Abstract
Longitudinal axons grow long distances along precise pathways to connect major CNS regions. However, during embryonic development, it remains largely undefined how the first longitudinal axons choose specific positions and grow along them. Here, we review recent evidence identifying a critical role for Slit/Robo signals to guide pioneer longitudinal axons in the embryonic brain stem. These studies indicate that Slit/Robo signals from the floor plate have dual functions: to repel longitudinal axons away from the ventral midline, and also to maintain straight longitudinal growth. These dual functions likely cooperate with other guidance cues to establish the major longitudinal tracts in the brain.
Key words: Slit, Robo, longitudinal axon, hindbrain, axon guidance
Longitudinal Axon Patterns Emerge Early in Brain Development
Regions of the CNS are connected by longitudinal tracts, which are bundles of nerve fibers that extend long distances along the rostral-caudal axis. Longitudinal tracts carry ascending sensory information from the spinal cord and brain stem up to higher brain centers, as well as descending impulses from brain to spinal cord, including motor signals. The tracts constitute a large part of the CNS fibers, particularly in the brain stem which is the major transit point for ascending and descending fibers. Specific populations of axons associate with each other to form tracts at particular positions. This commentary will review our recent study,1 along with related work from other labs, that have begun to define the molecular mechanisms of longitudinal axon guidance.
Very early in the development of the embryonic brain, longitudinal tracts emerge from early born neurons projecting axons to pioneer a simple pattern of fiber bundles.2–4 These early tracts form a scaffold, which is built upon by later axons, such as commissural axons which first cross the midline and then turn to grow longitudinally. Similar patterns arise in diverse embryos from lamphrey to fish to mammals, as well as in invertebrates such as Drosophila, and so are an evolutionarily-conserved feature of bilateral nervous systems. For a longitudinal tract to form in the proper position, the initial growth of longitudinal axons needs to be tightly regulated: the axons first need to orient longitudinally and then choose a specific dorsal-ventral (DV) position depending on their axon type. To form tracts, populations of axons need to maintain precise trajectories, form coherent tightly fasciculated tracts and efficiently reach their destination.
Strategies for longitudinal axon navigation: growing parallel to the ventral midline.
Longitudinal axons grow within neural tube tissue, but the identity of external cues and how they influence longitudinal trajectories remains unclear. Although intermediate target tissues provide instructive guide posts for many types of axons, longitudinal axons start at many different positions and share trajectories through many segments. Therefore, a continuous array of cues along the longitudinal axis of the neural tube seems a more likely guidance mechanism for longitudinal tracts. For example, longitudinal pioneers project accurately from forebrain to hindbrain tissue, even when intermediate midbrain tissue is deleted.5 In contrast, anterior-posterior cues may provide general directional information. For example, the midbrain-hindbrain boundary secretes diffusible cues that can inhibit hindbrain ascending axons in vitro.6 Other sources of cues may play region-specific roles, such as in the forebrain where local adhesion7 and diffusible signals8 influence longitudinal populations. Diffusible Slit repellent signals from the ventral midline have been implicated in guiding forebrain pioneers,9 and several major but relatively late forming forebrain tracts are disrupted in Slit and Robo mutant mouse embryos.10–12 Therefore, to identify whether this guidance system functions through the length of the neural tube and whether these signals guide pioneer longitudinal trajectories, we considered whether the floor plate might provide cues.
The floor plate, a strip of tissue along the ventral midline, extends from the forebrain through the spinal cord. The floor plate is a likely source of global longitudinal guidance because it directs commissural axon trajectories by providing key long-range cues. Commissural axons in the spinal cord first grow toward the floor plate, pulled by attractive Netrin and Sonic hedghog signals.13,14 Upon reaching the floor plate, commissural axons switch their responsiveness, thereby losing their attraction and instead becoming sensitive to repellent signals, primarily the family of secreted Slit proteins via the Robo receptors.15 This switch in responsiveness expels commissural axons from the ventral midline. Studies in Slit and Robo mutant mice and manipulations of Slit/Robo signals in chick embryos have provided evidence for an important Slit/Robo role for efficient midline crossing of spinal commissural axons.16,17 Furthermore, once contralateral projections are made, post-crossing commissural axons turn longitudinally and undertake trajectories at significant distances from the floor plate. These longitudinal trajectories also appear to rely on Slit/Robo signaling to project to specific positions.16–18 These studies suggest that the floor plate produces a field of diverse cues that act at a distance to guide pre- and post-crossing segments of commissural axons.
