Abstract
Mycoheterotrophic plants are achlorophyllous plants that obtain carbon from their mycorrhizal fungi. They are usually considered to associate with fungi that are (1) specific of each mycoheterotrophic species and (2) mycorrhizal on surrounding green plants, which are the ultimate carbon source of the entire system. Here we review recent works revealing that some mycoheterotrophic plants are not fungal-specific, and that some mycoheterotrophic orchids associate with saprophytic fungi. A re-examination of earlier data suggests that lower specificity may be less rare than supposed in mycoheterotrophic plants. Association between mycoheterotrophic orchids and saprophytic fungi arose several times in the evolution of the two partners. We speculate that this indirectly illustrates why transition from saprotrophy to mycorrhizal status is common in fungal evolution. Moreover, some unexpected fungi occasionally encountered in plant roots should not be discounted as ‘molecular scraps’, since these facultatively biotrophic encounters may evolve into mycorrhizal symbionts in some other plants.
Key words: endophytic fungi, evolution of mycorrhizae, mycoheterophy, mycorrhizae, saprophytic fungi, specificity
Considerable advances were recently made in the ecology of achlorophyllous, heterotrophic plants that obtain carbon from their mycorrhizal fungi (Fig. 1). Most plants have contact with soil through mycorrhizal symbioses, in which roots associate with a suitable fungal partner. Fungi utilize soil mineral nutrients, and while sharing them with host plants, they generally receive carbon as a reward. In contrast, some achlorophyllous plants living in the shaded forest understorey have reversed the process. They receive carbon from their mycorrhizal fungi exclusively, hence the designation ‘mycoheterotrophic’ (MH) plants.1 Mycoheterotrophy has appeared several times during the evolution of land plants, and more than 20 times among orchids that encompass half of all MH species.2 In the last decade, the development of molecular tools has enabled researchers to identify many fungal symbionts, which are often uncultivable. The fungi occurring in the densely colonized roots of MH species often produce a stronger PCR signal than any fungal contaminant, making molecular tools very effective for this field of study.
Figure 1.
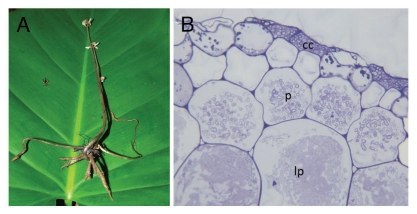
Wullschlaegelia aphylla, a mycoheterotrophic orchid unspecifically associated with saprotrophic Mycena and Gymnopus species. (A) Whole plant at flowering time, with reduced, tuberoid root system at that period. (B) Section of mycorrhizal root showing intracellular hyphal pelotons at early stage (p), or late stage (undergoing lysis, lp); among orchids, the colonization of dead cortical cell (cc) is a unique feature to some saprotrophic fungi (picture by A. Faccio, University of Torino).
Unexpectedly Diverse Fungal Partners
Although most MH species occur in tropical region, most MH plants currently investigated are from northern latitudes. In temperate MH plants, two main features were consistently found: high specificity and indirect below ground connection with nearby autotrophic plants.1,3 Regarding specificity, each of these temperate MH species only associate with a narrow range of closely related fungal taxa, and these taxa differ among MH species. High specificity is often assumed as an outcome of an arms race between coevolving fungi and MH plants; each MH species successfully exploits only one fungus, similar to specialized parasites (epiparasites4). Fungal partners of temperate MH species also form mycorrhizae with nearby autotrophic trees that represent the ultimate carbon source of the entire system.5 Direct evidence of a below-ground connection between a MH plant and surrounding trees was obtained by labelling photosynthates.6 In most studies, indirect evidence was provided by using stable isotopes;7 using 13C, natural abundances in MH plants were found to be similar to that of their mycorrhizal fungi but higher than that of nearby autotrophic plants and using 15N, natural abundances were found to increase from fungi to MH plants, as is expected along trophic chains.
