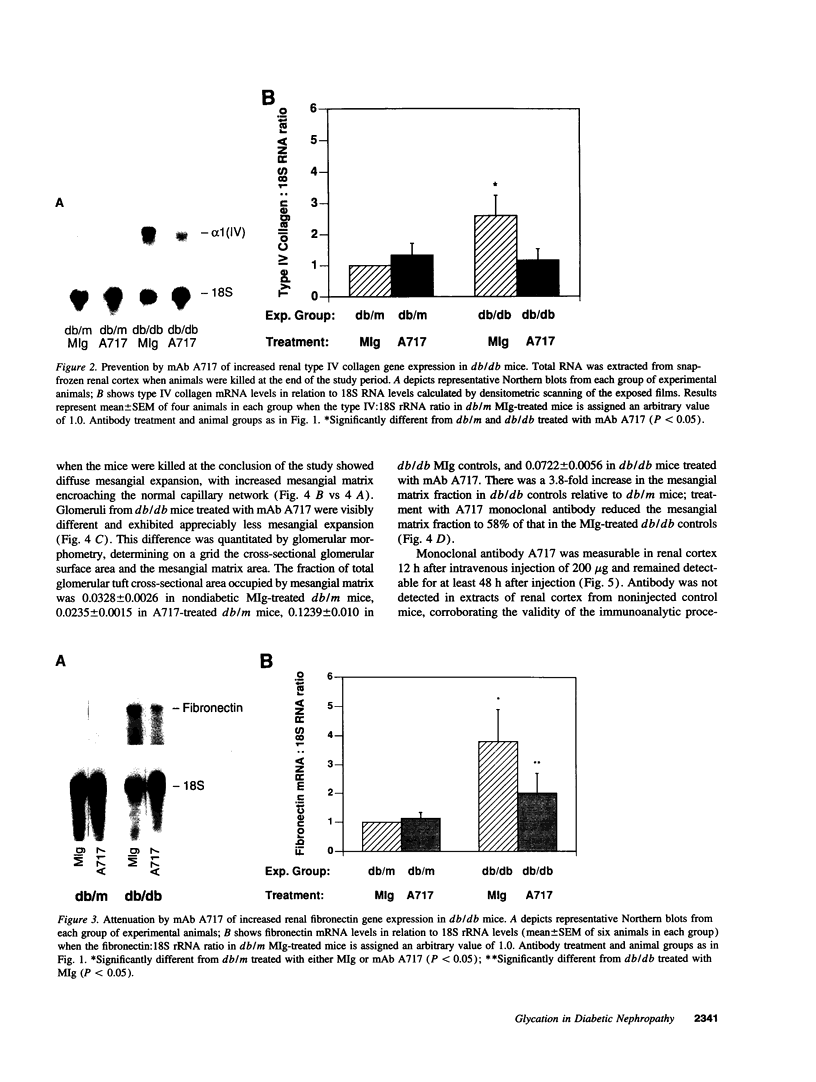
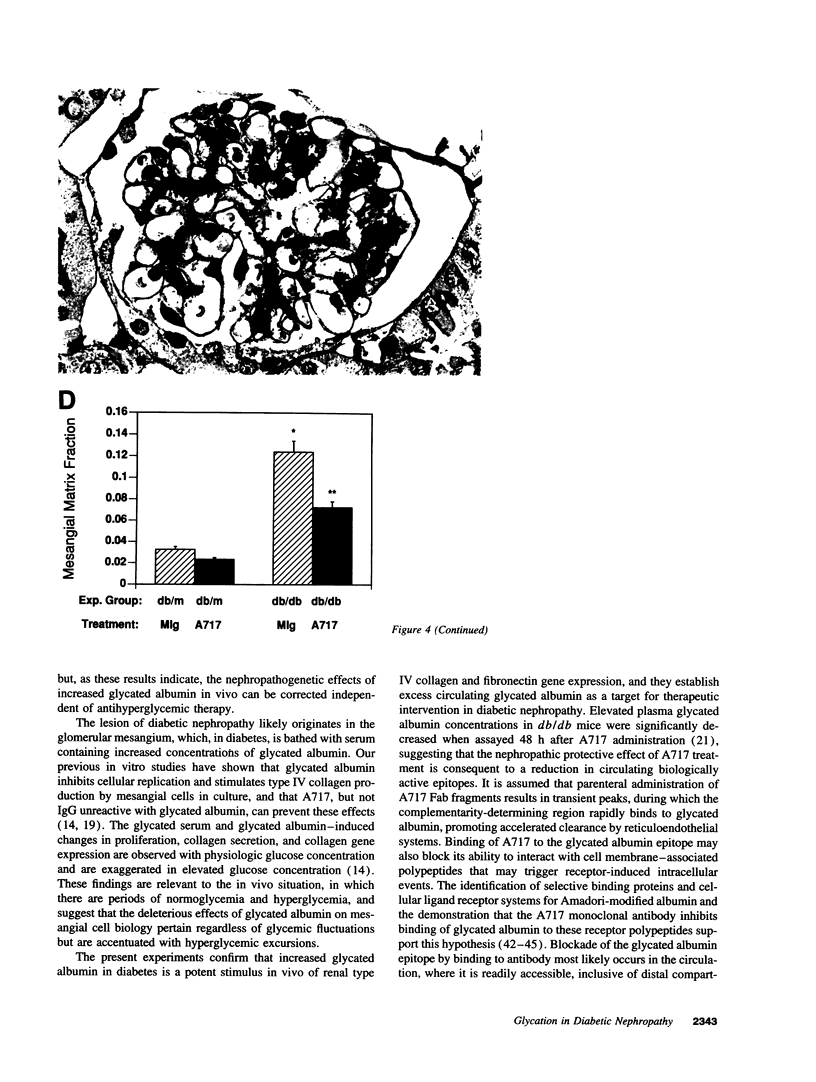
Accelerated protein glycation in diabetes has been mechanistically linked to the pathogenesis of diabetic nephropathy. Because glycated albumin induces abnormalities in cultured mesangial cells that resemble those characterizing the glomerular mesangium in diabetes, and monoclonal antibodies (A717) specific for Amadori-modified glycated albumin prevent these abnormalities, we postulated that in vivo administration of A717 could retard the progression of diabetic nephropathy. To test this hypothesis, diabetic db/db mice and their nondiabetic db/m littermates were treated with eight consecutive weekly injections of 150 micrograms of A717 (Fab fragments) to reduce the elevated plasma glycated albumin concentration, or with irrelevant murine IgG (MIg). Relative to nondiabetics, diabetic mice (MIg treated) manifested proteinuria (3.35 +/- 0.15 vs 0.87 +/- 0.1 mg albumin/mg creatinine), 3.8-fold increase in mesangial matrix fraction, and renal cortical overexpression of mRNAs encoding alpha 1(IV) collagen (2.6-fold increase) and fibronectin (3.8-fold increase). Treatment of db/db mice with A717 significantly reduced the proteinuria (1.52 +/- 0.3 mg/mg creatinine), inhibited mesangial matrix expansion, and attenuated overexpression of matrix mRNAs. The nephropathic protective effects of A717 were independent of any change in blood glucose concentrations. Antibodies unreactive with glycated albumin did not duplicate the beneficial effects of A717. Thus, abrogating the biologic effects of increased glycated albumin with A717 has a salutary influence on the pathogenesis of diabetic nephropathy and has novel therapeutic potential in its management.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrass C. K., Peterson C. V., Raugi G. J. Phenotypic expression of collagen types in mesangial matrix of diabetic and nondiabetic rats. Diabetes. 1988 Dec;37(12):1695–1702. doi: 10.2337/diab.37.12.1695. [DOI] [PubMed] [Google Scholar]
- Andersen A. R., Christiansen J. S., Andersen J. K., Kreiner S., Deckert T. Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983 Dec;25(6):496–501. doi: 10.1007/BF00284458. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Kooney A., Ulrich P., Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986 Jun 27;232(4758):1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Hud E. Measurement of plasma glycoalbumin levels with a monoclonal antibody based ELISA. J Immunol Methods. 