Abstract
The Aravan virus was isolated from a Lesser Mouse-eared Bat (Myotis blythi) in the Osh region of Kyrghyzstan, central Asia, in 1991. We determined the complete sequence of the nucleoprotein (N) gene and compared it with those of 26 representative lyssaviruses obtained from databases. The Aravan virus was distinguished from seven distinct genotypes on the basis of nucleotide and amino acid identity. Phylogenetic analysis based on both nucleotide and amino acid sequences showed that the Aravan virus was more closely related to genotypes 4, 5, and—to a lesser extent—6, which circulates among insectivorus bats in Europe and Africa. The Aravan virus does not belong to any of the seven known genotypes of lyssaviruses, namely, rabies, Lagos bat, Mokola, and Duvenhage viruses and European bat lyssavirus 1, European bat lyssavirus 2, and Australian bat lyssavirus. Based on these data, we propose a new genotype for the Lyssavirus genus.
Keywords: Lyssavirus, genotype, N gene, phylogenetic analysis, bat, central Asia, research
The Lyssavirus genus includes seven genotypes: rabies virus (RABV, genotype 1), Lagos bat virus (genotype 2), Mokola virus (genotype 3), Duvenhage virus (genotype 4), European bat lyssavirus 1 (EBLV-1, genotype 5), European bat lyssavirus 2 (EBLV-2, genotype 6), and Australian bat lyssavirus (ABLV, genotype 7) (1,2). Lagos bat virus was isolated from frugivorous bats (Eidolon helvum) in Nigeria in 1956 (3) and in 1974 from another bat (Micropterus pusillus) in the Central Africa Republic (4). Mokola virus was isolated from shrews (Crocidura sp.) and a child in Nigeria in 1968 (5,6), a girl in Nigeria in 1971 (7), and cats in Zimbabwe (8). Duvenhage virus was originally isolated from a human who died after being bitten by a bat in South Africa in 1970 (9) and from Miniopterus sp. bats in 1981 (10). EBLV-1 was isolated from bats (Eptesicus serotinus) in Germany in 1968 (11), in Poland in 1985 (12), in Denmark, Holland, and Spain in 1987, and in France in 1989 (13). Some isolates of EBLV-1 were obtained from bats in Ukraine and from one human case of bat origin in Russia in 1985 (14,15). EBLV-2 was isolated from a human in Finland in 1985 (16), and from bats in Holland, the Netherlands, Switzerland, and the U.K. EBLV-2 is mainly carried by bats of the Myotis genus (Myotis dasycneme and M. daubentonii) (17). ABLV was isolated from five species of flying fox bats, one species of an insectivorous bat, and two infected humans in 1996 (1,18,19).
Rabies viruses have been reported in Kazakhstan, central Asia (20). Terrestrial rabies viruses have been enzootic in all Central Asian countries and are mainly carried by dogs. Field rabies viruses have been isolated and characterized in Asia, specifically Pakistan, China, Indonesia, Thailand, the Philippines, Malaysia, India, and Sri Lanka (21–26). Isolation of lyssaviruses from bats has been reported only in India and Thailand; however, these viruses were reported as RABV (27,28). Recently, Arguin et al. detected neutralizing antibodies against ABLV in the serum of six bat species (Mineopterus schreibersi, Taphozous melanopogan, Philetor brachypteus, Scotophilus kuhli, Pteropus hypomelanus, and Rousettus amplexicaudatus) in the Philippines (29).
Aravan virus was originally isolated from the brain of a lesser mouse-eared bat (Myotis blythi) in Kyrghyzstan in 1991. The antigenic profile of the virus was analyzed by using two panels of antinucleocapsid (N) gene monoclonal antibodies developed at the Wistar Institute of Anatomy and Biology (USA) and the Central Veterinary Laboratory of Great Britain (Weybridge, U.K.) (30–32). These results demonstrated that the virus differed from rabies and serotypes 2 (Lagos bat virus), 3 (Mokola virus), 4 (Duvenhage virus), 5 (EBLV-1), and 6 (EBLV-2). Furthermore, 386 nucleotides (nt) of the N gene were determined from reverse transcription-polymerase chain reach (RT-PCR) product. Phylogenetic analysis suggested that the Aravan virus did not belong to the rabies virus group (33). In the present study, we determined the entire coding region of the N protein of Aravan virus and evaluated the phylogenetic relationships with other members of the Lyssavirus genus.
