Although the bleeding tendency of patients with renal failure is well known, the literature concerning the cause of the coagulation failure is both meager and confusing. The hemostatic abnormality has been said to be greater in acute than in chronic uremia, and even to occur by a different mechanism.21 A variety of clotting defects have been described in both types of patients. Various plasma clotting factors have been found to be moderately reduced 10 with inconstant hypofibrinogenemia,8–10 with or without involvement of the prothrombin complex.5,9 Abnormal thrombelastograms, reduced heparin tolerance,8 and prolonged thrombin time10 have also been seen in many, but not all, patients.
The most consistently reported coagulation abnormality of uremia has been a deficiency in prothrombin consumption.4,5,10,21,22 In some cases, this could be explained solely by the thrombocytopenia which has been thought by some to be a common 9,21 and by others to be a rare 6 complication of azotemia. In a significant number of patients, however, poor prothrombin consumption and other abnormalities of platelet function have occurred in the absence of thrombocytopenia. Cahalone et al 4 believed that there was inhibition or destruction of platelet f actor-3 activity in the uremic plasma of such patients with the consequent loss of platelet thromboplastic function. In a subsequent report the presence of any platelet abnormality was denied.1
In previous times, precise knowledge of clotting in the terminally uremic patient was of limited practical use since death was frequently imminent and unpreventable. With the development of renal dialysis and homotransplantation, the outlook is no longer hopeless and the avoidance and control of hemorrhage has assumed increased importance. In this report an attempt will be made to characterize the coagulation abnormalities of uremia, to enumerate the screening tests used for detection of potential bleeders, to evaluate the methods available for correction of the clotting defects, and to describe some effects of hemodialysis and renal homotransplantation upon coagulation.
Methods
Most of the uremic patients studied had been admitted to the hospital for hemodialysis; many ultimately became candidates for renal homotransplantation. The studies obtained at admission were considered as controls against which to judge the effect of subsequent therapy or of progression of disease. The following tests were performed: thrombin generation,16 recalcification time, and prothrombin consumption measured as serum prothrombin time,15 prothrombin complex measured as prothrombin time with the use of human brain thromboplastin,15 thrombelastograms,17 thrombin time,18 and euglobulin lysis time.13 Blood platelets were usually counted with the phase microscope,3 or, alternatively, estimated upon smear. Blood for all examinations was obtained with the two syringe technique using siliconized equipment and using citrate as anticoagulant (four parts blood to one part 3.8% sodium citrate). Centrifugation for separation of plasma was carried out in siliconized tubes at 1,470 g for five minutes; with this procedure all the platelets remained in the supernatant plasma.
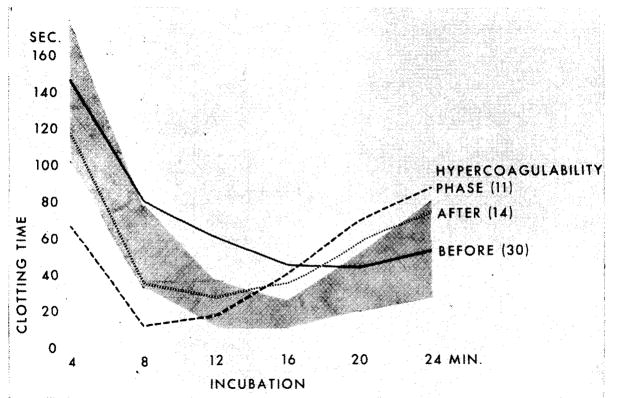
The thrombin generation test16 reflects the conversion of prothrombin to thrombin, a reaction which depends upon the activity of intrinsic blood thromboplastin. Freshly drawn plasma is recalcified and incubated and an aliquot added every four minutes to a standard fibrinogen solution. The clotting time of the fibrinogen solution is thus a measure of the speed and completeness of thrombin formation and ultimately for the amount of active thrombin remaining in the serum; the serial determinations provide data for graphs (Fig 1, 2, 3) which depict plasma incubation time (abscissa) and fibrinogen clotting time (ordinate).
Fig 1.
Thrombin generation pattern in uremic patients before and after homotransplantation of kidney. (Shaded Area) Range of value from 25 healthy individuals. (Black Curve) Average thrombin generation values of 34 uremic patients indicating deficient thrombin generation. (Dotted Curve) Average thrombin generation values of 14 patients one to ten days after transplantation showing normalization. (Dashed Curve) Average thrombin generation values of 11 patients 10 to 75 days after transplantation of the kidney, indicating hypercoagulability.
