Abstract
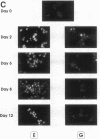
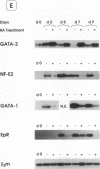
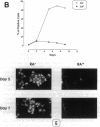
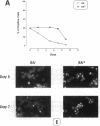
We have explored the expression of the transcription factors GATA-1, GATA-2, and NF-E2 in purified early hematopoietic progenitor cells (HPCs) induced to gradual unilineage erythroid or granulocytic differentiation by growth factor stimulus. GATA-2 mRNA and protein, already expressed in quiescent HPCs, is rapidly induced as early as 3 h after growth factor stimulus, but then declines in advanced erythroid and granulocytic differentiation and maturation. NF-E2 and GATA-1 mRNAs and proteins, though not detected in quiescent HPCs, are gradually induced at 24-48 h in both erythroid and granulocytic culture. Beginning at late differentiation/early maturation stage, both transcription factors are further accumulated in the erythroid pathway, whereas they are suppressed in the granulopoietic series. Similarly, the erythropoietin receptor (EpR) is induced and sustainedly expressed during erythroid differentiation, although beginning at later times (i.e., day 5), whereas it is barely expressed in the granulopoietic pathway. In the first series of functional studies, HPCs were treated with antisense oligomers targeted to transcription factor mRNA: inhibition of GATA-2 expression caused a decreased number of both erythroid and granulocyte-monocytic clones, whereas inhibition of NF-E2 or GATA-1 expression induced a selective impairment of erythroid colony formation. In a second series of functional studies, HPCs treated with retinoic acid were induced to shift from erythroid to granulocytic differentiation (Labbaye et al. 1994. Blood. 83:651-656); this was coupled with abrogation of GATA-1, NF-E2, and EpR expression and conversely enhanced GATA-2 levels. These results indicate the expression and key role of GATA-2 in the early stages of HPC proliferation/differentiation. Conversely, NF-E2 and GATA-1 expression and function are apparently restricted to erythroid differentiation and maturation: their expression precedes that of the EpR, and their function may be in part mediated via the EpR.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Erdjument-Bromage H., Davidson M. B., Tempst P., Orkin S. H. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993 Apr 22;362(6422):722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- Andrews R. G., Singer J. W., Bernstein I. D. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J Exp Med. 1989 May 1;169(5):1721–1731. doi: 10.1084/jem.169.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carè A., Testa U., Bassani A., Tritarelli E., Montesoro E., Samoggia P., Cianetti L., Peschle C. Coordinate expression and proliferative role of HOXB genes in activated adult T lymphocytes. Mol Cell Biol. 1994 Jul;14(7):4872–4877. doi: 10.1128/mcb.14.7.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Han X. L., Kan Y. W. Isolation of cDNA encoding the human NF-E2 protein. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11366–11370. doi: 10.1073/pnas.90.23.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T., Ikawa Y., Todokoro K. GATA-1 transactivates erythropoietin receptor gene, and erythropoietin receptor-mediated signals enhance GATA-1 gene expression. Nucleic Acids Res. 1991 Jul 25;19(14):3843–3848. doi: 10.1093/nar/19.14.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Craig W., Kay R., Cutler R. L., Lansdorp P. M. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993 May 1;177(5):1331–1342. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliason J. F. Granulocyte-macrophage colony formation in serum-free culture: effects of purified colony-stimulating factors and modulation by hydrocortisone. J Cell Physiol. 1986 Aug;128(2):231–238. doi: 10.1002/jcp.1041280214. [DOI] [PubMed] [Google Scholar]
- Fritsch G., Buchinger P., Printz D., Fink F. M., Mann G., Peters C., Wagner T., Adler A., Gadner H. Rapid discrimination of early CD34+ myeloid progenitors using CD45-RA analysis. Blood. 1993 May 1;81(9):2301–2309. [PubMed] [Google Scholar]
- Gabbianelli M., Sargiacomo M., Pelosi E., Testa U., Isacchi G., Peschle C. "Pure" human hematopoietic progenitors: permissive action of basic fibroblast growth factor. Science. 1990 Sep 28;249(4976):1561–1564. doi: 10.1126/science.2218497. [DOI] [PubMed] [Google Scholar]