Pioneer longitudinal axons also project within this field of cues emanating from the floor plate. In fact, in the brain, the pioneer longitudinal axons project before or concurrently with commissural trajectories.3,19 However, longitudinal trajectories do not project towards or away from the floor plate, but instead migrate parallel to the neuraxis as they maintain constant positions relative to the ventral midline (Fig. 1). This suggests that longitudinal trajectories cannot result from net attraction or repulsion from the midline and raises the question of whether the floor plate plays a role in their guidance. To begin to define basic longitudinal guidance mechanisms, we have recently undertaken a series of genetic and cellular studies to test the roles of floor plate and more specifically, Slit/Robo signaling.1
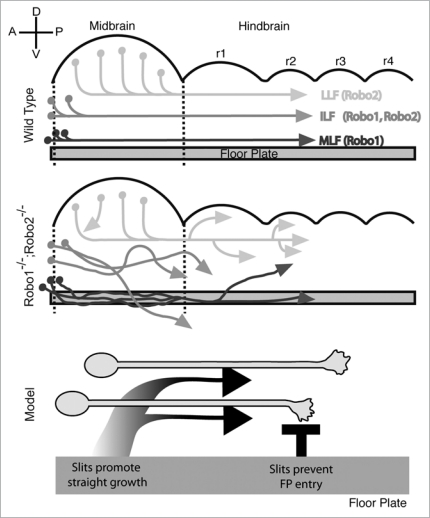
Figure 1.
Pioneer longitudinal axons and their guidance by Slit/Robo signals. Top: Schematic of longitudinal pioneer tracts in the mouse embryonic midbrain and hindbrain (r1-4, rhombomeres). Adapted from 1. Neurons (filled circles) give rise to populations of longitudinal axons, which descend through the hindbrain to form longitudinal fascicles (medial, intermediate, lateral: MLF, ILF, LLF). robo receptor expression has been shown in each population, as indicated. However, it is likely that both Robo1 and 2 are expressed in all or most longitudinal axons because double mutants for both receptors cause strong and widespread guidance errors. Upper left: the axes of the brain regions are indicated; A, anterior; P, posterior; D, dorsal; V, ventral. Middle: As an example of strongly disrupting Slit/Robo signaling, Robo1−/−; Robo2−/− double mutants show extensive errors in all pioneer longitudinal tracts. Adapted from 1. For example, many MLF axons enter and project within the floor plate, while other MLF axons wander dorsally away from the floor plate. Bottom: Slit signals from the floor plate have dual roles in guiding pioneer longitudinal axons. Slit signals prevent entry into the floor plate, possibly by repulsive signals (depicted by the black T). Slits also signal to direct growth along straight pathways, acting on several axon populations at considerable distances. The shaded arrows depict the presumed Slit protein gradients from the floor plate and this long range activity directs longitudinal projections straight and parallel to the floor plate.
The floor plate is important for longitudinal axon trajectories.
To determine whether longitudinal axons navigate using signals from the floor plate, we used gene inactivation and overexpression strategies in vivo to either remove or to create extra floor plate tissue.1 To reduce or remove floor plate tissue, we analyzed mouse embryos mutant for the transcription factor Gli2, which have a lack or reduction in differentiated floor plate cells in the midbrain and hindbrain. Gli2 mutants had profoundly disrupted longitudinal tracts, with axons projecting across the ventral midline, failing to maintain DV positions and, in some cases, switching to project anteriorly instead of posteriorly. An opposite strategy consisting of inducing ectopic floor plate tissue in the lateral neural tube by electroporation-mediated misexpression of Sonic Hedgehog was also used and resulted in ectopic floor-plate-like tissue that had a potent effect in diverting longitudinal trajectories. These observations point to floor plate signals as being important for longitudinal trajectories. The diverse behaviors of longitudinal axons suggest multiple guidance activities, potentially presenting positive and negative signals to influence DV positioning, as well as directional cues to set AP polarity of projections. A further implication is that these signals can act over long range, reaching from the ventral midline far up into dorsal tissue (alar plate) to influence the dorsal-most longitudinal axons.