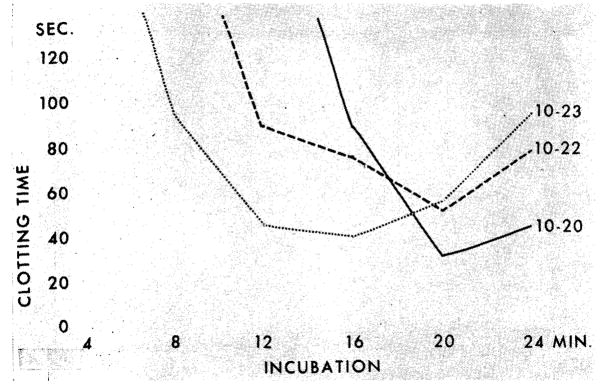
Fig 2.
Effect of hemodialysis upon thrombin generation in patient 9 (chronic glomerulonephritis). Oct 20: before dialysis. Activity of prothrombin complex 45%, platelets 163,000, BUN 316 mg/100 cc. Oct 22: after two dialyses. Platelets 91,250; BUN 262 mg/100 cc 10–23: BUN 160 mg/100 cc. Thrombin generation starts at normal time after third hemodialysis; yield, however, remains deficient.
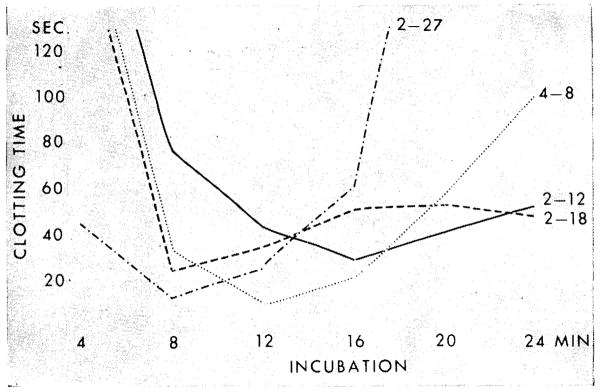
Fig 3.
Trend of thrombin generation pattern before and after homotransplantation of kidney (chronic glomerulonephritis). Patient 15. Curve, Feb 12: five days before transplantation. Platelets 244,000; BUN 174 mg/100 cc. Curve, Feb 18: one day after homotransplantation. Marked improvement. Platelets 285,000; BUN 43 mg/100 cc. Curve, Feb 27: hypercoagulability. Platelets 581,000; BUN 29 mg/100 cc. Curve, April 8: normal pattern. Platelets not done; BUN 22 mg/100 cc.
Plasma was used for substitution tests in two ways. First, blood was drawn from a healthy donor and centrifuged at 1,470 g for five minutes. All platelets remained in the supernatant; this “fresh plasma” was used within 30 minutes. Secondly, the above preparation was converted to “fresh frozen platelet-rich plasma” by first freezing (at −20 C) and then thawing. It was found that about half the platelets were destroyed by freezing and then thawing, thereby releasing platelet clotting factors; such plasma was used both for in vitro testing and for administration to patients. In both kinds of plasma, the original number of platelets was greater than in conventional blood bank preparations since the speed of centrifugation was less than that customarily used.
In vitro replacement therapy was also carried out with the human brain lipid prepared according to Bell and Alton,2 using a 1:100 dilution with buffered saline at pH 7.4. Serum prothrombin times were measured before and after the addition of the brain lipid.
Results
Untreated Uremic Patients
Studies performed shortly after admission revealed various clotting defects. Thrombin generation after 12 and 16 minutes incubation was abnormal in 82%, or 30 of 34 patients (Fig 1). In 22 of 26 cases (85%), the thrombin time was prolonged. Serum prothrombin time was short (below 30 seconds) in 14 of 24 (58%). In 16 of 33 patients, the number of platelets was reduced to one half to two thirds normal. The prothrombin complex (Quick one-stage prothrombin time) was reduced in 15 of 15 patients to an average of 40% (range 17% to 64%). None of 41 patients tested had reduced euglobulin lysis times; on the contrary, the values tended to be longer than normal.
With progression of uremia, further deterioration of all the above clotting parameters was observed. Serial thrombelastograms showed a progressive delay in the beginning and progression of the clot formation. Most severely affected were thrombin generation and the prothrombin complex. Thrombocytopenia of one fourth or less the normal value was seen. Even in such terminal patients, there was no increase in fibrinolytic activity.
