Abstract
B-cell lymphomas, which arise in lymphoid organs, can spread rapidly via the circulatory system and form solid tumors within multiple organs. Rate-limiting steps in this metastatic process may be the adhesion of lymphoma cells to vascular endothelial cells, their exit from the vasculature and their migration to tissue sites that will support tumor growth. Thus proteins that control B-cell adhesion and migration are likely to be key factors in lymphoma dissemination, and hence potential targets for therapeutic intervention. The Rap GTPases are master regulators of integrin activation, cell motility and the underlying cytoskeletal, adhesion and membrane dynamics. We have recently shown that Rap activation is critical for B-lymphoma cells to undergo transendothelial migration in vitro and in vivo. As a consequence, suppressing Rap activation impairs the ability of intravenously injected B-lymphoma cells to form solid tumors in the liver and other organs. We discuss this work in the context of targeting Rap, its downstream effectors, or other regulators of B-cell adhesion and migration as an approach for limiting the dissemination of B-lymphoma cells and the development of secondary tumors.
Key words: B-cell lymphomas, Rap GTPases, extravasation, chemokines, integrins, metastasis
B-cell lymphomas are frequently occurring malignancies that are often aggressive and difficult to treat. Abnormally proliferating B cells that acquire survival-promoting mutations originate within the bone marrow or the lymphoid organs but can traffic via the blood and lymphatic systems to other organs, where they can form solid tumors. A consequence of the genetic mechanisms that generate a large repertoire of antigen-detecting B-cell receptors (BCR) and antibodies is an increased frequency of chromosomal translocations and mutations that can lead to oncogenic transformation.1 During B-cell development in the bone marrow, the vast diversity of the BCR repertoire within an individual is generated by the random rearrangement of the VDJ gene segments that encode the BCR. Subsequent to antigen binding, highly proliferating B cells within the germinal centers of secondary lymphoid organs undergo somatic hypermutation of the genes encoding the immunoglobulin portion of the BCR in order to generate antibodies of higher affinity (“affinity maturation”). These cells can also undergo a second DNA rearrangement event associated with immunoglobulin class switching. Aberrant DNA rearrangements or somatic hypermutation can lead to oncogenic transformation. As examples, translocation of the c-myc gene into the IgH locus is characteristic of Burkitt's lymphoma whereas somatic hypermutation of genes that encode prosurvival proteins (e.g., pim-1) is associated with diffuse large B-cell lymphomas,2 the most common type of non-Hodgkin lymphoma.
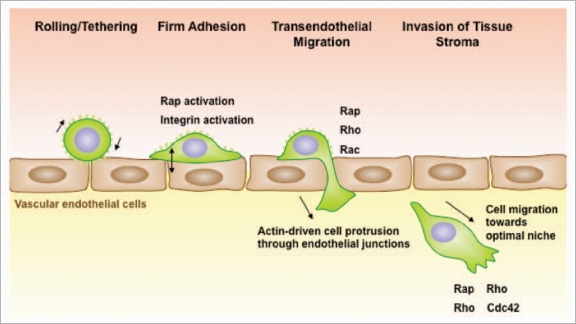
The ability of B-cell lymphomas to spread to multiple organs reflects the migratory capacity of their normal counterparts. B cells circulate continuously throughout the body via the blood and lymphatic systems. The extravasation of B cells out of the blood and into tissues is a multi-step process that requires selectin-mediated rolling on the surface of vascular endothelial cells, intergin-mediated firm adhesion to the endothelial cells, and migration across the endothelial cell monolayer that makes up the vessel wall (Fig. 1).3–6 These steps are orchestrated by chemokines and adhesion molecules that are displayed on the surface of the vascular endothelial cells. Chemokines initiate signaling within the B cell that results in integrin activation. The collaboration between chemokine receptor signaling and outside-in integrin signaling causes B cells to reorganize their cytoskeleton. This cytoskeletal reorganization allows B cells to spread on the surface of the vascular endothelial cells, migrate to sites suitable for extravasation (e.g., junctions between endothelial cells) and then deform themselves in order to move across the endothelial cell layer.7 The ability of B-cell lymphomas to follow these constitutive organ-homing cues allows them to spread to multiple organs throughout the body, making them difficult to combat. Diffuse large B-cell lymphomas are highly aggressive precisely for this reason and readily spread to the liver, kidneys and lungs.8 Thus, identifying key proteins that regulate the extravasation of B-cell lymphomas could suggest new therapeutic strategies for treating these malignancies.9
Figure 1.
