Abstract
Recombinant therapeutic proteins, including antibodies, contain a variety of chemical and physical modifications. Great effort is expended during process and formulation development in controlling and minimizing this heterogeneity, which may not affect safety or efficacy and, therefore, may not need to be controlled. Many of the chemical conversions also occur in vivo and knowledge about the alterations can be applied to assessment of the potential impact on characteristics and the biological activity of therapeutic proteins. Other attributes may affect the drug clearance and thereby alter drug efficacy. In this review article, we describe attribute studies conducted using clinical samples and how information gleaned from them is applied to attribute criticality assessment. In general, how fast attributes change in vivo compared to the rate of mAb elimination is the key parameter used in these evaluations. An attribute with more rapidly changing levels may have greater potential to affect safety or efficacy and thereby reach the status of a Critical Quality Attribute (CQA) that should be controlled during production and storage, but the effect will depend on whether compositional changes are due to chemical conversion or differential clearance.
Key words: quality by design, critical quality attributes, pharmacokinetics, biotransformations, microheterogeneity
Introduction
Therapeutic monoclonal antibodies (mAbs), like other therapeutic proteins, are enormously complex drugs typically produced in mammalian tissue culture cells through recombinant DNA technology.1,2 As a result of naturally-occurring molecular heterogeneity, imperfect cellular processing, chemical and enzymatic changes during manufacturing and additional changes upon storage, antibody drugs display a wide variety of minor chemical changes, collectively termed microheterogeneity.3–5 Common examples include glycan structural differences, deamidation, oxidation and glycation.3,6,7 Control of microheterogeneity within predefined analytical specifications has been used in quality control laboratories to guarantee consistent product quality during cGMP manufacturing.6,8
The recent Quality by Design (QbD) initiative for therapeutic biotechnology products, a joint pilot program between the US Food and Drug Administration and the biotechnology industry, is providing new guidance and expectations on QbD approaches in manufacturing for therapeutic proteins.9 As the name indicates, QbD encourages developers to build quality into the drug from the start. This approach requires significant knowledge of the drug's mechanism of action and how the drug's attributes affect quality. Physical or chemical changes known to affect the safety or efficacy of the drug are considered critical quality attributes or CQAs.10,11 Manufacturing is then designed to control the desired levels of CQAs within defined limits, providing a consistent product quality.12 With this new QbD paradigm, the process is defined by the target ranges for the CQAs which, in turn, provide assurance of consistent product quality.11
Prior to designing a cell culture and purification process for mAb manufacture, a quality target product profile provides a list of quality attributes (QA) and what levels are critical and hence need to be monitored and controlled.10,11 Such a list is obtained using a risk-based approach, with knowledge gained through clinical and animal studies on the molecule or related proteins.11,13 Since antibodies are a homologous class of molecules, knowledge gained through prior experience or from published studies may greatly aid in defining CQAs. The definition for quality attribute is fairly broad and can potentially include raw materials in addition to features of the drug molecule itself. In this review we have focused on those attributes specific to the product, which we term PQAs for product quality attributes. We discuss how tracking the PQA levels in clinical studies assists in the assessment of attribute criticality and provide examples of how results are interpreted. While these studies are unlikely to determine an attribute's impact by themselves, when combined with other types of information, they provide a critical tool to guide the developer in making appropriate assessments regarding drug development.
Effects of PQAs on Safety or Efficacy
A given product quality attribute has the potential to affect the safety or efficacy of therapeutic mAbs through a variety of mechanisms. Efficacy can be affected by changing the antibody's interaction with its target or an off-target ligand. Chemical modification of a critical complementarity-determining region (CDR) residue is an example of an attribute that exerts a direct effect on target binding. Such a change, which may occur at relatively low levels, may increase under varying cell culture or purification conditions, leading to variability in manufacturing lots. Changes may also be generated in vivo during the process of drug distribution or systemic circulation. Deamidation at Asn- 33 and oxidation at Trp-105 in the light chain and heavy chains, respectively, of two therapeutic mAbs represent examples of this class of attributes.14,15 Alternatively, a PQA may affect efficacy by changing the in vivo concentration by impacting clearance rates. Attributes that have been shown to impact FcRn binding, e.g., oxidation of Met-252 and -428, are candidates that may impact efficacy in this indirect fashion.16 Finally, a PQA may alter the safety profile of the therapeutic antibody either by causing an increase in potential immunogenicity or by causing an increase in off-target binding. Studies monitoring formation of potential safety-related PQAs can be used to estimate the impact of drug PQA levels on patient exposure. Ultimately, the impact of product quality attributes on safety may require appropriate toxicology and clinical studies.
Effects of PQA on Clearance
Two general approaches have been used to study the indirect impact of an attribute on clearance rates. In one approach, therapeutic protein preparations have been generated that differ in the attribute's levels, either through genetic manipulation,17 addition of metabolic inhibitors to cultures,18 purification strategies19 or enzymatic treatment.20 The samples differing in the levels of the attribute are then injected into animals to determine the impact on overall clearance rate. In another approach, the levels of the attribute are analyzed from patient serum over time after a single preparation of the therapeutic protein has been administered.21,22 Changes to the attribute levels with circulation time are interpreted as arising from differences in clearance rates.