There are older as well as more recent reports on associations between Asiatic tropical MH orchids and saprotrophic fungi, which were mainly identified by using in vitro isolation,8,9 a technique that often selects saprotrophic contaminants. Some MH orchids living in wet forests in Asia and Australia have been presumed to be associated with litter decaying fungi10,11 (reviewed in ref. 3). More recently, saprotrophic fungi were identified in situ using molecular tools on colonized roots or rhizomes. In the Asiatic Epipogium roseum, association with saprotrophic Psathyrellaceae12 (=Coprinaceae; Fig. 2) was further supported by in vitro reconstruction of the symbiosis that enabled successful development of the orchid.13 Psathyrellaceae were molecularly identified in the Asiatic MH Eulophia zollingeri14 (Fig. 2), Gymnopus-related fungi in the Australian MH Erythrorchis cassythoides,15 Mycenaceae in the Japanese Gastrodia confusa16 and the Caribbean Wullschlaegelia aphylla17 (Figs. 1A and 3), Marasmiaceae in the Australian Gastrodia sesamoides,18 and a Resinicium species (Hymenochaetales) in Gastrodia similis from La Réunion17 (Indian Ocean). Therefore, there are now multiple lines of evidence that several saprotrophic fungal lineages support some MH orchids, and that such symbioses are common in tropical latitudes.
Figure 2.
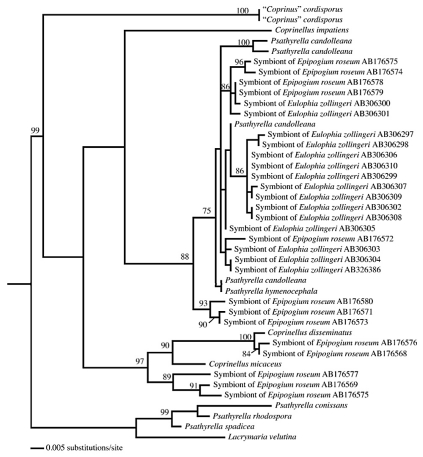
A phylogeny of Psathyrellaceae (maximum likelihood) shows that several lineages were recruited as mycorrhizal symbionts of the mycoheterotrophic orchids Eulophia zollingeri and Epipogium roseum. Mycorrhizal taxa fall within a clade consisting of Psathyrella candolleana representatives, while others fall within the Coprinellus clade (sequences from references 12 and 14; the support values on branches correspond to maximum likelihood bootstrap probabilities, and only values above 70% are shown).
Figure 3.
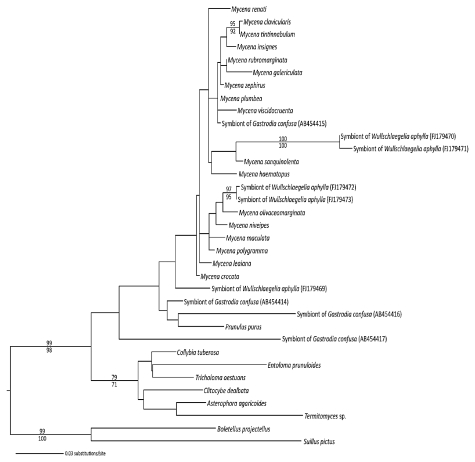
A phylogeny of Mycenaceae (maximum likelihood) shows that several lineages were recruited as mycorrhizal symbionts of the mycoheterotrophic orchids Gastrodia similis, G. confusa and Wullschlaegelia aphylla). Mycorrhizal taxa fall out among species of Mycena, while four additional isolates fall out with Mycena pura (= Prunulus purus), or in more basal positions within, or sister to, Mycenaceae (sequences from references 16 and 17; the support values on branches correspond to parsimony bootstrap/maximum likelihood bootstrap probabilities; only values above 70% are shown).
Specificity of Association: Less than Expected
Additionally, the MH orchid Wullschlaegelia aphylla associates with both Mycenaceae and Gymnopus-related fungi, and possibly with a species close to Psathyrellaceae.17 The former fungi were also identified by PCR from mycorrhizal pelotons—i.e., the fungal coils formed in mycorrhizal root cells (Fig. 1B)—supporting these taxa's ability to form orchid mycorrhizae. Some MH plants have thus non-specific associations with several fungal lineages. The recent finding of multiple fungal taxa within a plant or even within a root in two other MH species supports that specificity is not the rule: several fungi, all mycorrhizal on surrounding trees, occur in the orchid Aphyllorchis spp. from Thailand19 and in the North-American ericaceous Pyrola picta.20
Previous reports of specialized MH plants may need to be reinvestigated to determine whether they are truly non-specific. The African MH Afrothismia hydra (Thismiaceae) revealed mostly Glomus species and also an Acaulospora, which was perceived as a facultative partner with a reduced role in plant nutrition.21 In the MH orchid Gastrodia confusa, additional saprotrophic Marasmiaceae (Marasmiellus, Clitocybula) were occasionally recovered in individuals lacking the frequently amplified Mycena spp.;16 the authors hypothesized that some plant populations may differ in their mycorrhizal fungus, but Marasmiellus and Mycena fungi also co-existed in some plants. Therefore, fungi identified by molecular tools that have been discounted from relevant symbionts in the initial study (the “molecular scraps”, i.e., the fungal sequences that are not granted any relevant role) deserve to be reported. As shown in the analysis of the papers cited above, it is valuable to consider their putative mycorrhizal abilities. In the light of recent works, we could indeed find treasures among molecular scraps.