Rap activation is required for multiple steps in lymphoma dissemination. B-cell lymphomas exit the vasculature using the same mechanisms as normal B cells. Once B cells are tethered via selectin-mediated rolling, chemokines immobilized on the surface of vascular endothelial cells convert integrins to a high affinity state via a mechanism that involves activation of the Rap GTPases. This permits firm adhesion. Adhered B cells migrate across the endothelium and then send out actin-rich protrusions, which penetrate the endothelial barrier to reach the subendothelial matrix. The formation of these membrane processes, and the subsequent movement of the cells through the junctions, requires activation of the Rap, Rho and Rac GTPases. Once in the tissue, B-lymphoma cells assume a polarized morphology and can migrate towards optimal growth niches.
The ubiquitously-expressed Rap GTPases are master regulators of cell adhesion, cell polarity, cytoskeletal dynamics and cell motility.10 Receptor-induced conversion of the Rap GTPases to their active GTP-bound state (Rap-GTP) allows them to bind multiple effector proteins and thereby orchestrate their localization and function. These downstream effectors of Rap-GTP control integrin activation, actin polymerization and dynamics and the formation of protrusive leading edges in migrating cells (see below and Fig. 2). In both normal B cells and B-lymphoma cell lines, signaling via chemoattractant receptors, the BCR and integrins all activate Rap.11–13 Moreover, we have shown that chemokine-induced Rap activation is essential for the chemoattractants CXCL12 (SDF-1), CXCL13 and sphingosine-1-phosphate (S1P) receptors to stimulate B-cell migration and adhesion.12,14 Rap activation is also important for receptor-induced actin polymerization, cell spreading and cytoskeletal reorganization in both primary B cells and B-lymphoma cells.15 These findings suggested that Rap activation might be essential for the in vivo metastatic spread of B-cell lymphomas.
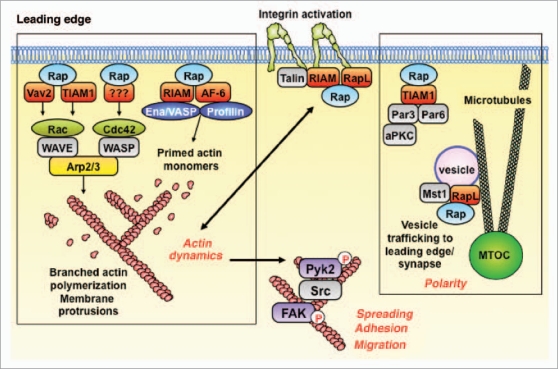
Figure 2.
The Rap GTPases are master regulators of actin dynamics, cell morphology, cell polarity and integrin-mediated adhesion. The Rap GTPases are activated subsequent to the binding of chemokines to their receptors or activated integrins to their ligands. The active GTP-bound form of Rap binds effector proteins that promote integrin activation, actin polymerization and membrane protrusion, as well as activation of the Pyk2 and FAK tyrosine kinases, which modulate cell spreading, adhesion and migration. Rap-GTP also plays a key role in establishing cell polarity and may direct membrane vesicles to the leading edge of the cell. See text for details. MTOC, microtubule-organizing center.