Both approaches have advantages and disadvantages. The first, the enrichment approach, has the advantage of directly measuring the drug clearance, not inferring clearance based on attribute level changes; however, an assumption is made that the specific attribute of interest is the sole significant difference between the samples. It may be possible that other attribute differences can account for the clearance differences between the two samples. Thus, studies not thoroughly demonstrating that samples are chemically and structurally comparable except for the attribute of interest may draw incorrect conclusions about the attribute's impact. Finally, these studies are limited to animals, typically rodents, though the goal is often to understand the effect in humans. Unfortunately, changes that impact human antibody clearance can be obscured in mice due to FcRn binding differences between species.23 A more relevant animal model involves expressing human FcRn in mice lacking the mouse version.24 This, however, carries additional complexities as the result of affinity differences of the endogenous mouse antibodies and the injected human antibody toward the human FcRn.25
The second, a post-administration collection approach, can follow a collection of attributes (microheterogeneity) simultaneously, which is not possible with the enrichment approach. Methodologies, such as peptide mapping with mass spectrometry detection, have the selectivity required to enable many attributes to be independently monitored. Since changes to a single administered sample are followed, the effect of specific microheterogeneity can be monitored without the confounding influence of multiple components of sample heterogeneity inherent to the enrichment approach. The potential for bias exists, however, when the drug is purified from biological samples using methods that may introduce bias specifically with respect to the attribute of interest, e.g., use of target-based affinity purification of a mAb used to study an attribute that decreases the affinity of the antibody for its target. Such an event can be identified with controls, but, on a practical level, both antibody binding sites would have to contain the attribute in order to escape affinity purification—a statistically low probability event given the typically low levels of attribute being followed. Although linkage of the change in attribute level to differential clearance is indirect, this approach has a major advantage of evaluating impact in humans, thus allowing analysis of human therapeutic mAb in human subjects. If other mechanisms besides clearance are responsible for the changes in attribute levels, such as metabolism of the attribute by in vivo mechanisms, the study might falsely interpret these changes as differential clearance, especially if proper in vitro controls are not performed.
Distinguishing Conversion from Clearance
A combination of experimentation and critical thinking is needed to distinguish chemical conversion from differential clearance. In a theoretical example of microheterogeneity, an antibody has two forms, A and B. A post-administration collection study has determined that over time in vivo, the level of the A form decreases in relation to the B form. Knowledge of the chemical mechanism that might be responsible for this change is needed to determine whether this change is due to conversion or clearance. Is the chemical or enzymatic conversion of A→B kinetically or thermodynamically possible? Can the conversion be recreated in vitro in either a buffer or serum sample? If a plausible pathway for in vivo A→B conversion exists and the conversion can be recreated in a closed in vitro system under physiological conditions, a good argument for in vivo conversion can be made. On the other hand, if, based on the chemistry, in vivo conversion of A→B seems implausible and the conversion cannot be duplicated in vitro, increased clearance of A offers an explanation. In this case, the existence of a logical mechanism by which attribute A might be cleared faster, such as the existence of known A-specific receptors, would lend credence to this explanation.
In Vitro Systems to Model In Vivo Conversion
In the simplest cases, a chemical conversion that occurs in vivo may be modeled by a pH-buffered, protein-free in vitro system such as PBS. Asparagine deamidation represents an example of a chemical conversion that can be observed to occur at comparable rates both in vivo and in vitro as it is primarily pH-controlled.21 In this case, there is no need to invoke differential clearance as the deamidation rate observed in vitro at physiological pH matches the observed in vivo conversion rate. In fact, the first-order deamidation rate constants at susceptible asparagine sites are so consistent at physiological pH that the degree of measured deamidation can be used to estimate the elimination rate constant and half-life of endogenous antibodies in vivo.21
In other cases, more complex in vitro models may be required to reproduce the in vivo conversion rates. Blood is composed of many types of cells, proteins and small molecules. Depending on the mechanism of conversion, any of these may have to be included or mimicked, in the in vitro system to achieve conversion rates similar to those observed in vivo. In some cases, it may be easier to utilize plasma or serum as a model system rather than attempt elaborate reconstitution experiments. Incubation of mAb in human serum at 37°C was used to demonstrate that trimming of high mannose (M6–M9) glycoforms occurred with similar rates in this in vitro model system as in vivo, attributed to the action of α-1,2 mannosidase activity in serum or blood.26 If a closed in vitro model system operating under physiological pH and temperature re-creates, with similar kinetics, an A→B conversion observed in vivo and the constituents of the model system logically explain the conversion, it can be argued that the changes observed in vivo are likely also due to conversions, not differential clearance.