1989 Sep 1;122(2):279–283. doi: 10.1016/0022-1759(89)90275-5. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Hud E. Production and characterization of monoclonal antibodies against human glycoalbumin. J Immunol Methods. 1989 Feb 8;117(1):121–129. doi: 10.1016/0022-1759(89)90126-9. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Hud E., Wu V. Y. Amelioration of diabetic nephropathy by treatment with monoclonal antibodies against glycated albumin. Kidney Int. 1994 Jun;45(6):1673–1679. doi: 10.1038/ki.1994.219. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Ziyadeh F. N. Amadori glucose adducts modulate mesangial cell growth and collagen gene expression. Kidney Int. 1994 Feb;45(2):475–484. doi: 10.1038/ki.1994.62. [DOI] [PubMed] [Google Scholar]
- Day J. F., Ingebretsen C. G., Ingebretsen W. R., Jr, Baynes J. W., Thorpe S. R. Nonenzymatic glucosylation of serum proteins and hemoglobin: response to changes in blood glucose levels in diabetic rats. Diabetes. 1980 Jul;29(7):524–527. doi: 10.2337/diab.29.7.524. [DOI] [PubMed] [Google Scholar]
- Durie F. H., Fava R. A., Foy T. M., Aruffo A., Ledbetter J. A., Noelle R. J. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993 Sep 3;261(5126):1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- Fabre J., Balant L. P., Dayer P. G., Fox H. M., Vernet A. T. The kidney in maturity onset diabetes mellitus: a clinical study of 510 patients. Kidney Int. 1982 May;21(5):730–738. doi: 10.1038/ki.1982.90. [DOI] [PubMed] [Google Scholar]
- Fukui M., Nakamura T., Ebihara I., Shirato I., Tomino Y., Koide H. ECM gene expression and its modulation by insulin in diabetic rats. Diabetes. 1992 Dec;41(12):1520–1527. doi: 10.2337/diab.41.12.1520. [DOI] [PubMed] [Google Scholar]
- Guthrow C. E., Morris M. A., Day J. F., Thorpe S. R., Baynes J. W. Enhanced nonenzymatic glucosylation of human serum albumin in diabetes mellitus. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4258–4261. doi: 10.1073/pnas.76.9.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton M. W., Richardson M., Winocour P. D. On glucose transport and non-enzymic glycation of proteins in vivo. J Theor Biol. 1993 Apr 21;161(4):481–490. doi: 10.1006/jtbi.1993.1068. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Bunn H. F. Kinetic analysis of the nonenzymatic glycosylation of hemoglobin. J Biol Chem. 1981 May 25;256(10):5204–5208. [PubMed] [Google Scholar]