Slit/Robo Signals are Critical Guides for Longitudinal Axons
With the floor plate having potent effects, the next step was to identify specific molecular signals for pioneer longitudinal axons. A starting point was the Slit family of repellents and their Robo receptors, chosen because Slits are expressed in the floor plate and have been implicated in guiding several axon populations, as previously mentioned. An additional rationale was provided by studies showing that Slit/Robo signaling in Drosophila has prominent midline signaling roles in longitudinal tract formation. One key observation from flies was that loss of midline Slit caused the entry of all longitudinal axons into the midline, implying that a role of Slit signaling is to keep longitudinal axons out of the midline.20,21 Tests of the fly Robo family of receptors further showed that longitudinal axons used Slit not simply to avoid entering the midline, but to choose tract positions at specific distances parallel to the midline by expressing certain combinations (or possibly levels) of the three Robo receptors, representing a “Robo code” that specified longitudinal tract positions.22,23 The extent to which vertebrate longitudinal axons use an analogous Slit/Robo positioning mechanism remained unclear. As mentioned above, spinal cord commissural axons require Slit/Robo signals to attain specific tract positions away from the floor plate.16,17
To extend this analysis, we studied mouse mutants for Slit1 and 2, and also for the two main Robo receptors, 1 and 2.1 Loss of both Slit1 and 2, or both Robo1 and 2, caused profound disruptions of all longitudinal tracts in midbrain and hindbrain (Fig. 1). The most strongly affected trajectories were the pioneers of the medial longitudinal fascicle (MLF), which normally projected adjacent to and parallel to the floor plate. Most MLF axons reacted to Slit or Robo loss by entering and crossing the floor plate. The main implication was that Slit/Robo signaling influences longitudinal trajectories by preventing midline entry, analogous to Slit-mediated repulsion in Drosophila. Thus, Slit signals from the floor plate have an important role in setting longitudinal trajectories by keeping pioneer axons out of the ventral midline.
Robo1/2 double mutants had the strongest phenotypes with widespread effects on all longitudinal pioneer tracts in the hindbrain (Fig. 1). This indicates that Robo1 and 2 play a major role in Slit reception. To test whether Robo1 and 2 had distinct roles in specifying the position of tracts, such as by a Robo code, we also examined mouse embryos that were homozygous mutant for either Robo1 or Robo2. Robo1 mutants showed a subtle defect in MLF projections, but otherwise had normal longitudinal tracts, and Robo2 mutants did not appear to affect any of the tracts. In comparison to the strong Robo1/2 double mutants, the mild single mutant phenotypes suggested that Robo1 and 2 are largely genetically redundant. Furthermore, in contrast to the Drosophila nerve cord, the single mutant phenotypes do not support a “Robo code” mechanism for positioning longitudinal tracts in the mammalian brain stem. While our results show an important long-range role for Slit/Robo signaling in setting longitudinal trajectories by keeping the axons ipsilateral, further experiments will be needed to test if Slit signals can act directly and in a graded manner, as would be predicted by a repulsive gradient.
Interestingly, the function of Slits in longitudinal axons does not appear to be limited to midline repulsion. Loss of Slit/Robo signaling also disrupted straight growth in all of the longitudinal tracts (Fig. 1). Many pioneer longitudinal trajectories made a distinct class of navigation errors, including wandering instead of growing straight longitudinally and, in some cases, diverging dorsally away from the floor plate. These observations indicate an unexpected role of Slit/Robo signaling in promoting straight growth of longitudinal axons. This function is particularly evident for axons projecting at dorsal or intermediate positions, which showed an increased variance in their direction of growth. Clearly this is not consistent with a simple repellent mechanism of Slit. Instead, these phenotypes provide evidence that even at significant distances from the floor plate, Slit signals are required for straight longitudinal trajectories. Together with long range effects previously observed for post-crossing commissural axons,17 it is clear that Slit signals act not merely as local inhibitors, but also provide long range positioning. The mechanism responsible for promoting straight growth remains unclear, but Slit/Robo signaling clearly is essential for efficient longitudinal projections.