Materials and Methods
Viruses
Aravan virus was isolated from the brain of one lesser mouse-eared bat (Myotis blythi) during a survey of 269 bats collected in the Osh region of Kyrghyzstan from 1988 to 1992 (30,32). A direct fluorescent antibody test was conducted. Aravan virus–infected mouse brains were impressed on glass slides, air-dried, and fixed with acetone. To detect the lyssavirus antigen, specimens were stained with fluorescein isothiocyanate (FITC)–labeled anti-rabies globulin (BBL, Cockeysville, MD) or FITC-labeled anti-rabies monoclonal globulin (Centocor Inc., Malvern, PA). FITC-labeled anti-nucleoprotein monoclonal antibodies (NC-MAbs, W502) cross- reactive to lyssaviruses were also used (19).
Amplification of Nucleoprotein cDNA and Direct Sequencing
Total RNA was extracted from virus-infected mouse brain emulsions with a commercial reagent (RNeasy Mini Kit, QIAGEN, Germany). cDNA was obtained with a T-Primed First-Strand kit (Amersharm Biosciences Corporation, Piscataway, NJ). PCR amplification and sequencing of the N gene were performed by using the sense primer AraN-S01 (5´-ATGTACCACCTCTACAATGG-3´, nt 55–74) and an antisense primer AraNC-1400 (5´-TCATGCTCAATTGTAAAAC-3´, nt 1456–1474). The cDNA template (2 μL) was amplified by using primers (AraN-S01 and AraNC-1400), according to the manufacturer’s instruction (Super Taq Premix Kit, Sawady Technology, Tokyo, Japan). PCR reactions were incubated at 94°C for 2 min, subjected to 40 cycles of 94°C for 30 s, 48° for 20 s, and 68°C for 2 min, and a final extension at 68°C for 7 min in a DNA thermal cycler (GeneAmpPCR System 9700 Applied Biosystems, Perkin-Elmer Corporation, Japan) (24,25). PCR products were purified by using a commercial kit (QIAquick PCR Purification Kit, QIAGEN). The sequences of the purified DNA products were determined on an automated sequencer (ABI model 310, Applied Biosystems, Foster City, CA) by using a PRIMS Ready Reaction Dyedeoxy Terminator Cycle Sequencing Kit (Applied Biosystems).
Phylogenetic Analysis
The 1350-nt and the deduced 450 amino acid (aa) sequences of the N gene of the Aravan virus were aligned with 26 lyssaviruses by using Clustal W program (34). A phylogenetic tree was constructed with the computer software MEGA 2 (35). Pairwise evolutionary nucleotide distances, including both transitions and transversions, were estimated according to Kimura’s two-parameter method. Phylogenetic trees were constructed by the neighbor-joining method with 1,000 replicates to generate bootstrap probabilities at each node (36).
Results and Discussion
Direct Fluorescent Antibody Assay
The three strains used in this study reacted against the Aravan virus infected mouse brain impressions. Fluorescence showed more scattered inclusions than those of the challenge virus standard in the acetone-fixed mouse brain smear (data not shown). The results confirmed that the Aravan virus is a lyssavirus.