Nevertheless, we still ignore whether all fungal partners actually provide carbon to the unspecialized MH orchids. To date, there is even no direct evidence or experimental design (e.g., by labelling experiments) showing that MH orchids associated with saprotrophs indirectly exploit carbon from dead organic matter. This is only supported by rhizomorphs (elongated bundles of fungal hyphae) that occasionally link dead leaves to mycorrhizal roots10,17 and 13C and 15N natural contents, consistent with the use of saprotrophic fungi as a food source.16,17 Wullschlaegelia aphylla is linked to rhizomorphs of Mycenaceae and Gymnopus-related fungi,17 but this does not entail that they all provide carbon; moreover, isotopic abundances only distinguish the ecology of the fungal species furnishing carbon, not its (or their) species.
Mycorrhizal Evolution: Promising Molecular Scraps?
The observation of simultaneous morphogenetic processes between plants and saprotrophic fungi (Fig. 1B), which usually do not colonize living material, is fascinating. This supports a lead role for MH orchids in the mycorrhizal morphogenetic process, since saprotrophic fungi were never selected for such a trait, and raises the question of how the orchids manipulate these fungi. Moreover, although investigating only a few models, current studies demonstrate that MH orchids recruited several independent saprotrophic lineages in the Hymenochaetales, Psathyrellaceae, Mycenaceae and Marasmiaceae (Fig. 2 and 3). Although some clades were targeted twice, such Psathyrellaceae in the unrelated orchids Epipogium roseum and Eulophia zollingeri (Fig. 2), no specific fungal clade is predisposed to associate with MH orchids. One can thus speculate that fungi do not need specific or rare predispositions to be driven to living roots by MH orchids. In other words, the transition to growing in living material might arise fairly easily in fungal evolution, possibly even without any specific genetic modification. Although the genomes of mycorrhizal fungi contain numerous genes specifically involved in symbiosis,22 it may be that the latter are only secondarily arisen as an adaptation to exploiting the biotrophic niche (indeed, saprotrophs from MH orchids grow in roots, but do not receive carbon from them). This may even explain why the transition to mycorrhizal status occurred so often in the evolution of fungi.23
In green orchids, in addition to the usual mycorrhizal symbionts (the rhizoctonias, i.e., members of Sebacinales, Ceratobasidiales and Tulasnellales, often considered as saprotrophs), other saprotrophs sometimes occur, e.g., Mycena species in Anoectochilus roxburghii24 or Cymbidium sinense.25 In these cases, they may be contaminants, or facultatively biotrophic encounters that either form mycorrhizal structures on very small root portions, or colonize the tissues as endophytes (i.e., grow within living plant tissues, causing an unapparent infection, but do not form true mycorrhizae, nor cause any disease symptoms). The latter cases may be on the pathway from which closer mycorrhizal relationships evolved. Indeed, evolution from saprotrophism to endophytism and then to mycorrhizal association is a possible pathway for the repetitive evolution of mycorrhizal associations seen in fungi.26 The same applies for the finding of tree mycorrhizal fungi in some rhizoctonia-associated green orchids, e.g., overlooked Russula species in Cypripedium.27 This may be the starting point for evolution to MH orchids connected by mycorrhizal fungi with surrounding autotrophs. Thus, when investigating green orchids, the report of minor ‘contaminant’ taxa, usually disregarded as molecular scraps, is also extremely relevant for our understanding of the evolution of the symbiosis! In this respect, a particular issue is that of the fungal shifts that occur in the evolution of some specific MH lineages, such as in Ericaceae, where closely related MH species specifically target different fungal taxa.28 How did the common ancestor shift to a new symbiont? Indeed, one of these facultatively biotrophic encounters may evolve into an exclusive symbiont.26
On the orchids' side, the ability to exploit fungal carbon arose several times2 using fungi mycorrhizal on other nearby autotrophs, or saprotrophic fungi. Even though some MH taxa only use one kind of fungus, such as tree mycorrhizal fungi in the Neottieae tribe or saprotrophs in the genus Gastrodia, there is evidence that associations with saprotrophic versus mycorrhizal fungi are not phylogenetically constrained in MH orchids. For example, although Epipogium roseum associates with saprotrophic Psathyrellaceae,12,13 the related E. aphyllum associates with tree mycorrhizal Inocybe.29 Strikingly, the saprotrophic fungi involved (Fig. 2 and 3) belong to taxa that are abundant in temperate ecosystems where MH plants also exist, however, to our knowledge, these taxa never interact with MH plants outside of the tropics (or very wet temperate forests). The ecological environment may be more relevant than the phylogenetic position of the orchid. A possibility is that a wetter and hotter climate may extend the period of growth and nutrition of saprotrophs, providing surplus carbon that can be provided to a MH plant at little or no cost to the fungus.17
Certainly, MH orchids associating with saprotrophic fungi are more tractable models for in vitro experimentation13 than those associating with mycorrhizal fungi. These taxa offer interesting models for testing the impact of MH orchids on associated fungi, the mechanisms of recognition between the partners or the exchanges at cell level. Beyond this, there is a great need for additional research on biological interactions in MH plants, and more generally on plant-microorganism relationships, in the tropics.