To test this hypothesis, we suppressed Rap activation in A20 murine B-lymphoma cells, a cell line derived from an aggressive diffuse large B-cell lymphoma. We blocked Rap activation in these cells by expressing a Rap-specific GTPase-activating protein (GAP), RapGAPII, which enzymatically converts Rap1 and Rap2 proteins to their inactive GDP-bound states. Injecting stable A20/RapGAPII and A20/empty vector transfectants intravenously into mice showed that Rap activation was required for these cells to form solid lymphomas within organs such as the liver.16 Solid tumor formation was delayed and reduced when A20/RapGAPII cells were injected instead of A20/control cells. Strikingly, the lymphoma cells isolated from the tumors that developed in mice injected with A20/RapGAPII cells had downregulated RapGAPII expression and regained the ability to activate Rap. Thus tumor formation reflected a strong in vivo selection for lymphoma cells capable of activating Rap. This indicates that Rap-dependent signaling is critical for the metastatic spread of B-cell lymphomas.
The ability of B-lymphoma cells to exit the vasculature and migrate into the underlying tissue is likely to be a rate-limiting step in the metastasis of B-cell lymphomas. We showed that this extravasation step is a Rap-dependent process for B-cell lymphomas. To do this, we performed competitive in vivo homing assays in which differentially-labeled A20/vector and A20/RapGAPII cells were co-injected into the tail veins of mice.16 Analyses performed 1–3 days after injecting the cells showed that A20/RapGAPII cells exhibited a greatly reduced ability to arrest and lodge in the liver, compared to control cells. The liver produces large amounts of the chemokine CXCL12 and is a major site of lymphoma homing and tumor formation. More detailed studies revealed that the control A20 cells that lodged in the liver had entered the liver parenchyma and had an elongated morphology, as expected for cells that are migrating within the tissue and interacting with resident cells. In contrast, a larger fraction of the A20/RapGAPII cells were round and appeared to still be within the vasculature. These findings suggest that Rap activation is required for efficient extravasation of lymphoma cells in vivo, as had previously been shown for in T cells in vitro.17
Leukocyte extravasation is a multi-step process that requires initial adhesion to the vascular endothelium followed by crawling on the luminal surface of the endothelial cells until a suitable site for migration through the endothelial barrier is located. We found that Rap activation was required for the initial adhesion of A20 cells to vascular endothelial cells in vitro.16 Whether integrin-mediated adhesion is an absolute requirement for tumor cells to arrest within organ vasculature remains an open question as tumor cells can be physically trapped in small vessels in a manner that is independent of integrins or other adhesion molecules (Freeman SA, unpublished data). In contrast, the ability of lymphoma cells to generate polarized membrane protrusions that invade junctions between vascular endothelial cells and then move through the junctions is likely to have a strong dependence on Rap-mediated integrin activation and Rap-mediated cell polarization and cytoskeletal reorganization. Indeed, we found that Rap activation was required for A20 B-lymphoma cells to form membrane projections that penetrated endothelial junctions in vitro, and for the subsequent transendothelial migration of A20 cells.16
In addition to this well-characterized paracellular mode of extravasation in which leukocytes crawl across endothelial cells until they arrive at cell-cell junctions and then migrate across the endothelial cell layer, leukocytes can also extravasate via a transcellular route.18 T cells can send invadopodia through endothelial cells, which upon contacting the subendothelial matrix pull the cell through and across the endothelial cell. The paracellular and transcellular routes of leukocyte extravasation may involve distinct modes of leukocyte motility and cytoskeletal reorganization. For example, activation of WASp and Src is required for transcellular extravasation of T cells, but dispensable for paracellular extravasation.18 Our data suggest that Rap activation is involved in the paracellular extravasation of B-cell lymphomas. It is not known if lymphoma cells, which are considerably larger than normal leukocytes, can undergo transcellular extravasation, and if so, whether Rap-dependent signaling is required. Determining the relative contributions of these two modes of extravasation, as well as their underlying molecular mechanisms, could facilitate the development of therapeutic approaches for reducing lymphoma cell extravasation and dissemination.