Nucleotide and Deduced Amino Acid Sequence Identities among the Aravan Virus and Other Lyssaviruses
The 1350-nt and the deduced 450 aa sequences of the Aravan virus were compared with 26 representative lyssaviruses belonging to seven genotypes (Table 1). We selected 16 representative rabies variants from the eight diverse groups, including rabies variants from geographic areas of Asia near Kyrghyzstan and from bats and raccoons in North and South America (25,37). The nucleotide and amino acid sequence identities among all 27 lyssaviruses, including Aravan virus, were calculated. Then genotype 1 was represented by seven rabies viruses (SRL1032, 86118BRE, 1500AFS, 9218TCH, 8738THA, insectivorous bat/Chile, and PA R89), and genotypes 2, 3, 4, 5, 6, and 7 were represented by Lagos bat virus (8619NGA), Mokola virus (MOK/U22843), Duvenhage virus (86132AS), EBLV-1 (8918FRA), EBLV-2 (9007FIN), and ABLV (Balina/AF006497), respectively (Table 2). The nucleotide sequence identity of Aravan virus with the genotypes 4, 5, 6, and 7 was 77% to 78%; with genotype 1, 75% to 77%; and with genotypes 2 and 3, 72% to 74%. The most extensive nucleotide sequence differences among isolates of genotype 1 were between the raccoon isolate (PA R89) and the African and Asian isolates (82.8% to 82.9% identity). The Aravan virus demonstrated 92% aa sequence identity with genotypes 4, 5, and 7; 89% with genotype 6; and 81% to 85% with genotypes 2 and 3. The maximum variation of amino acid sequences within genotype 1 was exhibited between a vampire bat isolate from Brazil and an African isolate (93.1% to 93.3% identity). Genotype 4 (Duvenhage virus) was most closely related to genotype 5 (EBLV-1) with nucleotide and amino acid sequence identities of 79.8% and 93.3%, respectively. ABLV (genotype 7) was closely related to SRL1032 (genotype 1, Sri Lankan rabies virus) with a 93.1% aa sequence identity. These values were almost same as maximum variation of genotype 1. Based on our present data, we determined that isolates sharing <79.8% nt and 93.1% to 93.3% aa sequence identities belonged to different genotypes. In several studies, thresholds of <80% nt and 92% or 93% aa sequence identities warranted the proposal of a new genotype (1,23,38). Hence, the nucleotide and amino acid percentage identity values demonstrated that Aravan virus should be regarded as a new lyssavirus genotype.
Table 1. Lyssavirus isolates used in this study.
| Genotypea | Yr isolated | Virus (strain) | Country of isolation | Host | Accession no. | ||
|---|---|---|---|---|---|---|---|
| 1 (Rabies) |
? |
CTN |
China |
? |
AF367863 |
|
|
| 1 (Rabies) |
1983 |
8738THA |
Thailand |
Human |
U22653 |
|
|
| 1 (Rabies) |
? |
? |
India |
? |
AF374721 |
|
|
| 1 (Rabies) |
1996 |
SRL1032 |
Sri Lanka |
Jackal |
AB041964 |
|
|
| 1 (Rabies) |
1992 |
9218TCH |
Chad |
Dog |
U22644 |
|
|
| 1 (Rabies) |
1988 |
9141RUS |
Russia |
Arctic fox |
U22656 |
|
|
| 1 (Rabies) |