- Ihm C. G., Lee G. S., Nast C. C., Artishevsky A., Guillermo R., Levin P. S., Glassock R. J., Adler S. G. Early increased renal procollagen alpha 1(IV) mRNA levels in streptozotocin induced diabetes. Kidney Int. 1992 Apr;41(4):768–777. doi: 10.1038/ki.1992.120. [DOI] [PubMed] [Google Scholar]
- Kesaniemi Y. A., Witztum J. L., Steinbrecher U. P. Receptor-mediated catabolism of low density lipoprotein in man. Quantitation using glucosylated low density lipoprotein. J Clin Invest. 1983 Apr;71(4):950–959. doi: 10.1172/JCI110849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig R. J., Blobstein S. H., Cerami A. Structure of carbohydrate of hemoglobin AIc. J Biol Chem. 1977 May 10;252(9):2992–2997. [PubMed] [Google Scholar]
- Krantz S., Brandt R., Gromoll B. Binding sites for short-term glycated albumin on peritoneal cells of the rat. Biochim Biophys Acta. 1993 May 8;1177(1):15–24. doi: 10.1016/0167-4889(93)90151-e. [DOI] [PubMed] [Google Scholar]
- Lane P. H., Steffes M. W., Fioretto P., Mauer S. M. Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int. 1993 Mar;43(3):661–667. doi: 10.1038/ki.1993.95. [DOI] [PubMed] [Google Scholar]
- Ledbetter S., Copeland E. J., Noonan D., Vogeli G., Hassell J. R. Altered steady-state mRNA levels of basement membrane proteins in diabetic mouse kidneys and thromboxane synthase inhibition. Diabetes. 1990 Feb;39(2):196–203. doi: 10.2337/diab.39.2.196. [DOI] [PubMed] [Google Scholar]
- Lee S. M., Bressler R. Prevention of diabetic nephropathy by diet control in the db/db mouse. Diabetes. 1981 Feb;30(2):106–111. doi: 10.2337/diab.30.2.106. [DOI] [PubMed] [Google Scholar]
- Lee S. M., Graham A. Early immunopathologic events in experimental diabetic nephropathy: a study in db/db mice. Exp Mol Pathol. 1980 Dec;33(3):323–332. doi: 10.1016/0014-4800(80)90030-1. [DOI] [PubMed] [Google Scholar]
- Lewis E. J., Hunsicker L. G., Bain R. P., Rohde R. D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993 Nov 11;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- Like A. A., Lavine R. L., Poffenbarger P. L., Chick W. L. Studies in the diabetic mutant mouse. VI. Evolution of glomerular lesions and associated proteinuria. Am J Pathol. 1972 Feb;66(2):193–224. [PMC free article] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Cerami A., Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992 Mar 15;267(8):5133–5138. [PubMed] [Google Scholar]