Rap GTPases are ubiquitously expressed and are involved in critical processes such as the formation of tight junctions between vascular endothelial cells.19 Therefore, targeting downstream effectors of Rap that mediate specific aspects of adhesion and migration may be a more reasonable way to limit lymphoma dissemination than targeting Rap activation. As shown in Figure 2 and reviewed by Bos,10 the effector proteins that are regulated either directly or indirectly by Rap-GTP control several modules that are critical for cell adhesion and migration.
Activated Rap is an essential component of the inside-out signaling pathway by which chemokine receptors activate integrins. Rap-GTP recruits the adaptor protein RapL as well as RIAM/talin complexes to the cytoplasmic domains of integrins.20,21 This results in conformational changes in the integrin extracellular domains that increase their affinity for adhesion molecules, such as those present on the surface of vascular endothelial cells. Actin-dependent intracellular forces exerted by talin on the integrin cytoplasmic domains also increase integrin affinity22 and may be regulated by Rap-GTP, which promotes actin polymerization (see below).
Effector proteins that bind Rap-GTP include upstream activators of Rac and Cdc42,23,24 GTPases that promote dynamic actin polymerization at the leading edge of migrating cells and at the growing ends of membrane protrusions. Activated Rac and Cdc42 act via the WASp and WAVE proteins to induce branching actin polymerization that drives membrane protrusion and the formation of lamellipodia and filopodia. Other Rap effectors, the RIAM25 and AF-6 adaptor proteins,26 allow Rap-GTP to recruit Ena/Vasp and profilin, proteins that prime actin monomers for incorporation into actin filaments, a rate-limiting step in actin filament assembly.
The Pyk2 and FAK tyrosine kinases are key regulators of cell adhesion, cell migration and cell morphology, and we have shown that they are also downstream targets of Rap-GTP signaling.27 Rap-dependent actin dynamics is critical for the activation of Pyk2 and FAK in B-lymphoma cells. Moreover the kinase activities of Pyk2 and FAK are required for B cell spreading, a key aspect of cell adhesion and motility.27 The importance of this Rap/Pyk2 signaling module is supported by the observation that B cells from Pyk2-deficient mice have a severe defect in chemokine-induced migration.28
Rap effectors also promote the establishment of cell polarity, another key aspect of cell motility. Rap-GTP binds the evolutionarily-conserved Par3/6 polarity complex29 and promotes the microtubule-dependent transport of vesicles containing integrins to the leading edge of migrating cells and to cell-cell contact sites such as immune synapses.30,31
A key question is whether modulating the expression or activity of individual targets of Rap signaling can effectively limit the dissemination of B-cell lymphomas. An exciting recent paper supports the idea that targeting proteins involved in cell motility may be an effective way to limit the spread and growth of B-cell lymphomas.9 Using a library of short hairpin RNAs (shRNAs) directed against 1,000 genes thought to be involved in lymphoma progression, Meachem et al. found that two regulators of the actin cytoskeleton, Rac2 and twinfilin (Twf1), were key determinants of lymphoma motility, invasiveness and progression. shRNA-mediated knockdown of either Rac2 or Twf1 expression dramatically inhibited the growth of Eµ-myc B-cell lymphomas in mice, a model for the development of human Burkitt lymphomas. The decreased lymphoma tumorgenicity, as well as the decreased ability of the lymphoma cells to engraft in the spleen and bone marrow and then metastasize to secondary sites such as the liver was associated with the cells' inability to migrate and crawl in vitro. This is consistent with our finding that inhibiting the in vitro migration and adhesion of B-lymphoma cells by suppressing Rap activation correlated with reduced extravasation and tumor formation in vivo.