? |
9196FX |
Canada |
Vulpes vulpes |
L20676 |
|
|
| 1 (Rabies) |
1987 |
1500AFS |
Rep.South Afr. |
Yellow mongoose |
U22628 |
|
|
| 1 (Rabies) |
1985 |
9142EST |
Estonia |
Racoon dog |
U22476 |
|
|
| 1 (Rabies) |
1986 |
8681IRA |
Iran |
Dog |
U22482 |
|
|
| 1 (Rabies) |
1985 |
86118BRE |
Brazil |
Vampire bat |
U22479 |
|
|
| 1 (Rabies) |
1992 |
BBCAN |
Canada |
Eptesicus fuscus
|
AF351833 |
|
|
| 1 (Rabies) |
1992 |
MYCAN |
Canada |
Myotis lucifugus
|
AF351839 |
|
|
| 1 (Rabies) |
? |
? |
Chile |
Tadarida brasiliensis
|
AF070450 |
|
|
| 1 (Rabies) |
1988 |
Insectivorous Bat |
Chile |
Insectivorous bat
|
AF351850 |
|
|
| 1 (Rabies) |
1989 |
PA R89 |
USA |
Raccoon |
U27221 |
|
|
| 2 (Lagos bat) |
1958 |
8619NGA |
Nigeria |
Eidolon helvum
|
U22842 |
|
|
| 3 (Mokola) |
? |
Y09762 |
? |
? |
Y09762 |
|
|
| 3 (Mokola) |
1981 |
MOK |
Zimbabwe |
Cat |
U22843 |
|
|
| 4 (Duvenhage) |
1986 |
86132AS |
Rep.South Africa |
Human |
U22848 |
|
|
| 5 (EBLV-1) |
1985 |
8615POL |
Poland |
Eptesicus serotinus
|
U22844 |
|
|
| 5 (EBLV-1) |
1989 |
8918FRA |
France |
E. serotinus
|
U22845 |
|
|
| 6 (EBLV-2) |
1986 |
9007FIN |
Finland |
Human |
U22846 |
|
|
| 6 (EBLV-2) |
1986 |
9018HOL |
Holand |
M. dasycneme
|
U22847 |
|
|
| 7 (ABLV |
1996 |
Ballina |
Australia |
Pteropid alecto
|
AF006497 |
|
|
| 7 (ABLV) |
1996 |
Insectivorous isolate |
Australia |
Insectivorous bat |
AF081020 |
|
|
| ? | 1991 | Aravan | Kyrghystan | M. blythi | AB094438 | ||
aEBLV-1, European bat lyssavirus 1; EBLV-2, European bat lyssavirus 2; ABLV, Australian bat lyssavirus; MOK, strain name in Mokola virus.
Table 2. Comparison of nucleotide and deduced amino acid sequences of Aravan virus with other 13 lyssaviruses.
| Amino acid sequence identity (%) | Nucleotide sequence identity (%) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Genotype 1 (rabies virus) |
Genotype 2 |
Genotype 3 |
Genotype 4 |
Genotype 5 |
Genotype 6 |
Genotype 7 |
||||||||||
| Aravan | SRL1032 | U22479BRE | U22628AFS | U22644CHAD | U22653THA | AF351850 | U27221 | LBU22842 | MKU22843 | 86132AS | 8918FRA | 9007FIN | AF006497 | ||||
| Aravan |
100.0 |
75.6 |
76.0 |
76.2 |
74.8 |
75.9 |
77.0 |
76.2 |
74.3 |
72.4 |
78.2 |
77.9 |
77.2 |
76.9 |
|||
| SRL1032 |
90.9 |
100.0 |
84.7 |
85.9 |
86.9 |
86.8 |
86.6 |
84.1 |
74.2 |
70.2 |
73.9 |
75.6 |
74.7 |
78.0 |
|||
| U22479BRE |
88.2 |
95.3 |
100.0 |
83.3 |
83.0a
|
83.3 |
89.9 |
83.7 |
73.8 |
69.6 |
73.8 |
75.3 |
74.2 |
77.2 |
|||
| U22628AFS |
89.3 |
96.7 |
93.3b
|
100.0 |
85.6 |
83.5 |
83.6 |
83.2 |
73.3 |
70.4 |
73.9 |
75.3 |
74.6 |
78.0 |
|||
| U22644CHAD |
88.7 |
96.9 |
93.1 b
|
94.4 |
100.0 |
86.0 |
84.0 |
82.9a
|
73.0 |
69.0 |
73.1 |
75.0 |
74.8 |
77.2 |
|||
| U22653THA |
89.8 |
97.1 |
94.2 |
95.1 |
95.3 |
100.0 |
84.2 |
82.8a
|
73.4 |
69.7 |
74.4 |
75.9 |
74.7 |
77.3 |
|||
|
AF351850 |
90.0 |
97.1 |
95.8 |
95.8 |
94.9 |
95.6 |
100.0 |
86.1 |
73.5 |
70.1 |
74.3 |
75.0 |
74.8 |
77.4 |
|||
|
U27221 |
89.6 |
