Abstract
Adiponectin is an adipocytokine involved in the pathogenesis of various obesity-related disorders. Also, it has been shown that adiponectin has therapeutic potential for metabolic syndrome, systemic insulin resistance, cardiovascular disease and more recently carcinogenesis. Adiponectin can modulate breast cancer cell growth and proliferation. Anti-metastatic effects of adiponectin have also been elucidated. It has been shown that adiponectin inhibits important metastatic properties such as adhesion, invasion and migration of breast cancer cells. Examination of the underlying molecular mechanisms has shown that adiponectin treatment increases AMP-activated protein kinase (AMPK) phosphorylation and activity. Adiponectin also increases phosphorylation of downstream target of AMPK, Acetyl-CoA Carboxylase (ACC) and decreases phosphorylation of p70S6 kinase (S6K). Importantly, adiponectin treatment increases the expression of tumor suppressor gene, LKB1 in breast cancer cells. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and more importantly, its biological functions including inhibition of adhesion, migration and invasion of breast cancer cells. Although further studies are required to analyze the effect of adiponectin on LKB1-AMPK-S6K axis, these data present a novel mechanism involving specific upregulation of tumor suppressor gene LKB1 by which adiponectin inhibits adhesion, invasion and migration of breast cancer cells. These results highlight a new role for LKB1 in adiponectin action and may have significant implication for development of novel therapeutic options.
Cancer research has largely focused on the molecular basis of oncogenic transformation and tumorigenesis for many years. Recent progress in cancer research has put the metastatic process at the center stage because higher metastatic potential of tumor cells is the major cause of mortality from solid tumors. Metastasis is a complex process that involves modulation of various molecular signaling networks. Tumor cells alter the microenvironment, attain greater cellular adhesion along with better ability to invade and migrate to gain access to circulation. These wandering tumor cells defy anoikis, survive in the circulation, exit into new permissive organ site and colonize distant organs.1 The microenvironment in which the tumor originates plays an important role in tumor initiation, progression and metastasis.
Key words: adiponectin, LKB1, invasion, migration, cancer, AMPK, S6K
Adipocytes Modulate Breast Tumor Growth and Metastasis
With epithelial and other cells accounting for only approximately 10% of human breast volume, adipocytes make up the bulk of the human breast. In many human breast cancers, there is reduced connective tissue separating the adipocytes from tumor cells. Also, carcinomas invade through the basement membrane and infiltrate fibrous tissue barriers, resulting in close positioning that allows more paracrine interactions between the adipocytes and breast epithelial cells which might directly affect breast tumorigenesis.2 In fact, paracrine effects of adipocytes on tumor cells have been demonstrated in various in vitro and in vivo pharmacological studies. Co-culture of mature rat adipocytes with breast carcinoma cells in three dimensional collagen matrix promotes proliferation of breast carcinoma cells.3 Treatment of MCF7 cells with conditioned media from murine adipocytes reveals upregulation of genes involved in invasion, proliferation and metastasis and downregulation of p18, a cell cycle checkpoint inhibitor and BARD1, a tumor suppressor protein in microarray analysis.4 Importantly, development of mammary tumors and metastasis is observed in mice injected s.c or i.p with the murine mammary carcinoma cell line.
SP1 and adipose tissue, while no tumor growth or metastasis is observed when SP1 cells are injected distant from any fat pad.5 In a direct comparison, 3T3-L1 murine adipocyte-secreted factors signifi- cantly stimulate MCF7 cell migration and proliferation when compared to fibroblastsecreted factors.6 The close relationship between adipocytes and breast cancer has also been shown by the finding that co-injection of SUM-159PT human breast adenocarcinoma cells and murine adipocytes results in the formation of three-times larger tumors in comparison to the smaller tumors resulting with co-injection of SUM-159PT cells with murine fibroblasts.4 Adipocytes have been considered as merely cargo spaces for fat storage, to be released during times of hardship, such as fasting or starvation. More recently, adipose tissue has been recognized as an important endocrine organ as adipocytes can produce various hormones, cytokines and growth factors, collectively called adipocytokines.7 Many adipocytokines such as leptin, interleukin 6 (IL6) and tumor necrosis factor alpha (TNFα) have been shown to increase breast cancer cell proliferation and tumorigenesis.2,8–11 Based on these studies, it is widely believed that adipocytokines can play key roles in mediating the stromal-breast epithelial crosstalk and in influencing the growth and proliferation of tumor stroma and breast tumor cells.