In Vitro Correction of the Clotting Abnormalities
Since deficient thrombin generation, abnormal prothrombin consumption (shortened serum prothrombin time), or both were the most common and most serious clotting defects, attempts were made to restore normal coagulation in the test tube by the addition of reagents. In this way, information was sought which could be applied for the planning of corrective systemic therapy.
The deficient prothrombin consumption was restored by the addition of brain lipid (Table 1) both in the samples containing normal platelets or in those with mild thrombocytopenia. Since brain lipid has similar clotting properties to those of platelet factor-3, this finding was compatible with the hypothesis that the deficient prothrombin consumption was due to platelet dysfunction and led to further studies with replacement of platelet factors. First, fresh plasma was added to the patient’s uremic plasma. Although improvement in prothrombin consumption occurred in some cases, there was no correction by fresh plasma in others (Table 1) at the standard 10% aliquot and sometimes even when up to 50% was added. The latter observation suggests that uremic plasma may exert an inhibitory influence on the prothrombin consumption of normal plasma.
Table 1.
Serum Prothrombin Time (Seconds) in Uremic Plasma Before and After Addition of Fresh Plasma, Fresh-Frozen Plasma, or Brain Lipid (Normal Values: Above 35 Seconds)
| Patient | Diagnosis | Without Addition | Plus 10 % Fresh Plasma | Plus 5% Fresh-Frozen Plasma | Plus 5 % Brain Lipid | Platelets |
|---|---|---|---|---|---|---|
| 1 | Polycystic kidney | 25.7 | 28.7 | 67.8 | 43.7 | 179,000 |
| 2 | Acute anuria (postsurgical) | 26.8 | 32.3* | 61.9 | 94.5 | 125,000 |
| 3 | Chronic glomerulonephritis | 22.6 | 50.6 | 61.5 | 145.0 | 127,000 |
| 4 | Chronic bilateral pyelonephritis | 29.6 | 28.8* | 86.3 | 168.6 | 162,000 |
| 5 | Chronic glomerulonephritis | 20.7 | 31.1 | 59.7 | 91.4 | 92,000 |
| 6 | Chronic glomerulonephritis | 22.2 | 28.2 * | 49.7 | 56.5 | 300,000 |
| 7 | Chronic glomerulonephritis | 29.2 | 58.5 | 108.0 | 52.0 | 112,000 |
Only partial correction on addition of 50 % fresh plasma.
In contrast, the addition of 5% fresh-frozen platelet-rich plasma obtained from the same donor restored prothrombin consumption in every case (Table 1). These findings support the possibility that the platelets, which are destroyed by freezing and thawing, release platelet factor-3 (or some other involved component) which in turn binds some substance in uremic plasma which inhibits thrombocyte function.
In Vivo Correction of the Clotting Abnormalities
In several patients, a clinically obvious bleeding diathesis seemed to be improved with infusion of 750 ml fresh-frozen platelet-rich plasma. In one patient with diffuse mucosal hemorrhage 750 ml fresh-frozen platelet-rich plasma was given concomitantly with a fractionated intravenous dose of 50 mg phytonadine. The bleeding stopped abruptly. Prothrombin complex rose from 7% to 45% and there was improvement of the thrombelastogram and thrombin generation for the ensuing two weeks.
Effect of Hemodialysis on Clotting Abnormalities
Six patients were studied. Five had poor prothrombin consumption and reduced thrombin generation; the sixth had deficient thrombin generation alone. After hemodialysis, all six patients had some improvement of prothrombin consumption, as indicated by subsequent prolongation of the serum prothrombin time (Table 2). Thrombin generation was also improved (Fig 2) in four of the five cases in which it was determined (Table 2). In all cases there was some shortening of the thrombin time (Table 2) after hemodialysis.
Table 2.