Estimating Quantitative Impact of PQAs
The final, but arguably most important, step in an in vivo study is to assess the impact of the conversion or clearance on the evaluation of criticality of the attribute. This appraisal, which includes a determination of the speed and degree of the changes, will be discussed separately for chemical or enzymatic conversion and for differential clearance. In both cases, however, changes that occur in vivo on a time scale shorter than overall drug clearance will have the largest effect on the degree of exposure to the attribute or total antibody clearance, respectively. The magnitude of these effects can be estimated by appropriate simulations once the rate constants for total drug clearance and attribute conversion or relative clearance have been determined.
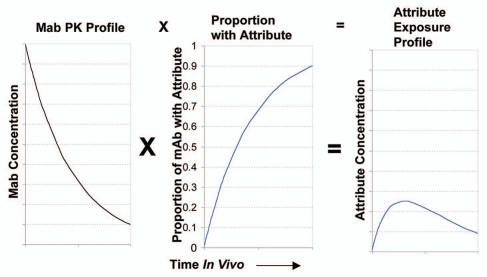
In the general case of an attribute B arising through A→B conversion in vivo, the patient exposure profile to the attribute will be given by the product of the antibody drug exposure profile and the proportion of attribute converted [B/(A + B)]. This is illustrated schematically in Figure 1. The magnitude of the patient exposure to the attribute will be determined by the relative rates for attribute conversion vs. antibody elimination. In the following discussion, an estimation of the quantitative impact on patient exposure to the attribute is provided using simple first-order kinetic simulations for both antibody elimination and attribute conversion. Although neither attribute conversion nor antibody elimination may be first order, these examples help illustrate how the relative rates of these two processes influence patient exposure to the attribute.
Figure 1.
Schematic of the relationship between mAb PK profile, growth of an attribute proportion over time in vivo and the resultant attribute exposure profile. The AUC of the attribute vs. time plot gives the patient exposure to the drug.
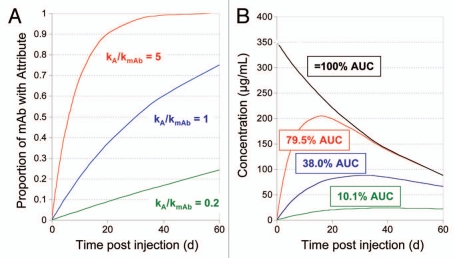
When an attribute is generated through chemical or enzymatic conversions in production or storage, the concern may be that the patient is exposed to an “unnatural” form, which may impact safety or efficacy. Yet, the fact that the attribute can be formed during cell culture conditions, conditions which are often not significantly different from those in circulation, hint that further formation of the PQA may occur in vivo. Figure 2 illustrates a theoretical example of A→B in vivo conversion after IV dosing where negligible B levels existed at the time of dosing, but changes in the B levels, as measured as the fraction of conversion, B/(A + B), were measured over time in circulation. In this simple example, the drug elimination rate is simulated by a single first order decay, C = Coe-kmAbt, where C = drug concentration, C0 is drug concentration at time of injection and kmAb = the drug elimination rate constant. Conversion of A→B is also simulated as a first order process where the fraction of mAb with attribute B is given as 1-e-kAt, where kA = the rate constant for A→B conversion. Patient exposure to B depends on the ratio of the A→B conversion rate to the drug elimination rate and is calculated as B = Coe-kmAbt (1- e-kAt), where B is concentration of attribute B. When both rate constants are identical at 0.023 d−1, corresponding to a half-life of 30 days and the injected drug contains no B form, 38% of the patient's exposure to the drug, as measured by pharmacokinetic area under the curve (AUC), will be to the B form over the time period corresponding to the first two half-lives of the drug. Thus, when the rate of conversion is similar in magnitude to the rate of elimination, a significant fraction of patient exposure will be to both drug forms. Patient exposure to the B form would increase with conversion rates increasing relative to elimination and would decrease concomitantly with decreased relative elimination rates (Fig. 2). Although numerical values for systemic exposure calculated from such a simple model will necessarily result in approximations, they are nevertheless useful in providing perspective on impact to patient exposure. These considerations acquire special importance when there is a difference in activity between A and B forms. In this case, exposure differences to the two forms may result in differences in drug efficacy; the relationship between patient exposure and clinical efficacy may be simple or complex and should be well-characterized for each molecule. Differences in activity between A and B forms greatly enhance the likelihood that these attributes would need to be well-controlled through specifications or in- process controls.
Figure 2.
Effect of different rates of attribute formation relative to rate of mAb elimination on patient exposure to an attribute forming in vivo. Calculated results, over two half-lives, for a mAb with an initial concentration of 350 µg/mL, zero attribute at time of injection and a first order clearance rate corresponding to a half-life of 30 days. (A) Proportion of mAb with attribute over time in vivo, calculated for an attribute formed with first order kinetics and three different hypothetical ratios of rate constant for attribute formation (kA) to rate constant for mAb elimination (kmAb). (B) mAb concentration (black line) and calculated attribute concentrations (colored lines) vs. time post injection. Numbers in boxes give the calculated degree of systemic exposure to the attribute, as estimated by partial AUC, relative to that of mAb.