- Giampaolo A., Sterpetti P., Bulgarini D., Samoggia P., Pelosi E., Valtieri M., Peschle C. Key functional role and lineage-specific expression of selected HOXB genes in purified hematopoietic progenitor differentiation. Blood. 1994 Dec 1;84(11):3637–3647. [PubMed] [Google Scholar]
- Gunji Y., Nakamura M., Hagiwara T., Hayakawa K., Matsushita H., Osawa H., Nagayoshi K., Nakauchi H., Yanagisawa M., Miura Y. Expression and function of adhesion molecules on human hematopoietic stem cells: CD34+ LFA-1- cells are more primitive than CD34+ LFA-1+ cells. Blood. 1992 Jul 15;80(2):429–436. [PubMed] [Google Scholar]
- Heberlein C., Fischer K. D., Stoffel M., Nowock J., Ford A., Tessmer U., Stocking C. The gene for erythropoietin receptor is expressed in multipotential hematopoietic and embryonal stem cells: evidence for differentiation stage-specific regulation. Mol Cell Biol. 1992 Apr;12(4):1815–1826. doi: 10.1128/mcb.12.4.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E., Toki T., Ishihara H., Ohtani H., Gu L., Yokoyama M., Engel J. D., Yamamoto M. Erythroid transcription factor GATA-1 is abundantly transcribed in mouse testis. Nature. 1993 Apr 1;362(6419):466–468. doi: 10.1038/362466a0. [DOI] [PubMed] [Google Scholar]
- Jones S. S., D'Andrea A. D., Haines L. L., Wong G. G. Human erythropoietin receptor: cloning, expression, and biologic characterization. Blood. 1990 Jul 1;76(1):31–35. [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990 Apr 20;248(4953):378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Labbaye C., Valtieri M., Testa U., Giampaolo A., Meccia E., Sterpetti P., Parolini I., Pelosi E., Bulgarini D., Cayre Y. E. Retinoic acid downmodulates erythroid differentiation and GATA1 expression in purified adult-progenitor culture. Blood. 1994 Feb 1;83(3):651–656. [PubMed] [Google Scholar]
- Leary A. G., Zeng H. Q., Clark S. C., Ogawa M. Growth factor requirements for survival in G0 and entry into the cell cycle of primitive human hemopoietic progenitors. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4013–4017. doi: 10.1073/pnas.89.9.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. E., Temizer D. H., Clifford J. A., Quertermous T. Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J Biol Chem. 1991 Aug 25;266(24):16188–16192. [PubMed] [Google Scholar]
- Leonard M., Brice M., Engel J. D., Papayannopoulou T. Dynamics of GATA transcription factor expression during erythroid differentiation. Blood. 1993 Aug 15;82(4):1071–1079. [PubMed] [Google Scholar]
- Martin D. I., Zon L. I., Mutter G., Orkin S. H. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990 Mar 29;344(6265):444–447. doi: 10.1038/344444a0. [DOI] [PubMed] [Google Scholar]
- Meri S., Morgan B. P., Wing M., Jones J., Davies A., Podack E., Lachmann P. J. Human protectin (CD59), an 18-20-kD homologous complement restriction factor, does not restrict perforin-mediated lysis. J Exp Med. 1990 Jul 1;172(1):367–370. doi: 10.1084/jem.172.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Mignotte V., Eleouet J. F., Raich N., Romeo P. H. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6548–6552. doi: 10.1073/pnas.86.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthon M. A., Bernard O., Mitjavila M. T., Romeo P. H., Vainchenker W., Mathieu-Mahul D. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood. 1993 Feb 1;81(3):647–655. [PubMed] [Google Scholar]
- Ney P. A., Sorrentino B. P., McDonagh K. T., Nienhuis A. W. Tandem AP-1-binding sites within the human beta-globin dominant control region function as an inducible enhancer in erythroid cells. Genes Dev. 1990 Jun;4(6):993–1006. doi: 10.1101/gad.4.6.993. [DOI] [PubMed] [Google Scholar]
- Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993 Jun 1;81(11):2844–2853. [PubMed] [Google Scholar]
- Orkin S. H. GATA-binding transcription factors in hematopoietic cells. Blood. 1992 Aug 1;80(3):575–581. [PubMed] [Google Scholar]
- Peschle C., Gabbianelli M., Testa U., Pelosi E., Barberi T., Fossati C., Valtieri M., Leone L. c-kit ligand reactivates fetal hemoglobin synthesis in serum-free culture of stringently purified normal adult burst-forming unit-erythroid. Blood. 1993 Jan 15;81(2):328–336. [PubMed] [Google Scholar]