Serum Prothrombin Time, Thrombin Time, and Thrombin Generation Before and After Hemodialysis*
| Patient | Diagnosis | Serum Prothrombin Time | Thrombin Time | Thrombin Generation After Dialysis | |||
|---|---|---|---|---|---|---|---|
| Before | (…) Days | After | Before | After | |||
| 3 | Chronic glomerulonephritis | 22.6 | 1 | 25.0 | 33.6 | 30.7 | Improved |
| 8 | Chronic glomerulonephritis | 26.3 | 1 | 44.0 | 25.2 | 23.2 | Not improved |
| 9 | Chronic glomerulonephritis | 35.2 | 1 | 44.2 | 33.0 | 25.0 | Improved |
| 6 | Chronic glomerulonephritis | 22.3 | 1 | 48.0 | 28.0 | 23.0 | Improved |
| 1 | Polycystic kidney | 29.1 | 4 | 29.8 | 18.8 | 16.8 | Slightly improved |
| 10 | Chronic pyelonephritis | 19.8 | 5 | 44.6 | 17.3 | 17.0 | Not done |
Thrombin generation tests were carried out in the blood samples drawn for serum prothrombin time.
The Influence of Homotransplantation
Clot formation improved in each of 14 patients who received a well-functioning renal homograft. Serum prothrombin times rapidly returned to normal (Table 3). In six of six patients the thrombin times were shortened and eventually normalized (Table 4). Serial thrombelastograms showed marked improvement of the timing and development of the clot, often within a day or two (Fig 4). Thrombin generation was equally improved in each of 14 patients (Fig 1). The correction was essentially complete by the first to tenth posttransplant day. Moreover, the acute increase in thrombin generation actually exceeded the normal pattern in most instances inasmuch as it started too early, provided a premature maximum yield, or both (Fig 1); the resultant hypercoagulability was in general greatest from 10 to 17 days after operation.
Table 3.
Serum Prothrombin Time Before and After Homotransplantation of the Kidney (Normal Values: Above 35 Seconds)
| Patient | Diagnosis | Serum Prothrombin Time |
Platelets Before | Platelets After | ||
|---|---|---|---|---|---|---|
| Before | (…) Days | After | ||||
| 11 | Chronic glomerulonephritis | 24.7 | 3 | 67.0 | Average | 201,000 |
| 12 | Chronic glomerulonephritis | 19.0 | 17 | 69.0 | 100,000 | 209,000 |
| 13 | Chronic glomerulonephritis | 27.4 | 60 | 71.8 | 217,000 | 466,000 |
| 14 | Chronic glomerulonephritis | 20.2 | 130 | 77.4 | 163,000 | average |
Table 4.
Thrombin Time (Seconds) Before and After Homotransplantation of The Kidney (Normal Range: 14–16 Seconds)
| Patient | Before Transplantation | After Transplantation |
|
|---|---|---|---|
| 1–5 Days | 1 Month | ||
| 15 | 37.0 | 17.6 | 17.7 |
| 16 | 20.5 | 16.0 | 18.4 |
| 17 | 28.4 | 21.5 | 15.4 |
| 18 | 22.4 | 18.4 | 16.0 |
| 12 | 30.0 | 17.1 | 16.0 |
| 19 | 25.0 | 18.0 | 14.1 |
| Average | 27.2 | 18.1 | 16.3 |
Fig 4.
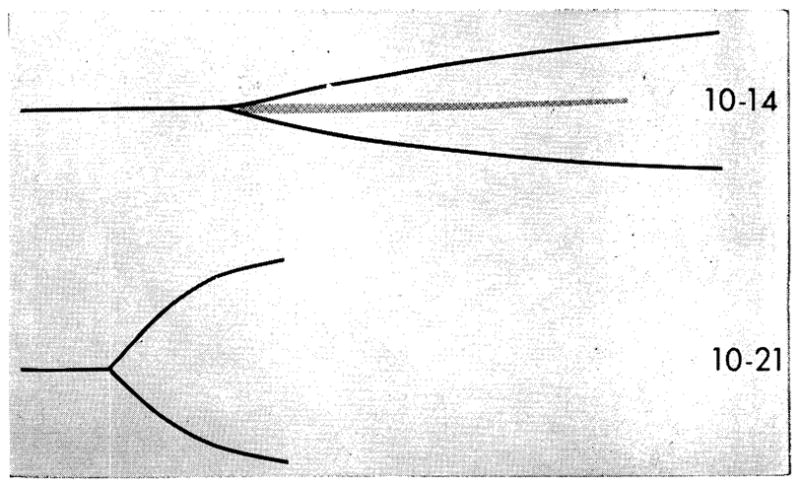
Thrombelastograms before and after homotransplantation of kidney (chronic glomerulonephritis. (Patient 20: thrombelastogram on Oct 14, four days before transplantation, platelets 62,000, BUN 148 mg/100 cc. Thrombelastogram on Oct 21, three days after transplantation, platelets 95,000, BUN 20 mg/100 cc. This thrombelastogram is normal. Center strip of the presurgical thrombelastogram indicating severe platelet insufficiency has disappeared and fibrin formation starts at normal time.