95.8 |
93.6 |
94.2 |
93.8 |
94.9 |
95.1 |
100.0 |
73.1 |
70.2 |
74.2 |
74.6 |
75.7 |
77.1 |
|||
|
LBU22842 |
84.7 |
82.9 |
81.8 |
81.1 |
80.4 |
81.8 |
83.1 |
82.2 |
100.0 |
74.8 |
73.4 |
74.4 |
72.5 |
72.6 |
|||
| MKU22843 |
80.9 |
78.2 |
77.6 |
77.3 |
76.2 |
77.6 |
78.4 |
77.8 |
84.4 |
100.0 |
71.6 |
69.9 |
69.2 |
71.0 |
|||
| 86132AS |
91.8 |
88.9 |
86.9 |
87.6 |
87.1 |
87.6 |
88.7 |
87.8 |
85.8 |
80.7 |
100.0 |
79.8c
|
75.9 |
77.0 |
|||
| 8918FRA |
92.0 |
88.9 |
86.7 |
87.8 |
87.8 |
88.4 |
88.2 |
88.2 |
83.8 |
79.1 |
93.3d
|
100.0 |
78.0 |
76.9 |
|||
| 9007FIN |
88.9 |
88.0 |
86.2 |
87.1 |
86.9 |
88.0 |
87.3 |
87.3 |
79.1 |
76.2 |
86.2 |
88.0 |
100.0 |
77.2 |
|||
| AF006497 | 92.0 | 93.1d | 91.1 | 91.6 | 91.1 | 91.8 | 91.8 | 91.3 | 82.0 | 79.8 | 90.0 | 89.8 | 87.8 | 100.0 | |||
aThe values were shown as maximum variation of nucleotide sequence identities (%) within genotype 1. bThe values were shown as maximum variation of amino acid sequence identities (%) within genotype 1. cThresholds of nucleotide sequence identities % as different genotypes. dThresholds of amino acid sequence identities % as different genotypes.
Phylogenetic Analysis
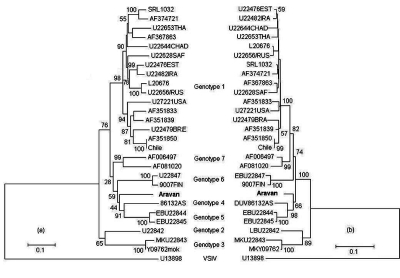
A phylogenetic tree of 27 lyssaviruses, including the Aravan virus, based on the 1350-nt sequence of the N gene was constructed by using the vesicular stomatitis Indiana virus (VSIV, tsW16B/U13898) as an outgroup (Figure, a). The lyssaviruses divided into two groups: one group consisted of genotypes 2 and 3, and the other consisted of genotypes 1, 4, 5, 6, 7, and the Aravan virus. The latter group was divided into six distinct clusters corresponding to genotypes 1, 7, 6, and 5 (high bootstrap values of 98%, 99%, 100%, and 100%, respectively), then Aravan virus and genotype 4. Moreover, the Aravan virus clustered with genotypes 4, 5, and 6 (low bootstrap value of 59%). Duvenhage virus (genotype 4) and EBLV-1 (genotype 5) formed the same cluster (high bootstrap value of 91%), and are therefore closely related. The Aravan virus occupied the phylogenetic position between genotype 6 and the cluster of genotypes 4 and 5. We also constructed a phylogenetic tree based on the deduced 450-aa sequences of the N gene (Figure, b). Similar to the nucleotide data, the amino acid sequences divided into two large groups and further subdivided into eight groups. One group consisted of genotypes 2 and 3 (bootstrap value of 89%), and the other group consisted of genotypes 1, 7, 6, 4, and 5, and the Aravan virus (high bootstrap value of 100%). The latter group had three distinct clusters corresponding to genotypes 1, 7, and 6 (high bootstrap values of 100%, 99%, and 100%, respectively), genotypes 4 and 5 (same cluster with a high bootstrap value of 98%), and the Aravan virus. The Aravan virus did not group with any other genotypes and is located at a position close to the cluster of genotypes 4 and 5 (bootstrap value of 66%).