The involvement of both Rap and Rac2 in lymphoma motility and dissemination may reflect the fact that these two GTPases lie in the same pathway. Rap-GTP has been shown to bind the Rac activator Vav2 and promote Rac activation.23 Conversely, Batista and colleagues showed that Rac2 acts upstream of Rap to promote Rap activation and modulate B-cell adhesion and immune synapse formation.32 Although the interrelationship of Rap and Rac2 in B-cell lymphomas remains to be clarified, the Rac2/Rap signaling module is a potential target for limiting the spread of B-cell lymphomas. Inhibiting this Rac2/Rap module that controls B-cell motility and adhesion may reduce both the extravasation of lymphoma cells into organs as well as the ability of B-lymphoma cells to crawl to sites within the organ where they can establish a suitable metastatic niche. Migration through the subendothelial stroma to find optimal growth niches is a rate-limiting step in the dissemination of many types of tumors.33 Blocking Rap-dependent adhesion may also prevent B-lymphoma cells from forming critical adhesive interactions with tissue-resident stromal cells. In vitro, the survival of many B-cell lymphomas depends on integrin engagement34,35 and the subsequent activation of pro-survival signaling pathways (e.g., the PI 3-kinase/Akt pathway) by integrin signaling.36 It is not known whether Rap-dependent adhesion and the ensuing integrin-mediated survival signaling are required for B-cell lymphomas to form solid tumors at secondary sites in vivo.
A series of recent papers has identified the hematopoietic lineage-restricted adaptor protein kindlin-3 as a key regulator of integrin activation in leukocytes. Kindlin-3 is required for leukocyte adhesion in vitro and for in vivo extravasation,37–39 making it a potential target for limiting the spread of B-cell lymphomas. Kindlin-3 binds to the cytoplasmic domain of several integrin beta subunits but the mechanism by which it promotes integrin activation is not known. An interesting question is whether Rap-GTP, or the RapL/RIAM/talin complexes that are recruited to integrins by Rap-GTP, regulate the localization or function of kindlin-3. Whether or not Rap and kindlin-3 act in the same pathway, it would be interesting to test whether knocking down the expression of kindlin-3 reduces the dissemination of B-cell lymphomas in either the A20 cell model we have used or the Eµ-myc B-cell lymphoma model used by Meachem et al.9
Although we have thus far referred to the Rap GTPases collectively as “Rap,” there are five Rap GTPases in humans and mice, Rap1a, Rap1b, Rap2a, Rap2b and Rap2c, each encoded by a separate gene. Several reports have suggested distinct functions for Rap1 versus Rap2,14,40 but it is not known to what extent the functions of the five Rap proteins are redundant or unique. Although many studies have not assessed Rap2 activation, loss-of-function approaches such as overexpressing Rap-specific GAPs or expressing the dominant-negative Rap1N17 protein may suppress the activation of all Rap proteins. Nevertheless, the possibility that different Rap proteins have distinct functions, coupled with cell type-specific differences in the expression of the Rap proteins, may present additional opportunities for targeting Rap signaling in tumor cells. Rap1b is much more abundant than Rap1a in B cells and recent work has shown that Rap1b-deficient murine B cells exhibit impaired migration and adhesion in vitro, as well as impaired in vivo homing.41,42 If B-lymphoma cells also express much more Rap1b than Rap1a, then Rap1b could be a target for limiting the spread of these malignant B cells. An important caveat is that Rap1b is also the most abundant Rap1 isoform in platelets and plays a critical role in platelet aggregation and clotting.43,44
As master regulators of cell adhesion and migration, the Rap GTPases and the signaling pathways they control are obvious therapeutic targets for limiting the spread of B-cell lymphomas. Other signaling pathways that impact B-cell migration and adhesion, perhaps independently of Rap, are also attractive targets. Our in vivo experiments and those of Meachem et al.9 provide direct evidence that interfering with key regulators of adhesion and migration can dramatically limit the dissemination of B-cell lymphomas and the development of secondary tumors in critical organs. Further studies are needed to determine if this approach would be a useful therapeutic strategy for patients with B-cell lymphoma.
Finally, it will be of interest to determine whether gain-of-function mutations that increase Rap signaling, or activate other pathways that promote B cell migration and adhesion, contribute to the aggressiveness of certain types of B-cell lymphomas. Increased Rap activation is associated with enhanced invasiveness in several types of tumors.45,46 If this were true for B-cell lymphomas, then Rap-GTP levels could be a useful prognostic marker for aggressive lymphomas, in addition to being a potential therapeutic target.