The effects of restoration of renal function are even more evident in the study of individual cases such as that depicted in Fig 4 and 5. In these patients the typical deficiency of thrombin generation existed before operation. Within one to three days after transplantation, this was dramatically restored toward normal; there had been essentially no change in the platelet count. In the patient shown in Fig 4, there was slight hypercoagulability after nine days of good homograft function, but by the seventh postoperative week, the pattern was normal.
Fig 5.
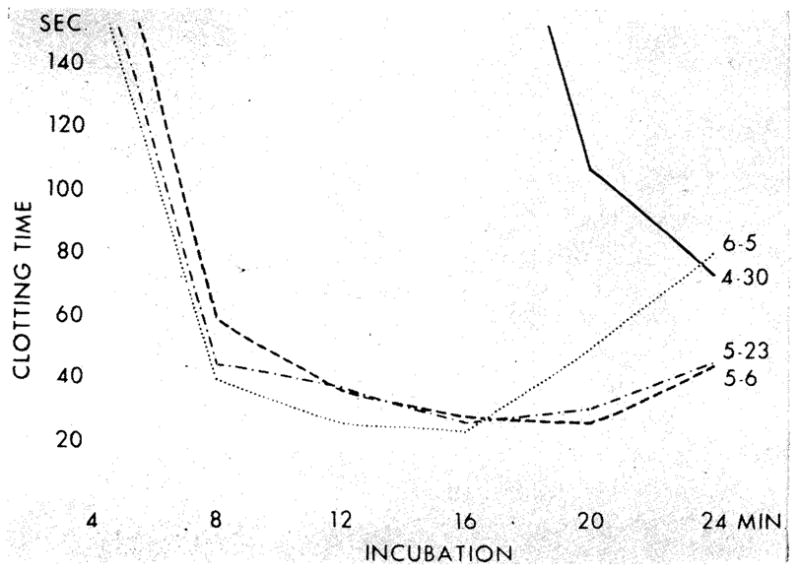
Trend of thrombin generation pattern before and after homotransplantation of kidney (chronic glomerulonephritis). Patient 11. Curve, April 30: very deficient thrombin generation. Adequate platelets on smear; BUN 230 mg/100 cc. Curve, May 6: three days after transplantation. Marked improvement. Platelets 201,000; BUN 25 mg/100 cc Curve, May 23: no change. Platelets 569,000; BUN 37mg/100cc. Curve, June 5: further improvement but not completely normal thrombin generation. Platelets 553,000; BUN 46 mg/100 cc.
In those patients who achieved long-term survival, the correction of the clotting abnormalities was parallel to the quality of homograft function. However, when rejection occurred, the coagulation defects reverted to the typical uremic pattern.
All patients who received a successful homotransplantation had a marked rise of antithrombin III activity.19 In no case has this returned to normal, even after observation for more than two years. The details and possible significance of this observation will be reported separately.
Comment
The foregoing data suggest that the specific coagulation deficiencies in uremia are essentially due to platelet disorders; the abnormal serum prothrombin times and the retarded thrombin generation are explicable on this basis since in vitro correction is possible by addition of material with platelet factor-3 function. The poor clotting is apparently not due primarily to a reduction in the number of platelets, since significant thrombocytopenia was a relatively uncommon finding except in terminal cases. Furthermore, in vitro correction was accomplished less effectively with fresh plasma with its normal platelet count than with fresh-frozen plasma or brain lipid which either contained a reduced number of platelets or none at all, respectively.
Instead, the findings in the present study support the contention of Cahalone et al 4 who believed that some substance was present in uremic plasma which inhibited platelet function; they demonstrated that such plasma abolished the coagulating function of normal platelets within 18 hours. Inasmuch as the clotting abnormality may be improved by hemodialysis it might be contended that the hypothetical material can cross the membrane of the artificial kidney, although such an interpretation is highly speculative since the homologous blood used to prime the extracorporeal circuit may theoretically have contributed. The beneficial effect of agents such as fresh-frozen platelet-rich plasma and brain lipid raise the possibility that platelet factor-3 may bind with and inactivate the inhibitory factor.