How widespread is Slit/Robo guidance for longitudinal axons, and are other cue systems involved?
The important role of Slit/Robo guidance for longitudinal pioneers suggests new directions for axon guidance. A first question is whether Slit/Robo guidance is generally important for all longitudinal axons. During fetal forebrain development, several major tracts are missing or dispersed in Slit and Robo mutant mice.10–12 However, because these tracts are relatively late forming, it remains unclear whether the Slit/Robo mutant phenotypes may be indirect consequences of errors made by earlier pioneers. Additional evidence of Slit/Robo longitudinal guidance is emerging from other systems, particularly zebrafish, including a population of forebrain pioneers,9 as well as a group of tyrosine hydroxylase-expressing descending axons.24 Based on these and other studies in progress, as well as the studies on post-crossing commissural trajectories, an emerging model is that Slit/Robo signals are broadly important in many regions of the CNS for setting longitudinal trajectories.
For a wider view of longitudinal guidance mechanisms, it will be important to identify other guidance signals. First, are Slit/Robo signals the only floor plate-derived repellents for longitudinal axons? They do appear to be predominant for the MLF because in Robo1/2 double mutants most of these axons find the floor plate an attractive tissue for entry and growth.1 This implies that in the absence of Slit/Robo signaling, the floor plate has a net attractive activity for MLF axons. Floor plate-derived attractants, such as Netrin1, likely also influence trajectories, as recently shown for longitudinal axons in zebrafish.24 An intriguing idea is that a balance between attractant and repellent midline signals might orient axons and set their specific longitudinal trajectories. On another level, even when longitudinal axons make strong errors in Slit/Robo mutants, they still grow in the anterior-posterior direction. This implies an independent anterior-posterior directional guidance system. Wnt and Shh signals are likely candidates. Wnts serve as important directional cues for anterior-posterior guidance in mouse,25,26 as well as in C. elegans.27 For example, a longitudinal gradient of Wnt signals acts through Frizzled receptors on the post-crossing segments of spinal cord commissural axons to set their anterior direction of growth.26 Much later in development, Wnt signals direct the descending projections of corticospinal axons through the spinal cord, in this case through Ryk receptors.25 Shh also provides an anterior-posterior gradient in the spinal cord to direct post-crossing commissural trajectories.28 These Wnt and Shh guidance systems share features, including cues expressed in anterior-posterior gradients over long distances, as well as axonal receptors that direct growth either up or down the gradients. Interestingly, both Wnt and Shh expression are prominent in brain floor plate during longitudinal pioneer outgrowth. Overall, longitudinal axons may have mechanisms to simultaneously interpret separate cue systems for anterior-posterior directionality, floor plate-derived attractants and repellents for DV positioning, as well Slit/Robo signals promoting straight growth.
In conclusion, Slit/Robo signals have emerged as important guides for pioneer longitudinal axons. These signals have strong and widespread roles across multiple early longitudinal axon populations, and likely continue to guide later axon types, as well. These roles include repulsion from the ventral midline, as predicted from Slit/Robo effects on many other types of migrating axons and cells. Our results also identify novel functions in promoting straight growth along longitudinal pathways. Future studies to discover how longitudinal growth cones integrate Slit/Robo signals with other cues may prove to be a long journey, but one that is critical to understanding brain development.