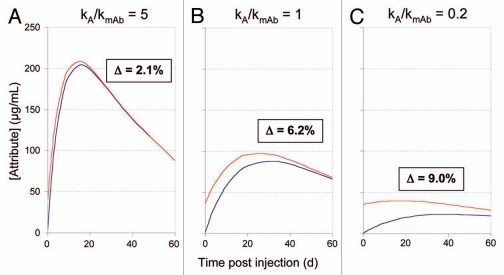
Simulated changes in patient exposure based on samples initially differing in the amount of attribute B, where attribute A→B conversion also occurs over time in vivo are shown in Figure 3. The general effect is that A→B conversion in vivo leads to diminished differences in patient exposure to B as compared to the initial lot-to-lot differences; the significance decreases as the drug is eliminated. The patient's exposure to B varies with both the initial levels of B and the rate of B formation relative to the rate of drug elimination. If attribute formation is fast relative to drug clearance, exposure to the attribute is relatively high, but variations in initial attribute level have minimal impact on overall attribute exposure (Fig. 3A). Initial level differences impact patient exposure more significantly when slow attribute formation is coupled to relatively fast clearance (Fig. 3C). As shown in Figure 3A, an example with fast attribute B formation relative to drug clearance, a 10% lot-to-lot variation in attribute B generates only a small (2.1%) difference in patient exposure. Process and storage controls of the attribute levels would provide little benefit because they would have little influence on patient exposure and hence minimal impact on the safety or efficacy of the drug. Conversely, as shown in Figure 3C, when lot to lot variations in attribute B are large compared to the amount of attribute B that can form in vivo over the time period in question, larger differences in exposure to attribute will result. In this case greater attention might be paid to process and storage control. Other information, including potential impact on efficacy, theoretical safety concerns regarding attribute B, as well as prior clinical exposure, can factor into the decision making.
Figure 3.
Patient exposure to attribute as a function of attribute level of injection calculated for three different rates of attribute formation. Assumed numerical parameters are the same as those for Figure 2. Blue trace, 0% attribute B at time of injection; red trace, 10% attribute B at time of injection. Δ refers to the difference in area under the two curves expressed as a percentage of the total mAb AUC. kA = first order rate constant for attribute A→B conversion in vivo, kmAb = first order rate constant for mAb elimination.
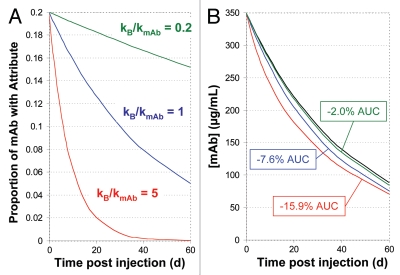
An attribute that alters the rate of elimination could also affect the drug's efficacy. In such cases, how the relative rate of attribute elimination (rate of change in the proportion of mAb containing the attribute) compares to the absolute rate of mAb elimination will determine the quantitative impact on systemic exposure to drug. Again, using a model that employs first order rate constants for both mAb elimination (kmAb) and relative attribute elimination (kB), we can calculate the mAb concentration at any time t as C = Coe-kmAbt(1 − B0/C0(1 − e-kBt)), where B0/C0 represents the proportion of mAb with attribute B at injection. The impact of this on AUC is illustrated in Figure 4 for a hypothetical mAb example where an attribute, present at a proportion of 0.2 relative to total mAb at time of injection, is cleared more quickly than bulk mAb. When the rate constants for relative attribute elimination and bulk mAb elimination are identical, systemic exposure to mAb is decreased by 7.6% over the first two elimination half-lives. Although modest in numerical terms, a difference of this magnitude may lead to a failure of the bioequivalence criteria in human studies. Attribute B might be deemed a critical quality attribute based on these considerations. In contrast, a numerically larger proportional exposure to an increasing attribute, such as discussed in the context of Figure 2, need not have and, in practice, frequently does not have, any impact on safety or efficacy provided the clearance of the attribute is similar to that of bulk mAb.
Figure 4.
Effect of different relative attribute clearance rates on patient exposure to mAb. Calculated results, over two half-lives, for a mAb with an initial concentration of 350 µg/mL, 20% attribute B at time of injection and a first order mAb clearance rate corresponding to a half-life of 30 days. (A) Proportion of mAb containing attribute B as a function of rate constant for reduction in the proportion of attribute B (kB) and rate constant for mAb elimination (kmAb). (B) Calculated decrease in patient exposure (partial AUC) to mAb as a result of three different first order relative elimination rates of the attribute. The black curve represents mAb elimination in the absence of faster attribute elimination. Colored curves show mAb elimination kinetics with different relative attribute elimination rates matching the respective colors in (A).
Information from Endogenous Antibodies
Information gleaned from the analysis of attributes on the endogenous antibodies of healthy subjects can provide additional clues about criticality. Therapeutic antibody product quality attributes that are also found in significant levels on endogenous human antibodies would seem less likely to represent a safety concern. Myeloma proteins, such as the multiple commercially available human IgG1 and IgG2 forms,27 represent another potential source of purified human antibodies for attribute evaluation, as long as the atypical background of these molecules and potential effect on attributes, is kept in mind. The monoclonal nature of the myeloma proteins allows site specific modifications in the Fab region to be studied, which would be difficult with polyclonal pools of endogenous antibodies.