Other changes in coagulation were minor and probably relatively nonspecific. Prolonged thrombin times similar to those in the present report have been seen in patients with heart disease15,20 and other chronic illnesses. The moderate reduction in prothrombin complex (factors II, V, VII, X) reported in the present study is probably due at least in part to the liver disease which often accompanies uremia.8 That the hepatic injury was not severe is indicated by the facts that plasma fibrinogen tended to be higher than normal and that there was no increased fibrinolytic activity.
The retarded thrombin generation could be easily restored to normal in the test tube, but the effects of systemic therapy or renal hemodialysis were far less predictable. Although examples of apparent improvement of this test were seen both with infusion of fresh-frozen platelet-rich plasma (in combination with phytonadine), or with hemodialysis, the correction was at best partial and transient. In contrast, successful homotransplantation restored effective coagulation rapidly, for as long as adequate renal function was maintained, and without fail. An improvement, if not a complete correction, was often present within 24 hours. Conceivably, some of the immediate alterations could have been attributable to the nonspecific effects of surgery, to blood transfusions used at this time, to the splenectomy which was also performed in all of these patients,11 or to the administration of corticosteroids in many cases. Nevertheless, the principal benefit was clearly the consequence of reestablishment of adequate renal excretion. It deteriorated only with the return of renal failure. Control studies performed prior to transplantation indicated that azathioprine was without influence on the results of the coagulation studies.
In actuality, the intensity of clotting not only reached, but exceeded, normal values during the first few weeks, rendering the patients hypercoagulable from a laboratory point of view. Whether the degree of hypercoagulability is unique in renal homotransplantation, is the consequence of immunosuppressive drugs other than azathioprine, splenectomy, or both, or is only an exaggeration of the well-known increased tendency to accelerated clotting after any operation cannot be answered with certainty. It is, however, worth noting that the incidence of pulmonary embolization in cases of homotransplantation has been materially greater than in the average postoperative patient.11
In the course of this study, a battery of examinations was carried out not only in order to characterize the abnormalities of clotting which might be present, but also to define which tests would have the greatest practicality for the study and management of future cases. In view of the findings herein reported, it is thought that the ideal analysis should be sensitive for platelet function and endogenous inhibitors, should measure both hypercoagulability and hypocoagulability, and should be suitable for visual inspection in graphs or curves so that the changing kinetics of coagulation can be followed during the course of the disease and its therapy. Although the thrombin generation test and the thrombelastogram measure both overlapping but different clotting parameters, both examinations partially fulfill all three criteria. Since the thrombin time measures both heparin and heparin-like thrombin inhibitors, it adds a potentially useful additional dimension. None of the three examinations requires plasma dilution, so that the natural relation between factors inhibiting and promoting coagulation is not disrupted. Other tests can be considered as ancillary and used for the answer of specific detailed questions.
Summary
Blood coagulation was studied in patients with chronic renal failure in order to characterize the clotting deficiency in such patients and to assess the effects of therapy. The most common abnormalities of uremia seemed attributable to platelet dysfunction with consequent reduction of the conversion of prothrombin to thrombin; reduced thrombin generation and shortened serum prothrombin times resulted. This defect was apparently not usually caused by thrombocytopenia. Rather, it appeared that some substance may have been present in azotemic plasma which inhibited platelet function. In vitro addition of substances such as brain lipid or fresh-frozen platelet-rich plasma corrected the coagulation deficiency. Systemic therapy with fresh-frozen platelet-rich plasma seemed of some benefit.
Hemodialysis was shown to improve clotting in several cases but this effect was inconstant and transient. In contrast, renal homotransplantation rapidly restored good coagulation in direct parallel with and for as long as the persistence of good renal function. The correction usually exceeded normal, leading to a state of hypercoagulability primarily from the first to third postoperative weeks; this transient finding could help explain the heightened incidence of pulmonary embolization during this time.
Acknowledgments
This study was supported by grant HE 5538 and 5 T1 5638 from the National Heart Institute, US Public Health Service; and AM 06283, AM 06344, HE 07735, AM 07772, AI 04152, FR 00051, and FR 00069 from the US Public Health Service.