And, please, let's never forget reporting on the molecular scraps!
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10791
References
- 1.Leake JR. Myco-heterotroph/epiparasitic plant interactions with ectomycorrhizal and arbuscular mycorrhizal fungi. Curr Opin Plant Biol. 2004;7:422–428. doi: 10.1016/j.pbi.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Molvray M, Kores PJ, Chase MW. Polyphyly of mycoheterotrophic orchids and functional influence on floral and molecular characters. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Melbourne, Australia: CSIRO; 2000. pp. 441–447. [Google Scholar]
- 3.Taylor DL, Bruns TD, Leake JR, Read D. Mycorrhizal specificity and function in myco-heterotrophic plants. In: Van der Heijden MGA, Sanders I, editors. Mycorrhizal Ecology. Berlin, Germany: Springer-Verlag; 2002. pp. 375–413. [Google Scholar]
- 4.Merckx V, Bidartondo MI, Hynson NA. Mycoheterotrophy: when fungi host plants. Ann Bot. 2009;104:1255–1261. doi: 10.1093/aob/mcp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selosse M-A, Roy M. Green plants that feed on fungi: facts and questions about mixotrophy. Trends Plant Sci. 2009;14:64–70. doi: 10.1016/j.tplants.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 6.McKendrick SL, Leake JR, Read DJ. Symbiotic germination and development of myco-heterotrophic plants in nature: transfer of carbon from ectomycorrhizal Salix repens and Betula pendula to the orchid Corallorhiza trifida through shared hyphal connections. New Phytol. 2000;145:539–548. doi: 10.1046/j.1469-8137.2000.00592.x. [DOI] [PubMed] [Google Scholar]
- 7.Trudell SA, Rygiewicz PT, Edmonds RL. Nitrogen and carbon stable isotope abundances support the myco-heterotrophic nature and host-specificity of certain achlorophyllous plants. New Phytol. 2003;160:391–401. doi: 10.1046/j.1469-8137.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 8.Umata H. In vitro symbiotic association of an achlorophyllous orchid, Erythrorchis ochobiensis, with orchid and non-orchid fungi. Memoirs of the Faculty of Agriculture, Kagoshima University. 1998a;34:97–107. [Google Scholar]
- 9.Umata H. A new biological function of shiitake mushroom, Lentinula edodes, in a myco-heterotrophic orchid, Erythrorchis ochobiensis. Mycoscience. 1998b;39:85–88. [Google Scholar]
- 10.Kusano S. Gastrodia elata and its symbiotic association with Armillaria mellea. J Coll Agric Japan. 1911;9:1–73. [Google Scholar]
- 11.Burgeff H. Studien an tropischen Orchideen. Jena, Germany: Gustav Fischer; 1932. Saprophytismus und Symbiose. [Google Scholar]
- 12.Yamato M, Yagame T, Suzuki A, Iwase K. Isolation and identification of mycorrhizal fungi associating with an achlorophyllous plant, Epipogium roseum (Orchidaceae) Mycoscience. 2005;46:73–77. [Google Scholar]
- 13.Yagame T, Yamato M, Mii M, Suzuki A, Iwase K. Developmental processes of achlorophyllous orchid, Epipogium roseum: from seed germination to flowering under symbiotic cultivation with mycorrhizal fungus. J Plant Res. 2007;120:229–236. doi: 10.1007/s10265-006-0044-1. [DOI] [PubMed] [Google Scholar]
- 14.Ogura-Tsujita Y, Yukawa T. High mycorrhizal specificity in a widespread mycoheterotrophic plant, Eulophia zollingeri (Orchidaceae) Am J Bot. 2008;95:93–97. doi: 10.3732/ajb.95.1.93. [DOI] [PubMed] [Google Scholar]