Figure.
Rooted phylogenetic tree showing genetic relationships among Aravan virus and 26 lyssaviruses. Phylogenetic relationships were determined by comparing the 1350-nucleotide sequences of the nucleoprotein (N) gene (a) and the deduced 450-amino-acid sequences (b) by the neighbor-joining method (36). The sequences used were those of genotypes 1, 2, 3, 4, 5, 6, and 7 shown in Table 1 by using vesicular stomatitis Indiana virus (VSIV) as an outgroup (tsW16B/U13898).
These results, along with those in Table 2 and the Figure, suggest that the Aravan virus does not belong to any of the seven Lyssavirus genotypes (rabies, Lagos bat, Mokola, Duvenhage, EBLV-1, EBLV-2, and ABLV). Thus, we propose that the Aravan virus forms an independent cluster and is a new member of the Lyssavirus genus.
In this article, we have reported the first lyssavirus distinct from rabies virus originating on the Asian continent. The Aravan virus was more closely related to genotypes 4, 5, and, to a lesser extent, 6, which circulates among insectivorus bats in Europe and Africa. The lesser mouse-eared bat, from which the Aravan virus was isolated, is widely distributed in northern Africa, the Mediterranean, southern Europe, Crimea, Caucasus, Palestine, southwest Asia, and parts of central and eastern Asia. This information should be considered in the discussion of lyssavirus classification and evolution, as it suggests the possibility of a broader geographic distribution of the Aravan virus. We have no information about human rabies caused by bat exposure from central Asia, and rabies surveillance in this area is not known well. Based on this information and the virus’ misdiagnoses as rabies, we consider that transmission of Aravan virus to humans is possible. Indeed, this finding stimulates interest in new genotypes of lyssaviruses and is important from the viewpoint of public health, necessitating further lyssavirus surveillance of bats on the Asian continent.
Acknowledgments
We thank C. E. Rupprechtt for providing the fluorescein/isothiocyanate–labeled W502 and I. Kurane for helpful advice in preparing the manuscript. We also thank an anonymous reviewer, whose constructive criticism and many useful suggestions improved the manuscript.
The work was partly supported by the Russian Foundation for Basic Research, grant (00-04-48004).
Biography
Dr. Arai is a research scientist at Department of Virology, National Institute of Infectious Diseases, Tokyo, Japan. Her research interests focus on the molecular epidemiology of lyssaviruses and the quality control of rabies vaccines.
References
- 1.Gould AR, Hyatt AD, Lunt R, Kattenbelt JA, Hengstberger S, Blacksell SD. Characterization of a novel lyssavirus isolated from Pteropid bats in Australia. Virus Res. 1998;54:165–87. 10.1016/S0168-1702(98)00025-2 [DOI] [PubMed] [Google Scholar]
- 2.Badrane H, Bahloul C, Perrin P, Tordo N. Evidence of two lyssavirus phylogroups with distinct pathogenicity and immunogenicity. J Virol. 2001;75:3268–76. 10.1128/JVI.75.7.3268-3276.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulger LR, Porterfield JS. Isolation of a virus from Nigerian fruit bats. Trans R Soc Trop Med Hyg. 1958;52:421–4. 10.1016/0035-9203(58)90127-5 [DOI] [PubMed] [Google Scholar]
- 4.Sureau P, Tignor GH, Smith AL. Antigenic characterization of the Bangui strain (ANCB-672d) of Lagos bat. Ann Virol. 1980;131:25–32. [Google Scholar]