Using In Vivo Results to Evaluate Quality Attribute Criticality
Clinical study data can be used together with other relevant information to assess an attribute's criticality. Specifically how such an evaluation is done is outside the scope of this review, but it could include various in vitro activity data, clinical experience and previous experience with related molecules containing the attribute of interest. Two examples are discussed to illustrate the connection between data obtained from clinical attribute studies and evaluation of quality attribute criticality. In the first, deamidation was studied in vivo and in vitro for three (both IgG1 and IgG2) injected therapeutic mAbs.21 Among the conserved sites, only Asn 384 was found to be deamidated at an appreciable rate and all mAbs exhibited similar deamidation kinetics, both in vivo and in vitro, suggesting that deamidation is primarily pH controlled. Endogenous IgG1 and IgG2 were collectively found to be 23% deamidated at this site. This value was then used to calculate a very reasonable circulating half-life of 30 days for the endogenous antibodies, using the measured therapeutic mAb deamidation rate constant. Significant conversion rates in vivo raises the question of the importance of controlling lot-to-lot variability with respect to this PQA. In view of the large degree of deamidation occurring in vivo, relatively smaller deamidation differences among drug lots appear less important. Additionally, the authors demonstrated quantitatively how significant initial lot-to-lot differences in degree of deamidation result in much smaller patient exposure differences to this attribute, similar to what is simulated in Figure 3C. Since deamidation at Asn 384 is not known to affect safety or efficacy, it may be reasonable to assign this as a non-critical PQA. A practical consequence might be that specifications with respect to degree of deamidation be broadened or potentially eliminated. Because this attribute behaves similarly among IgG1 and IgG2 antibodies, this information can be applied to specification setting on new antibody drugs that have yet to enter clinical studies. This feedback loop using “lessons learned” is an essential element of QbD.
A second example is represented by the reshuffling, in vivo, of IgG2 disulfide isoforms, which may exhibit different activities.22 The fact that disulfide variants occur among endogenous IgG2 antibodies and that reshuffling, to an apparent equilibrium state, occurs in vivo over a relevant time scale, reduces the significance of initial lot-to-lot differences in disulfide isoform distribution. In vivo reshuffling rate constants may be used to evaluate the potential impact quantitatively, which may factor into assessing the criticality of this attribute. However, in this case, activity differences that might exist between the different disulfide forms would be weighed in conjunction with the reshuffling information. Viewed this way, the in vivo information becomes critical to placing any initial lot-to-lot isoform and perhaps activity, differences into the proper context, leading to a more relevant assessment of attribute criticality. Using QbD principles, this information should then feed back to influence the possible control or specification strategy around this attribute.
Analytical Methodology and Controls for In Vivo Attribute Characterization
PQA analyses from in vivo studies first require purification of the antibody drug from patient or animal serum. Best results can be obtained through affinity purification with the target ligand or anti-drug antibodies, which can provide a high degree of purification with relative ease of use. Concentrations of serum-purified therapeutic antibody can range from hundreds of micrograms per milliliter to sub-microgram per milliliter levels, depending on the dosing, route of administration and time after injection.21,22 These low concentrations of mAb must first be purified from a matrix of endogenous antibodies and other proteins present at total concentrations over 50 mg/mL, so care must be taken to ensure that the proper degree of purification has been achieved for all the samples analyzed. In one study, we observed that approximately 70% of a mAb can be recovered from human serum by such procedures, with purity estimated as greater than 95% by microchip CE-SDS, for our lowest purity sample.22 In addition, proper controls should be performed demonstrating that the isolation procedure does not alter the attribute levels.
Many analytical methods have been employed to study attribute changes in vivo. Two of the most prevalent tools for obtaining detailed information regarding mAb structure and hence PQAs, high performance liquid chromatography (HPLC) and mass spectrometry (MS), are also suited for in vivo analysis. The investigator must decide whether site specific information is needed or whether whole molecule changes should be monitored instead. Site specific methods, such as RP-HPLC peptide mapping with MS identification, improve selectivity by identifying and quantifying only those attributes on specific peptides from sites on the protein of interest.28 For example, in the study of specific Fc glycans as attributes, methods that rely on glycan release from sample are, in principle, subject to interference from any contaminating serum glycoprotein. Peptide mapping methods also allow sites of concern to be monitored independent of interference from sites deemed unimportant. Deamidation on a CDR that impacts antibody activity might be an example of this type of modification.29 More selective peptide map-based methods suffer from potential interference only when the modified peptide in question exists in multiple proteins or at multiple sites in the protein under investigation. As an example, IgG1 and IgG2 Fc glycosylation can be monitored independently in complex samples by trypsin or Lys-C peptide mapping, since these Fc glycopeptides differ at multiple residues between IgG1 and IgG2 and correspondingly have different molecular weights and MS2 fragmentation patterns. This site-specific approach can serve to increase the specificity and accuracy significantly, but may do so at the expense of sensitivity, since all similar modifications are not measured in aggregate. Nevertheless, site-specific information often is a key factor in the evaluation of attribute criticality. For example, Huang et al. used peptide mapping to follow deamidation of a specific CDR Asn residue of a therapeutic antibody in vivo.30 Although other Asn sites deamidate in vivo on the same time scale, peptide mapping allowed the site of interest to be monitored in isolation. Methods monitoring global changes to the antibody may be more sensitive because they sum similar modifications at multiple sites. Modifications occurring at many sites at similar rates would be expected to provide the greatest sensitivity enhancement by using a global method. Examples of such methods include mass analysis and chromatography on the intact molecule. Glycation occurring at low levels at multiple lysines can be effectively quantified by whole mass analysis of (deglycosylated, if necessary) antibody chains, though additional site information might be needed to properly evaluate the criticality of this attribute.