Acknowledgements
Our laboratory is funded by grants from the Canadian Institutes of Health Research and the Cancer Research Society (Canada). K.B.L.L. has been supported by a graduate fellowship from the Michael Smith Foundation for Health Research. S.A.F. is supported by graduate fellowships from the University of British Columbia and the Michael Smith Foundation for Health Research.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11114
References
- 1.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 2.Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Kuppers R, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 3.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 4.Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004;4:360–370. doi: 10.1038/nri1354. [DOI] [PubMed] [Google Scholar]
- 5.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 6.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 7.Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9:953–959. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]
- 8.de Leval L, Hasserjian RP. Diffuse large B-cell lymphomas and Burkitt lymphoma. Hematol Oncol Clin North Amer. 2009;23:791–827. doi: 10.1016/j.hoc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Meacham CE, Ho EE, Dubrovsky E, Gertler FB, Hemann MT. In vivo RNAi screening identifies regulators of actin dynamics as key determinants of lymphoma progression. Nat Genet. 2009;41:1133–1137. doi: 10.1038/ng.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 11.McLeod SJ, Ingham RJ, Bos JL, Kurosaki T, Gold MR. Activation of the Rap1 GTPase by the B cell antigen receptor. J Biol Chem. 1998;273:29218–29223. doi: 10.1074/jbc.273.44.29218. [DOI] [PubMed] [Google Scholar]
- 12.Durand CA, Westendorf J, Tse KW, Gold MR. The Rap GTPases mediate CXCL13- and sphingosine 1-phosphate-induced chemotaxis, adhesion and Pyk2 tyrosine phosphorylation in B lymphocytes. Eur J Immunol. 2006;36:2235–2249. doi: 10.1002/eji.200535004. [DOI] [PubMed] [Google Scholar]
- 13.Lin KBL, Freeman SA, Zabetian S, Brugger H, Weber M, Lei V, et al. The Rap GTPases regulate B cell morphology, immune-synapse formation and signaling by particulate B cell receptor ligands. Immunity. 2008;28:75–87. doi: 10.1016/j.immuni.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 14.McLeod SJ, Li AH, Lee RL, Burgess AE, Gold MR. The Rap GTPases regulate B cell migration toward the chemokine stromal cell-derived factor-1 (CXCL12): potential role for Rap2 in promoting B cell migration. J Immunol. 2002;169:1365–1371. doi: 10.4049/jimmunol.169.3.1365. [DOI] [PubMed] [Google Scholar]
- 15.McLeod SJ, Shum AJ, Lee RL, Takei F, Gold MR. The Rap GTPases regulate integrin-mediated adhesion, cell spreading, actin polymerization and Pyk2 tyrosine phosphorylation in B lymphocytes. J Biol Chem. 2004;279:12009–12019. doi: 10.1074/jbc.M313098200. [DOI] [PubMed] [Google Scholar]
- 16.Lin KBL, Tan P, Freeman SA, Lam M, McNagny KM, Gold MR. The Rap GTPases regulate the migration, invasiveness and in vivo dissemination of B-cell lymphomas. Oncogene. 2010;29:608–615. doi: 10.1038/onc.2009.345. [DOI] [PubMed] [Google Scholar]
- 17.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, et al. Rap1 translates chemokine signals to integrin activation, cell polarization and motility across vascular endothelium under flow. J Cell Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 20.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, et al. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 23.Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwamborn JC, Puschel AW. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci. 2004;7:923–929. doi: 10.1038/nn1295. [DOI] [PubMed] [Google Scholar]