- 5.Shope RE, Murphy FA, Harrison AK, Causey OR, Kemp GE, Simpson DI, et al. Two African viruses serologically and morphologically related to rabies virus. J Virol. 1970;6:690–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp GE, Causey OR, Moore DL, Odelola A, Fabiyi A. Mokola virus. Further studies on IBAN 27377, a new rabies-related etiologic agent of zoonosis in Nigeria. Am J Trop Med Hyg. 1972;21:356–9. [PubMed] [Google Scholar]
- 7.Familusi JB, Osunkoya BO, Moore DL, Kemp GE, Fabiyi A. A fatal human infection with Mokola virus. Am J Trop Med Hyg. 1972;21:959–63. [DOI] [PubMed] [Google Scholar]
- 8.Von Teichman BF, de Koker WC, Bosch SJE, Bishop GC, Meredith CD, Bingham J. Mokola virus infection: description of recent South African cases and a review of the virus epidemiology. J S Afr Vet Assoc. 1998;69:169–71. [DOI] [PubMed] [Google Scholar]
- 9.Meredith CD, Rossouw AP, Koch HVP. An unusual case of human rabies thought to be chiropteran origin. S Afr Med J. 1971;45:767–9. [PubMed] [Google Scholar]
- 10.Van der Merwe M. Bats as vectors of rabies. S Afr J Sci. 1982;78:421–2. [Google Scholar]
- 11.Schneider LG, Cox JH. Bat lyssaviruses in Europe. In: Rupprecht CE, Dietzschold B, Koprowski H, editors. Lyssaviruses. Berlin: Springer-Verlag;1994. p. 207–18. [Google Scholar]
- 12.Lafon M, Herzog M, Sureau P. Human rabies vaccines induce neutralizing antibodies against the European bat rabies virus (Duvenhage). Lancet. 1986;2:515. 10.1016/S0140-6736(86)90384-3 [DOI] [PubMed] [Google Scholar]
- 13.Bourhy H, Kissi B, Lafon M, Sacramento D, Tordo N. Antigenic and molecular characterization of bat rabies virus in Europe. J Clin Microbiol. 1992;30:2419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selimov MA, Tatarov AG, Botvinkin AD, Klueva EV, Kulikova LG, Khismatullina NA. Rabies-related Yuli virus; identification with a panel of monoclonal antibodies. Acta Virol. 1989;33:542–5. [PubMed] [Google Scholar]
- 15.Selimov MA, Smekhov AM, Antonova LA, Shablovskaya EA, King AA, Kulikova LG. New strains of rabies-related viruses isolated from bats in the Ukraine. Acta Virol. 1991;35:226–31. [PubMed] [Google Scholar]
- 16.Lumio J, Hillbom M, Roine R, Ketonen L, Haltia M, Valle M, et al. Human rabies of bat origin in Europe. Lancet. 1986;1:378. 10.1016/S0140-6736(86)92336-6 [DOI] [PubMed] [Google Scholar]
- 17.King AA, Meredith CD, Thomson GR. The biology of Southern African Lyssavirus variants. In: Rupprecht CE, Dietzschold B, Koprowski H, editors. Lyssaviruses. Berlin: Springer-Verlag; 1994. p. 267–95. [DOI] [PubMed] [Google Scholar]
- 18.Bradley JM, Epstein JH, Neill AS, Heel K, Field H, Barret J, et al. Potential exposure to Australian bat lyssavirus, Queensland, 1996–1999. Emerg Infect Dis. 2000;6:259–64. 10.3201/eid0603.000305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser GC, Hooper PT, Lunt RA, Gould AR, Gleeson LJ, Hyatt AD, et al. Encephalitis caused by a lyssavirus in fruit bats in Australia. Emerg Infect Dis. 1996;2:327–31. 10.3201/eid0204.960408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansyzbaev BK. Isolation of rabies virus from Siberian polecats in Kazakhstan. [in Russian]. Vopr Virusol. 1975;4:485. [PubMed] [Google Scholar]
- 21.Smith JS, Orciari LA, Yager PA, Seidel HD, Warner CK. Epidemiologic and historical relationships among 87 rabies virus isolates as determined by limited sequence analysis. J Infect Dis. 1992;166:296–307. [DOI] [PubMed] [Google Scholar]