- Peschle C., Testa U., Valtieri M., Gabbianelli M., Pelosi E., Montesoro E., Sposi N. M., Fossati C., Camagna A., Caré A. Stringently purified human hematopoietic progenitors/stem cells: analysis of cellular/molecular mechanisms underlying early hematopoiesis. Stem Cells. 1993 Sep;11(5):356–370. doi: 10.1002/stem.5530110503. [DOI] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Romeo P. H., Prandini M. H., Joulin V., Mignotte V., Prenant M., Vainchenker W., Marguerie G., Uzan G. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990 Mar 29;344(6265):447–449. doi: 10.1038/344447a0. [DOI] [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Dai C. H., Koury S. T., Horn S. T., Glick A. D., Civin C. I. Purification of human blood burst-forming units-erythroid and demonstration of the evolution of erythropoietin receptors. J Cell Physiol. 1990 Feb;142(2):219–230. doi: 10.1002/jcp.1041420202. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Pevny L., Wiles M. V., Keller G., Costantini F., Orkin S. H. Rescue of erythroid development in gene targeted GATA-1- mouse embryonic stem cells. Nat Genet. 1992 May;1(2):92–98. doi: 10.1038/ng0592-92. [DOI] [PubMed] [Google Scholar]
- Sposi N. M., Zon L. I., Carè A., Valtieri M., Testa U., Gabbianelli M., Mariani G., Bottero L., Mather C., Orkin S. H. Cell cycle-dependent initiation and lineage-dependent abrogation of GATA-1 expression in pure differentiating hematopoietic progenitors. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6353–6357. doi: 10.1073/pnas.89.14.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S., Inazawa J., Nakahashi Y., Abe T., Tokunaga R. Structure of the human ferrochelatase gene. Exon/intron gene organization and location of the gene to chromosome 18. Eur J Biochem. 1992 Apr 1;205(1):217–222. doi: 10.1111/j.1432-1033.1992.tb16771.x. [DOI] [PubMed] [Google Scholar]
- Testa U., Pelosi E., Gabbianelli M., Fossati C., Campisi S., Isacchi G., Peschle C. Cascade transactivation of growth factor receptors in early human hematopoiesis. Blood. 1993 Mar 15;81(6):1442–1456. [PubMed] [Google Scholar]
- Trainor C. D., Evans T., Felsenfeld G., Boguski M. S. Structure and evolution of a human erythroid transcription factor. Nature. 1990 Jan 4;343(6253):92–96. doi: 10.1038/343092a0. [DOI] [PubMed] [Google Scholar]
- Tsai F. Y., Keller G., Kuo F. C., Weiss M., Chen J., Rosenblatt M., Alt F. W., Orkin S. H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994 Sep 15;371(6494):221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Strauss E., Orkin S. H. Functional analysis and in vivo footprinting implicate the erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Genes Dev. 1991 Jun;5(6):919–931. doi: 10.1101/gad.5.6.919. [DOI] [PubMed] [Google Scholar]
- Udomsakdi C., Lansdorp P. M., Hogge D. E., Reid D. S., Eaves A. C., Eaves C. J. Characterization of primitive hematopoietic cells in normal human peripheral blood. Blood. 1992 Nov 15;80(10):2513–2521. [PubMed] [Google Scholar]
- Valtieri M., Gabbianelli M., Pelosi E., Bassano E., Petti S., Russo G., Testa U., Peschle C. Erythropoietin alone induces erythroid burst formation by human embryonic but not adult BFU-E in unicellular serum-free culture. Blood. 1989 Jul;74(1):460–470. [PubMed] [Google Scholar]
- Valtieri M., Schirò R., Chelucci C., Masella B., Testa U., Casella I., Montesoro E., Mariani G., Hassan H. J., Peschle C. Efficient transfer of selectable and membrane reporter genes in hematopoietic progenitor and stem cells purified from human peripheral blood. Cancer Res. 1994 Aug 15;54(16):4398–4404. [PubMed] [Google Scholar]
- Valtieri M., Venturelli D., Caré A., Fossati C., Pelosi E., Labbaye C., Mattia G., Gewirtz A. M., Calabretta B., Peschle C. Antisense myb inhibition of purified erythroid progenitors in development and differentiation is linked to cycling activity and expression of DNA polymerase alpha. Blood. 1991 Mar 15;77(6):1181–1190. [PubMed] [Google Scholar]
- Weiss M. J., Keller G., Orkin S. H. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 1994 May 15;8(10):1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ko L. J., Leonard M. W., Beug H., Orkin S. H., Engel J. D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- Zon L. I., Youssoufian H., Mather C., Lodish H. F., Orkin S. H. Activation of the erythropoietin receptor promoter by transcription factor GATA-1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10638–10641. doi: 10.1073/pnas.88.23.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]