- 25.Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, et al. RIAM, an Ena/VASP and profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7:585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc Natl Acad Sci USA. 2000;97:9064–9069. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tse KW, Dang-Lawson M, Lee RL, Vong D, Bulic A, Buckbinder L, et al. B cell receptor-induced phosphorylation of Pyk2 and focal adhesion kinase involves integrins and the Rap GTPases and is required for B cell spreading. J Biol Chem. 2009;284:22865–22877. doi: 10.1074/jbc.M109.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 29.Gerard A, Mertens AE, van der Kammen RA, Collard JG. The Par polarity complex regulates Rap1- and chemokine-induced T cell polarization. J Cell Biol. 2007;176:863–875. doi: 10.1083/jcb.200608161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita H, Fukuhara S, Sakurai A, Yamagishi A, Kamioka Y, Nakaoka Y, et al. Local activation of Rap1 contributes to directional vascular endothelial cell migration accompanied by extension of microtubules on which RAPL, a Rap1-associating molecule, localizes. J Biol Chem. 2005;280:5022–5031. doi: 10.1074/jbc.M409701200. [DOI] [PubMed] [Google Scholar]
- 31.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 32.Arana E, Vehlow A, Harwood NE, Vigorito E, Henderson R, Turner M, et al. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity. 2008;28:88–99. doi: 10.1016/j.immuni.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 34.Weekes CD, Kuszynski CA, Sharp JG. VLA-4 mediated adhesion to bone marrow stromal cells confers chemoresistance to adherent lymphoma cells. Leuk Lymphoma. 2001;40:631–645. doi: 10.3109/10428190109097661. [DOI] [PubMed] [Google Scholar]
- 35.Taylor ST, Hickman JA, Dive C. Survival signals within the tumour microenvironment suppress druginduced apoptosis: lessons learned from B lymphomas. Endocrine-Related Cancer. 1999;6:21–23. doi: 10.1677/erc.0.0060021. [DOI] [PubMed] [Google Scholar]
- 36.Uddin S, Hussain AR, Siraj AK, Manogaran PS, Al-Jomah NA, Moorji A, et al. Role of phosphatidylinositol 3′-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood. 2006;108:4178–4186. doi: 10.1182/blood-2006-04-016907. [DOI] [PubMed] [Google Scholar]
- 37.Malinin NL, Zhang L, Choi J, Ciocea A, Razorenova O, Ma YQ, et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat Med. 2009;15:313–318. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, et al. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009;15:300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 39.Svensson L, Howarth K, McDowall A, Patzak I, Evans R, Ussar S, et al. Leukocyte adhesion deficiency- III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med. 2009;15:306–312. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miertzschke M, Stanley P, Bunney TD, Rodrigues-Lima F, Hogg N, Katan M. Characterization of interactions of adapter protein RAPL/Nore1B with RAP GTPases and their role in T cell migration. J Biol Chem. 2007;282:30629–30642. doi: 10.1074/jbc.M704361200. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Yu M, Podd A, Wen R, Chrzanowska-Wodnicka M, White GC, et al. A critical role of Rap1b in B-cell trafficking and marginal zone B-cell development. Blood. 2008;111:4627–4636. doi: 10.1182/blood-2007-12-128140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu H, Awasthi A, White GC, Chrzanowska-Wodnicka M, Malarkannan S. Rap1b regulates B cell development, homing and T cell-dependent humoral immunity. J Immunol. 2008;181:3373–3383. doi: 10.4049/jimmunol.181.5.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crittenden JR, Bergmeier W, Zhang Y, Piffatgh CL, Liang Y, Wagner DD, et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 44.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC. Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:680–687. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey CL, Kelly P, Casey PJ. Activation of Rap1 promotes prostate cancer metastasis. Cancer Res. 2009;69:4962–4968. doi: 10.1158/0008-5472.CAN-08-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Chenwei L, Mahmood R, van Golen K, Greenson J, Li G, et al. Identification of a putative tumor suppressor gene Rap1GAP in pancreatic cancer. Cancer Res. 2006;66:898–906. doi: 10.1158/0008-5472.CAN-05-3025. [DOI] [PubMed] [Google Scholar]