- Mauer S. M., Steffes M. W., Ellis E. N., Sutherland D. E., Brown D. M., Goetz F. C. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984 Oct;74(4):1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVerry B. A., Fisher C., Hopp A., Huehns E. R. Production of pseudodiabetic renal glomerular changes in mice after repeated injections of glucosylated proteins. Lancet. 1980 Apr 5;1(8171):738–740. doi: 10.1016/s0140-6736(80)91234-9. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984 Feb 9;310(6):356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. Prediction of clinical diabetic nephropathy in IDDM patients. Alternatives to microalbuminuria? Diabetes. 1990 Jul;39(7):761–767. doi: 10.2337/diab.39.7.761. [DOI] [PubMed] [Google Scholar]
- Neuman R. G., Hud E., Cohen M. P. Glycated albumin: a marker of glycaemic status in rats with experimental diabetes. Lab Anim. 1994 Jan;28(1):63–69. doi: 10.1258/002367794781065663. [DOI] [PubMed] [Google Scholar]
- Noth R. H., Krolewski A. S., Kaysen G. A., Meyer T. W., Schambelan M. Diabetic nephropathy: hemodynamic basis and implications for disease management. Ann Intern Med. 1989 May 15;110(10):795–813. doi: 10.7326/0003-4819-110-10-795. [DOI] [PubMed] [Google Scholar]
- Osterby R., Parving H. H., Hommel E., Jørgensen H. E., Løkkegaard H. Glomerular structure and function in diabetic nephropathy. Early to advanced stages. Diabetes. 1990 Sep;39(9):1057–1063. doi: 10.2337/diab.39.9.1057. [DOI] [PubMed] [Google Scholar]
- Predescu D., Simionescu M., Simionescu N., Palade G. E. Binding and transcytosis of glycoalbumin by the microvascular endothelium of the murine myocardium: evidence that glycoalbumin behaves as a bifunctional ligand. J Cell Biol. 1988 Nov;107(5):1729–1738. doi: 10.1083/jcb.107.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell M., Stephen P. M., Paulsen R., Valentine J. L., Rasbold K., Hestorff T., Eastberg S., Shint D. C. An interspecies comparison of normal levels of glycosylated hemoglobin and glycosylated albumin. Comp Biochem Physiol B. 1985;81(4):819–822. doi: 10.1016/0305-0491(85)90072-0. [DOI] [PubMed] [Google Scholar]
- Sabbatini M., Sansone G., Uccello F., Giliberti A., Conte G., Andreucci V. E. Early glycosylation products induce glomerular hyperfiltration in normal rats. Kidney Int. 1992 Oct;42(4):875–881. doi: 10.1038/ki.1992.363. [DOI] [PubMed] [Google Scholar]