Because antibodies are composed of multiple polypeptide chains, information is lost when attribute levels are measured by techniques that don't retain the intact antibody structure. Peptide maps quantify the level of attribute per heavy or light chain, but have lost information regarding the combinatorial association of the attribute-containing chains. For a low-level attribute found at fraction X on heavy chains, the proportion of intact antibodies containing the attribute could range from X (preferential pairing) to (2X-X2) (random pairing). Fc glycoforms represent examples of attributes that have previously been shown to be present on intact antibody molecules in ratios that differ from what is expected based on random pairing.31 Therefore, in order to more accurately estimate the effect, it may be more relevant to calculate the fraction of antibodies with such an attribute as opposed to the fraction of heavy chains containing this attribute. The pairing preference of Fc-glycans can often be determined by intact mass measurement of mAbs, which, in turn, can be used to calculate the fraction of antibody containing a particular glycan-related attribute.
Purity Considerations and Potential Artifacts
As the concentration of therapeutic mAb declines post-dosing and the ratio of therapeutic IgG to endogenous IgG in serum samples declines, a decrease in the purity of mAb samples purified from corresponding serum samples is possible due to non-specific binding of endogenous IgGs to the affinity resin. Peptide map-based analytics can decipher such interferences, so long as peptides generated from the proteinaceous impurities differ in sequence from those from therapeutic mAb of interest. Copurification of endogenous IgGs of the same subclass as the therapeutic mAb may therefore lead to errors in interpretation in situations where attribute levels differ significantly between therapeutic IgG and endogenous IgGs. To illustrate, therapeutic mAbs produced in CHO cell culture may contain both glycan structures not found in measurable levels on endogenous human IgG antibodies,32 as well as specific glycoforms in proportions quite different from those found on endogeneous IgG of the same subclass.32 A change in the level of such an attribute over time in vivo may be attributed to altered rates of clearance, but could also arise artifactually from dilution with the identical peptide from endogenous antibodies of the same subclass, due to decreasing sample purity.
In addition, as the sensitivity of available methods improves, special consideration should be given to avoiding spurious conclusions based on mis-interpretation of data. The ability to analyze moderate to minor changes in specific attribute levels in low concentration and relatively impure samples is a tribute to the ongoing improvements made in analytical technologies; however, every observed change may not translate into an actual conversion in vivo. In the analysis of therapeutic IgGs, co-purification of endogenous IgGs can lead to spurious mass shifts found on particular peptides (data not shown), which are actually related to amino acid substitutions between variable regions or the constant regions of IgG subclasses. For example, in the heavy chain, the Lys-C generated peptide T393-K409 differs only by a V397M substitution between IgG1 and IgG2.16 If one were analyzing a therapeutic IgG1 purified from serum with a low level of non-specifically purified endogenous IgG2, this sequence difference would lead to the observation of a peptide with a mass shift of 32 Da, which increased in concentration relative to the level of “un-modified” peptide over time. Depending on the presence or quality of the obtained MS2 spectrum, this apparent mass shift could be misinterpreted as increasing levels of double oxidation of the peptide or, alternatively, as arising from a mutant version of the mAb with a different clearance rate. In this case, constant region sequence examination between IgG1 and IgG2 subclasses would offer a clue as to the presence of this potential artifact.
Other analytical artifacts may arise from concentration, reactant ratio or enzyme-substrate ratio differences between samples. When a change in PQA level correlates with the length of time the drug was in a trial subject, the possibility that the change was due to differing ex-vivo concentrations of mAb throughout the experimental procedures may need to be ruled out. If changes in methionine (Met) oxidation levels over time are observed in vivo using peptide mapping, at least two mechanisms, in addition to chemical conversion in vivo, could account for the apparent changes. Solvent exposure of Met residues within the intact protein is known to correlate with oxidation sensitivity.16,33–36 If the same amount of proteolytic enzyme is used to digest the re-purified mAb regardless of concentration, a greater percentage of low concentration mAb is fully digested prior to the higher concentration mAb. Since the proteolytic peptides are solvent-exposed, the product of (solvent exposure x time) is increased in the low concentration sample due to the change in enzyme substrate ratio. Alternatively, a simple change in reactant ratios caused by the decreased mAb peptide concentration could increase the ratio of oxidized to non-oxidized peptide. Many concentration or purity-related artifactual attribute changes can be ruled out with a simple serum spiking experiment. In such an experiment, the therapeutic mAb is spiked into serum at concentrations similar to those observed in trial subjects at various time points. Since the serum-spiked mAb is immediately re-purified and analyzed, any attribute artifacts related to purity or concentration can be identified.