- 22.Mccoll KA, Gould AR, Selleck PW, Hooper PT, Westbury HA, Smith JS. Polymerase chain reaction and other laboratory techniques in the diagnosis of long incubation rabies in Australia. Aust Vet J. 1993;70:84–9. 10.1111/j.1751-0813.1993.tb03282.x [DOI] [PubMed] [Google Scholar]
- 23.Kissi B, Tordo N, Bourhy H. Genetic polymorphism in the rabies virus nucleoprotein gene. Virology. 1995;209:526–37. 10.1006/viro.1995.1285 [DOI] [PubMed] [Google Scholar]
- 24.Arai YT, Yamada K, Kameoka Y, Horimoto T, Yamamoto K, Yabe S, et al. Nucleoprotein gene analysis of fixed and street rabies virus variants using RT-PCR. Arch Virol. 1997;142:1787–96. 10.1007/s007050050197 [DOI] [PubMed] [Google Scholar]
- 25.Arai YT, Takahashi H, Kameoka Y, Shiino T, Wimalaratne O, Lodmell DL. Characterization of Sri Lanka rabies virus isolates using nucleotide sequence analysis of nucleoprotein gene. Acta Virol. 2001;45:327–33. [PubMed] [Google Scholar]
- 26.Ito N, Sugiyama M, Oraveerakul K, Piyaviriyakul P, Lumlertdacha B, Arai YT, et al. Molecular epidemiology of rabies in Thailand. Microbiol Immunol. 1999;43:551–9. [DOI] [PubMed] [Google Scholar]
- 27.Pal SR, Arora B, Chuttani PN, Broor S, Choudhury S, Joshi RM, et al. Rabies virus infection of a flying fox bat, Pterous policephalus in Chandigarh, North India. Trop Geogr Med. 1980;32:265–7. [PubMed] [Google Scholar]
- 28.Smith PC, Lawhaswasdi K, Vick WE, Stanton JS. Isolation of rabies virus from fruit bats in Thailand. Nature. 1967;216:384. 10.1038/216384a0 [DOI] [PubMed] [Google Scholar]
- 29.Arguin PM, Murray-Lillibridge K, Miranda MEG, Smith JS, Calaor AB, Rupprecht CE. Serologic evidence of lyssavirus infections among bats, the Philippines. Emerg Infect Dis. 2002;8:258–62. 10.3201/eid0803.010330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuzmin IV, Botvinkin AD, Rybin SN, Bayaliev AB. Lyssavirus with usual antigenic structure isolated from bat in Southern Kyrghyzstan. [in Russian.]. Vopr Virusol. 1992;7:256–9. [PubMed] [Google Scholar]
- 31.Kuzmin IV, Botvinkin AD. The behaviour of bats Pipistrellus pipistrellus after experimental inoculation with rabies and rabies-like viruses and some aspects of the pathogenesis. Myotis. 1996;34:939. [Google Scholar]
- 32.Botvinkin AD, Kuzmin IV, Rybin SN. The unusual bat lyssavirus Aravan from Central Asia. Myotis. 1996;34:101–4. [Google Scholar]
- 33.Arai YT, Kuzmin IV, Kurane I. Phylogenetic analysis of Aravan virus isolated from the bat in Central Asia: natural foci infections of human. Proceedings of the Conference on the 80th Anniversary of the Institute for Natural Foci Infections, Omsk, Russia, 2001. p. 65–9. [Google Scholar]
- 34.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–82. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: Molecular evolutionary genetics analysis software. Tempe (AZ): Arizona State University; 2001. [DOI] [PubMed] [Google Scholar]
- 36.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. [DOI] [PubMed] [Google Scholar]
- 37.Badrane H, Tordo N. Host switching in Lyssavirus history from the Chroptera to the Carnivora orders. J Virol. 2001;75:8096–104. 10.1128/JVI.75.17.8096-8104.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourhy H, Kissi B, Tordo N. Molecular diversity of the Lyssavirus genus. Virology. 1993;194:70–81. 10.1006/viro.1993.1236 [DOI] [PubMed] [Google Scholar]