Acknowledgements
Funding was provided to G.S.M. by the March of Dimes grant #1-FY06-387 and by NIH grant NS054740.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11219
References
- 1.Farmer WT, Altick AL, Nural HF, Dugan JP, Kidd T, Charron F, et al. Pioneer longitudinal axons navigate using floor plate and Slit/Robo signals. Development. 2008;135:3643–3653. doi: 10.1242/dev.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Easter SS, Jr, Burrill J, Marcus RC, Ross LS, Taylor JS, Wilson SW. Initial tract formation in the vertebrate brain. Prog Brain Res. 1994;102:79–93. doi: 10.1016/S0079-6123(08)60533-6. [DOI] [PubMed] [Google Scholar]
- 3.Chedotal A, Pourquie O, Sotelo C. Initial tract formation in the brain of the chick embryo: selective expression of the BEN/SC1/DM-GRASP cell adhesion molecule. Eur J Neurosci. 1995;7:198–212. doi: 10.1111/j.1460-9568.1995.tb01056.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson SW, Ross LS, Parrett T, Easter SS., Jr The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development. 1990;108:121–145. doi: 10.1242/dev.108.1.121. [DOI] [PubMed] [Google Scholar]
- 5.Mastick GS, Fan CM, Tessier-Lavigne M, Serbedzija GN, McMahon AP, Easter SS., Jr Early deletion of neuromeres in Wnt-1−/− mutant mice: evaluation by morphological and molecular markers. J Comp Neurol. 1996;374:246–258. doi: 10.1002/(SICI)1096-9861(19961014)374:2<246::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Montiel HL, Melendez-Herrera E, Cepeda-Nieto AC, Mejia-Viggiano C, Larriva-Sahd J, Guthrie S, et al. Diffusible signals and fasciculated growth in reticulospinal axon pathfinding in the hindbrain. Dev Biol. 2003;255:99–112. doi: 10.1016/s0012-1606(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 7.Andrews GL, Mastick GS. R-cadherin is a Pax6-regulated, growth-promoting cue for pioneer axons. J Neurosci. 2003;23:9873–9880. doi: 10.1523/JNEUROSCI.23-30-09873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ringstedt T, Braisted JE, Brose K, Kidd T, Goodman C, Tessier-Lavigne M, et al. Slit inhibition of retinal axon growth and its role in retinal axon pathfinding and innervation patterns in the diencephalon. J Neurosci. 2000;20:4983–4991. doi: 10.1523/JNEUROSCI.20-13-04983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devine CA, Key B. Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Dev Biol. 2008;313:371–383. doi: 10.1016/j.ydbio.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, et al. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- 11.Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, et al. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, et al. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci. 2007;27:3395–3407. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 14.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 15.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 16.Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, et al. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- 17.Reeber SL, Sakai N, Nakada Y, Dumas J, Dobrenis K, Johnson JE, et al. Manipulating Robo expression in vivo perturbs commissural axon pathfinding in the chick spinal cord. J Neurosci. 2008;28:8698–8708. doi: 10.1523/JNEUROSCI.1479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeber SL, Kaprielian Z. Leaving the midline: how Robo receptors regulate the guidance of post-crossing spinal commissural axons. Cell Adh Migr. 2009;3:300–304. doi: 10.4161/cam.3.3.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Easter SS, Jr, Ross LS, Frankfurter A. Initial tract formation in the mouse brain. J Neurosci. 1993;13:285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S. Slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4:2169–2187. doi: 10.1101/gad.4.12a.2169. [DOI] [PubMed] [Google Scholar]
- 21.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 22.Simpson JH, Bland KS, Fetter RD, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors controls lateral position. Cell. 2000;103:1019–1032. doi: 10.1016/s0092-8674(00)00206-3. [DOI] [PubMed] [Google Scholar]
- 23.Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell. 2000;103:1033–1045. doi: 10.1016/s0092-8674(00)00207-5. [DOI] [PubMed] [Google Scholar]
- 24.Kastenhuber E, Kern U, Bonkowsky JL, Chien CB, Driever W, Schweitzer J. Netrin-DCC, Robo-Slit, and heparan sulfate proteoglycans coordinate lateral positioning of longitudinal dopaminergic diencephalospinal axons. J Neurosci. 2009;29:8914–8926. doi: 10.1523/JNEUROSCI.0568-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song X, et al. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- 26.Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 27.Pan C, Howell J, Clark S, Hilliard M, Cordes S, Bargmann C, et al. Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev Cell. 2006;10:367–377. doi: 10.1016/j.devcel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardo M, et al. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci. 2005;8:297–304. doi: 10.1038/nn1396. [DOI] [PubMed] [Google Scholar]