Summary
Traditional manufacturing of biotherapeutics has placed a high degree of importance on achieving product consistency at time of lot release with respect to multiple quality attributes, each of which will fall somewhere along a continuum of criticality with respect to drug safety and efficacy. Consistency based specifications, however, have been used only as surrogate markers of product quality, the purpose of which is to assure proper safety and efficacy of the drug in the human body. Importantly, PQAs are not necessarily static after administration, but may change in ways that affect the impact and hence potential criticality, of the PQA. A valid assessment of the criticality of attributes, as required by the QbD paradigm, would therefore benefit from an evaluation of attribute impact in vivo.
Our goal here has been to outline and evaluate practical ideas regarding how tracking PQA levels in clinical studies can ultimately aid in assessing attribute criticality. The important role played by in vivo studies of attribute stability toward this end, which will help to deliver on the promise of QbD, dictates that it be employed during the key decision-making phases of therapeutic mAb development. In vivo studies can directly measure or help model, systemic exposure to an attribute which is a prerequisite for determining its impact. In addition, information gained about comparative systemic exposure to or clearance of, an attribute can be key to understanding bioequivalence data. Ultimately, a thorough understanding of product quality attribute behavior in the patient's body should contribute to the development of safer and more efficacious mAb therapeutics.
Acknowledgements
We wish to acknowledge Edward Lee for helpful discussions regarding pharmacokinetic concepts, Zhonqi Zhang for guidance on MassAnalyzer software and Drew Kelner for support and critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/12897
References
- 1.Birch JR, Racher AJ. Antibody production. Adv Drug Deliv Rev. 2006;58:671–685. doi: 10.1016/j.addr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 3.Kozlowski S, Swann P. Current and future issues in the manufacturing and development of monoclonal antibodies. Adv Drug Deliv Rev. 2006;58:707–722. doi: 10.1016/j.addr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Gaza-Bulseco G, Faldu D, Chumsae C, Sun J. Heterogeneity of monoclonal antibodies. J Pharm Sci. 2008;97:2426–2447. doi: 10.1002/jps.21180. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Singh S, Zeng DL, King K, Nema S. Antibody structure, instability and formulation. J Pharm Sci. 2007;96:1–26. doi: 10.1002/jps.20727. [DOI] [PubMed] [Google Scholar]
- 6.Chirino AJ, Mire-Sluis A. Characterizing biological products and assessing comparability following manufacturing changes. Nat Biotechnol. 2004;22:1383–1391. doi: 10.1038/nbt1030. [DOI] [PubMed] [Google Scholar]
- 7.Harris RJ. Heterogeneity of recombinant antibodies: linking structure to function. Dev Biol. 2005;122:117–127. [PubMed] [Google Scholar]
- 8.ICH Q6B, author. Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products. http://wwwichorg/LOB/media/MEDIA432pdf.
- 9.US Food and Drug Administration, author. Notice of pilot program for submission of quality information for biotechnology products in the Office of Biotechnology Products FDA, docket number FDA-2008-N-03551. 2008. http://www.fda.gov/OHRMS/DOCKETS/98fr/FDA-2008-N-0355-n.pdf.
- 10.ICH Q8(R2), author Pharmaceutical Development. http://wwwichorg/LOB/media/MEDIA4986pdf.