- 15.Dearnaley JDW. The fungal endophytes of Erythrorchis cassythoides—is this orchid saprophytic or parasitic? Aust Mycol. 2006;25:51–57. [Google Scholar]
- 16.Ogura-Tsujita Y, Gebauer G, Hashimoto T, Umata H, Yukawa T. Evidence for novel and specialized mycorrhizal parasitism: the orchid Gastrodia confusa gains carbon from saprotrophic Mycena. Proc Royal Soc Lond B. 2009;276:761–767. doi: 10.1098/rspb.2008.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martos F, Dulormne M, Pailler T, Bonfante P, Faccio A, Fournel J, et al. Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytol. 2009;184:668–681. doi: 10.1111/j.1469-8137.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 18.Dearnaley JWD, Bougoure JJ. Molecular identification of Marasmiaceae mycobionts in rhizomes of Gastrodia sesamoïdes—evidence for direct orchid parasitism. Fungal Ecol. 2009 In press. [Google Scholar]
- 19.Roy M, Whatthana S, Richard F, Vessabutr S, Selosse M-A. Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biol. 2009a;7:51. doi: 10.1186/1741-7007-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynson N, Bruns TD. Evidence of a myco-heterotroph in the plant family Ericaceae that lacks mycorrhizal specificity. Proc Royal Soc Lond B. 2009;276:4053–4059. doi: 10.1098/rspb.2009.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke T, Beenken L, Döring M, Kocyan A, Agerer R. Arbuscular mycorrhizal fungi of the Glomus-group A lineage (Glomerales; Glomeromycota) detected in myco-heterotrophic plants from tropical Africa. Mycol Progr. 2006;5:24–31. [Google Scholar]
- 22.Martin F, Selosse M-A. The Laccaria genome: a symbiont blueprint decoded. New Phytol. 2008;180:296–310. doi: 10.1111/j.1469-8137.2008.02613.x. [DOI] [PubMed] [Google Scholar]
- 23.Hibbett DS, Matheny PB. Relative ages of ectomycorrhizal mushrooms and their plant hosts. BMC Biol. 2009;7:13. doi: 10.1186/1741-7007-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo S-X, Fan L, Cao W-Q, Xu J-T, Xiao P-G. Mycena anoectochila sp. Nov. isolated from mycorrhizal roots of Anoectochilus roxburghii from Xishuangbanna, China. Mycologia. 1997;89:952–954. [Google Scholar]
- 25.Fan L, Guo S, Cao W, Xiao P, Xu J, Fan L, et al. Isolation, culture, identification and biological activity of Mycena orchidicola sp. nov. in Cymbidium sinense (Orchidaceae) Acta Mycol Sin. 1996;15:251–255. [Google Scholar]
- 6.Selosse M-A, Dubois M-P, Alvarez N. Are Sebacinales common root endophytes? Mycol Res. 2009;113:1062–1069. doi: 10.1016/j.mycres.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Shefferson RP, Taylor DL, Garnica S, McCormick MK, Adams S, Gray HM, et al. The evolutionary history of mycorrhizal specificity among lady's slipper orchids. Evolution. 2007;61:1380–1390. doi: 10.1111/j.1558-5646.2007.00112.x. [DOI] [PubMed] [Google Scholar]
- 28.Bidartondo MI, Bruns TD. Fine-level mycorrhizal specificity in the Monotropoideae (Ericaceae): specificity for fungal species groups. Mol Ecol. 2002;11:557–569. doi: 10.1046/j.0962-1083.2001.01443.x. [DOI] [PubMed] [Google Scholar]
- 29.Roy M, Yagame T, Yamato M, Iwase K, Heinz C, Faccio A, et al. Ectomycorrhizal Inocybe species associate with the mycoheterotrophic orchid Epipogium aphyllum Sw., but not with its asexual propagules, throughout its Eurasiatic range. Ann Bot. 2009b;104:595–610. doi: 10.1093/aob/mcn269. [DOI] [PMC free article] [PubMed] [Google Scholar]