References
- 1.Altschueler G, Marcus J, Ullman HL. Platelets and Platelet Phosphatides in Uremia. Blood. 1960;16:1439. [PubMed] [Google Scholar]
- 2.Bell WN, Alton HG. A Brain Extract as a Substitute for Platelet Suspension in the Thromboplastin Generation Test. Nature. 1954;174:880. doi: 10.1038/174880a0. [DOI] [PubMed] [Google Scholar]
- 3.Brecher C, Schneiderman M, Cronkite EP. The Reproducibility and Constancy of Platelet Count. Amer J Clin Path. 1953;33:15. doi: 10.1093/ajcp/23.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Cahalone SF, et al. Acquired Thrombopathia. Amer J Clin Path. 1958;30:507. [Google Scholar]
- 5.Castellanos H. Alteracions en la hemostasia en la uremia cronica. Pren Med Argent. 1964;51:24. [PubMed] [Google Scholar]
- 6.Cheney K, Bonnin JA. Coagulation Defect in Uremia. Brit J Haemat. 1962;8:215. doi: 10.1111/j.1365-2141.1962.tb06514.x. [DOI] [PubMed] [Google Scholar]
- 7.Geiger MT, Sander JM, Rath CE. Evidence for a Qualitative Platelet Defect in Uremia, abstracted. Blood. 1960;15:428. [Google Scholar]
- 8.Gross R, Nieth H, Mammen E. Blutungsbereitschaft und Gerinnungsstoerungen bei Uraemie. Klin Wschr. 1958;36:107. doi: 10.1007/BF01500451. [DOI] [PubMed] [Google Scholar]
- 9.Kendall AG, Lowenstein L, Morgan RO. The Hemorrhagic Diathesis in Renal Disease. Canad Med Assoc J. 1961;85:405. [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JH, Zucker MB, Ferguson JH. Bleeding Tendency in Uremia. Blood. 1956;11:1073. [PubMed] [Google Scholar]
- 11.Starzl TE. Experience in Renal Transplantation. Philadelphia: W. B. Saunders Co; 1964. [Google Scholar]
- 12.von Kaulla KN. Zur klinischen Bedeutung und Technik des Heparintoleranztestes in vitro. Deutsch Med Wschr. 1953;78:1075. [PubMed] [Google Scholar]
- 13.von Kaulla KN, Schultz RL. Methods for Evaluation of Human Fibrinolysis: Studies With Two Combined Techniques. Amer J Clin Path. 1958;29:104. doi: 10.1093/ajcp/29.2.104. [DOI] [PubMed] [Google Scholar]
- 14.von Kaulla KN, Swan H. Clotting Deviations in Man Associated With Open-Heart Surgery in Hypothermia. J Thorac Surg. 1958;36:857. [PubMed] [Google Scholar]
- 15.von Kaulla KN, Swan H, Paton B. Variations of the Thrombin Time in Certain Patients Undergoing Open-Heart Surgery: Discussion of Its Prognostic Significance. J Thorac Cardiov Surg. 1960;40:260. [Google Scholar]
- 16.von Kaulla KN, von Kaulla E. Thrombin Generation in Normal Subjects and in Cardiac Patients. Circ Res. 1964;14:436. doi: 10.1161/01.res.14.5.436. [DOI] [PubMed] [Google Scholar]
- 17.von Kaulla KN, von Kaulla E. Thrombelastography: A Method of Continuous Recording of Fibrin Formation and Fibrinolysis. In: Tocantins LM, Kazal LA, editors. Blood Coagulation, Hemorrhage, and Thrombosis. New York: Grune & Stratton; 1964. pp. 34–40. [Google Scholar]
- 18.von Kaulla KN, von Kaulla E. Estimation of the Thrombin Time of Plasma. In: Tocantins LM, Kazal LA, editors. Blood Coagulation, Hemorrhage and Thrombosis. New York: Grune & Stratton; 1964. pp. 335–340. [Google Scholar]
- 19.von Kaulla KN, von Kaulla E. Thrombin Generation and Antithrombin III Activity in Homotransplantation of the Human Kidney. Tenth Congress of the International Society of Hematology; Stockholm. 1964. Abstract G-35. [Google Scholar]
- 20.von Kaulla KN, von Kaulla E. Observations With the Thrombin Time of Plasma in Health and Disease. to be published. [Google Scholar]
- 21.Rath E, Maillard JA, Schreiner GE. Bleeding Tendency in Uremia. New Eng J Med. 1957;257:808. doi: 10.1056/NEJM195710242571704. [DOI] [PubMed] [Google Scholar]
- 22.Wurzel HA. The Coagulation Defects in Uremia. Proceedings of the VI International Congress, International Society of Hematology; Rome. 1958; New York: Grune & Stratton; 1960. pp. 1187–1190. [Google Scholar]