- 11.Rathore AS, Mhatre R, editors. Quality by Design for Biopharmaceuticals. Hoboken, NJ: John Wiley & Sons, Inc; 2009. [Google Scholar]
- 12.Rathore AS, Branning R, Cecchini D. Quality: Design space for biotech products. BioPharm Int. 2007:36–40. [Google Scholar]
- 13.Rathore AS. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009;27:546–553. doi: 10.1016/j.tibtech.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Vlasak J, Bussat MC, Wang S, Wagner-Rousset E, Schaefer M, Klinguer-Hamour C, et al. Identification and characterization of asparagine deamidation in the light chain CDR1 of a humanized IgG1 antibody. Anal Biochem. 2009;392:145–154. doi: 10.1016/j.ab.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 15.Wei Z, Feng J, Lin HY, Mullapudi S, Bishop E, Tous GI, et al. Identification of a single tryptophan residue as critical for binding activity in a humanized monoclonal antibody against respiratory syncytial virus. Anal Chem. 2007;79:2797–2805. doi: 10.1021/ac062311j. [DOI] [PubMed] [Google Scholar]
- 16.Pan H, Chen K, Chu L, Kinderman F, Apostol I, Huang G. Methionine oxidation in human IgG2 Fc decreases binding affinities to protein A and FcRn. Protein Sci. 2009;18:424–433. doi: 10.1002/pro.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright A, Morrison SL. Effect of altered CH2-associated carbohydrate structure on the functional properties and in vivo fate of chimeric mouse-human immunoglobulin G1. J Exp Med. 1994;180:1087–1096. doi: 10.1084/jem.180.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Q, Shankara S, Roy A, Qiu H, Estes S, McVie-Wylie A, et al. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol Bioeng. 2008;99:652–665. doi: 10.1002/bit.21598. [DOI] [PubMed] [Google Scholar]
- 19.Millward TA, Heitzmann M, Bill K, Langle U, Schumacher P, Forrer K. Effect of constant and variable domain glycosylation on pharmacokinetics of therapeutic antibodies in mice. Biologicals. 2008;36:41–47. doi: 10.1016/j.biologicals.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Newkirk MM, Novick J, Stevenson MM, Fournier MJ, Apostolakos P. Differential clearance of glycoforms of IgG in normal and autoimmune-prone mice. Clin Exp Immunol. 1996;106:259–264. doi: 10.1046/j.1365-2249.1996.d01-847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YD, van Enk JZ, Flynn GC. Human antibody Fc deamidation in vivo. Biologicals. 2009;37:313–322. doi: 10.1016/j.biologicals.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Liu YD, Chen X, Enk JZ, Plant M, Dillon TM, Flynn GC. Human IgG2 antibody disulfide rearrangement in vivo. J Biol Chem. 2008;283:29266–29272. doi: 10.1074/jbc.M804787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung YA, Wu X, Reyes AE, 2nd, Vernes JM, Lien S, Lowe J, et al. A therapeutic anti-VEGF antibody with increased potency independent of pharmacokinetic half-life. Cancer Res. 2010;70:3269–3277. doi: 10.1158/0008-5472.CAN-09-4580. [DOI] [PubMed] [Google Scholar]
- 24.Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18:1759–1769. doi: 10.1093/intimm/dxl110. [DOI] [PubMed] [Google Scholar]
- 25.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Liu YD, Flynn GC. The effect of Fc glycan forms on human IgG2 antibody clearance in humans. Glycobiology. 2009;19:240–249. doi: 10.1093/glycob/cwn120. [DOI] [PubMed] [Google Scholar]
- 27.Dillon TM, Ricci MS, Vezina C, Flynn GC, Liu YD, Rehder DS, et al. Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. J Biol Chem. 2008;283:16206–16215. doi: 10.1074/jbc.M709988200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z. Large-scale identification and quantification of covalent modifications in therapeutic proteins. Anal Chem. 2009;81:8354–8364. doi: 10.1021/ac901193n. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Lu J, Wroblewski VJ, Beals JM, Riggin RM. In vivo deamidation characterization of monoclonal antibody by LC/MS/MS. Anal Chem. 2005;77:1432–1439. doi: 10.1021/ac0494174. [DOI] [PubMed] [Google Scholar]
- 30.Huang HZ, Nichols A, Liu D. Direct identification and quantification of aspartyl succinimide in an IgG2 mAb by RapiGest assisted digestion. Anal Chem. 2009;81:1686–1692. doi: 10.1021/ac802708s. [DOI] [PubMed] [Google Scholar]
- 31.Masuda K, Yamaguchi Y, Kato K, Takahashi N, Shimada I, Arata Y. Pairing of oligosaccharides in the Fc region of immunoglobulin G. FEBS Lett. 2000;473:349–357. doi: 10.1016/s0014-5793(00)01557-x. [DOI] [PubMed] [Google Scholar]
- 32.Flynn GC, Chen X, Liu YD, Shah B, Zhang Z. Naturally occurring glycan forms of muman immunoglobulins G1 and G2. Mol Immunol. 2010;47:2074–2082. doi: 10.1016/j.molimm.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Frelinger AL, 3rd, Zull JE. The role of the methionine residues in the structure and function of parathyroid hormone. Arch Biochem Biophys. 1986;244:641–649. doi: 10.1016/0003-9861(86)90632-6. [DOI] [PubMed] [Google Scholar]
- 34.Teh LC, Murphy LJ, Huq NL, Surus AS, Friesen HG, Lazarus L, et al. Methionine oxidation in human growth hormone and human chorionic somatomammotropin. Effects on receptor binding and biological activities. J Biol Chem. 1987;262:6472–6477. [PubMed] [Google Scholar]
- 35.Sasaoki K, Hiroshima T, Kusumoto S, Nishi K. Oxidation of methionine residues of recombinant human interleukin 2 in aqueous solutions. Chem Pharm Bull. 1989;37:2160–2164. doi: 10.1248/cpb.37.2160. [DOI] [PubMed] [Google Scholar]
- 36.Lam XM, Yang JY, Cleland JL. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody HER2. J Pharm Sci. 1997;86:1250–1255. doi: 10.1021/js970143s. [DOI] [PubMed] [Google Scholar]