- Seyer-Hansen K., Hansen J., Gundersen H. J. Renal hypertrophy in experimental diabetes. A morphometric study. Diabetologia. 1980 Jun;18(6):501–505. doi: 10.1007/BF00261707. [DOI] [PubMed] [Google Scholar]
- Steffes M. W., Bilous R. W., Sutherland D. E., Mauer S. M. Cell and matrix components of the glomerular mesangium in type I diabetes. Diabetes. 1992 Jun;41(6):679–684. doi: 10.2337/diab.41.6.679. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Witztum J. L. Glucosylation of low-density lipoproteins to an extent comparable to that seen in diabetes slows their catabolism. Diabetes. 1984 Feb;33(2):130–134. doi: 10.2337/diab.33.2.130. [DOI] [PubMed] [Google Scholar]
- Wiklund O., Witztum J. L., Carew T. E., Pittman R. C., Elam R. L., Steinberg D. Turnover and tissue sites of degradation of glucosylated low density lipoprotein in normal and immunized rabbits. J Lipid Res. 1987 Sep;28(9):1098–1109. [PubMed] [Google Scholar]
- Winegrad A. I. Banting lecture 1986. Does a common mechanism induce the diverse complications of diabetes? Diabetes. 1987 Mar;36(3):396–406. doi: 10.2337/diab.36.3.396. [DOI] [PubMed] [Google Scholar]
- Wu V. Y., Cohen M. P. Identification of aortic endothelial cell binding proteins for Amadori adducts in glycated albumin. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1131–1136. doi: 10.1006/bbrc.1993.1743. [DOI] [PubMed] [Google Scholar]
- Wu V. Y., Cohen M. P. Receptors specific for Amadori-modified glycated albumin on murine endothelial cells. Biochem Biophys Res Commun. 1994 Jan 28;198(2):734–739. doi: 10.1006/bbrc.1994.1106. [DOI] [PubMed] [Google Scholar]
- Ziyadeh F. N., Cohen M. P. Effects of glycated albumin on mesangial cells: evidence for a role in diabetic nephropathy. Mol Cell Biochem. 1993 Aug 11;125(1):19–25. doi: 10.1007/BF00926830. [DOI] [PubMed] [Google Scholar]
- Ziyadeh F. N. Renal tubular basement membrane and collagen type IV in diabetes mellitus. Kidney Int. 1993 Jan;43(1):114–120. doi: 10.1038/ki.1993.19. [DOI] [PubMed] [Google Scholar]
- Ziyadeh F. N., Snipes E. R., Watanabe M., Alvarez R. J., Goldfarb S., Haverty T. P. High glucose induces cell hypertrophy and stimulates collagen gene transcription in proximal tubule. Am J Physiol. 1990 Oct;259(4 Pt 2):F704–F714. doi: 10.1152/ajprenal.1990.259.4.F704. [DOI] [PubMed] [Google Scholar]
- Ziyadeh F. N. The extracellular matrix in diabetic nephropathy. Am J Kidney Dis. 1993 Nov;22(5):736–744. doi: 10.1016/s0272-6386(12)80440-9. [DOI] [PubMed] [Google Scholar]