Adiponectin Functions as a Protective Adipocytokine
Adiponectin12–15 is an important adipocytokine that has been known as a “guardian angel adipocytokine” owing to its protective role against the pathogenesis of various obesity-related disorders (Fig. 1) and the metabolic syndrome, particularly type 2 diabetes and cardiovascular disease.16–18 Therapeutic potential of adiponectin has been shown in various studies where replenishment of adiponectin in animal models reduces body weight, improves glucose/lipid homeostasis, increases insulin sensitivity, prevents atherosclerosis and fatty liver diseases.17 Adiponectin suppresses proliferation and activation of immune cells,19 downregulates vascular adhesion molecules in endothelial cells and inhibits smooth muscle migration.20 Adiponectin controls the bioavailability of certain growth factors by directly binding them hence preventing their interactions with respective receptors.21 More recent epidemiological, biochemical and molecular biological studies have established an important role for adiponectin in cancer.22 Adiponectin mediates its cellular functions in a tissue-dependent manner via its membrane bound receptors; AdipoR1 and AdipoR2. Recently, T-cadherin has been identified as an adiponectin receptor.23–25 Low levels of plasma adiponectin associated with obesity have been linked with many common forms of cancer in various epidemiological studies.22,26 Low serum adiponectin levels has been linked with higher risk of breast cancer in both postmenopausal and premenopausal women, independent of age, menopause status, hormone receptor status, lymph node metastasis, status of ER and Her2/neu.27,28 Breast tumors arising in women with low-serum adiponectin levels may have a more aggressive phenotype (large size of tumor, high histological grade and increased metastasis).27–29 Adiponectin exerts an anti-proliferative response in human breast cancer cells.30–35 Expression of adiponectin in breast tumor samples and adjacent tissues suggests that adiponectin secretion from mammary adipocytes might affect the malignant properties of breast cancer cells in the breast tumor microenvironment via paracrine interactions.36
Figure 1.
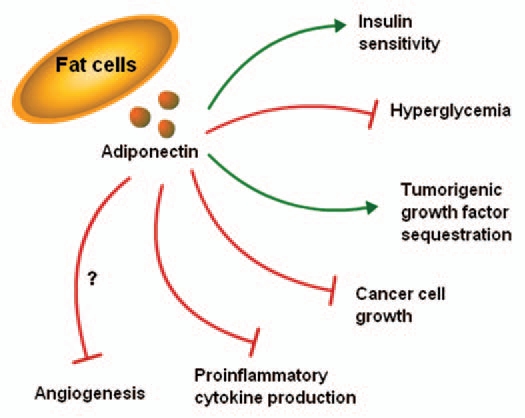
Adiponectin modulates the pathogenesis of various obesity-related disorders.
Molecular Mechanisms Underlying Adiponectin Function
Recent studies have shown the negative effect of adiponectin on the malignant properties of breast cancer cells. Taliaferro-Smith et al. show that adiponectin treatment inhibits malignant properties such as adhesion, invasion and migration of breast cancer cells.37 How does adiponectin produce this profound change in metastatic properties of breast cancer cells? Investigating the underlying molecular mechanisms, they show that adiponectin stimulates AMPK phosphorylation and activity while reducing mTOR activity. Adiponectin reduces phosphorylation of S6K, a known target of mTOR. A very important aspect of this study is that adiponectin treatment increases the expression of tumor suppressor LKB1 (Fig. 2).37 More importantly, LKB1 is not only required for adiponectin-mediated modulation of AMPK-S6K axis but also for adiponectin-mediated inhibition of adhesion, invasion and migration of breast cancer cells. This conclusion is supported by the findings that treatment of stable LKB1-null-MCF7 cells with adiponectin shows no adiponectin-mediated increase in AMPK phosphorylation or reduction in pS6K phosphorylation while LKB1-expressing MCF7 cells exhibit adiponectin-mediated increase in AMPK phosphorylation and decrease in pS6K phosphorylation. In addition, it is shown that adiponectin treatment does not affect migration and invasion potential of stable LKB1 nullMCF7. Hence, adiponectin treatment significantly inhibits malignant properties of breast cancer cells via modulation of LKB1-AMPK-S6K axis.
Figure 2.
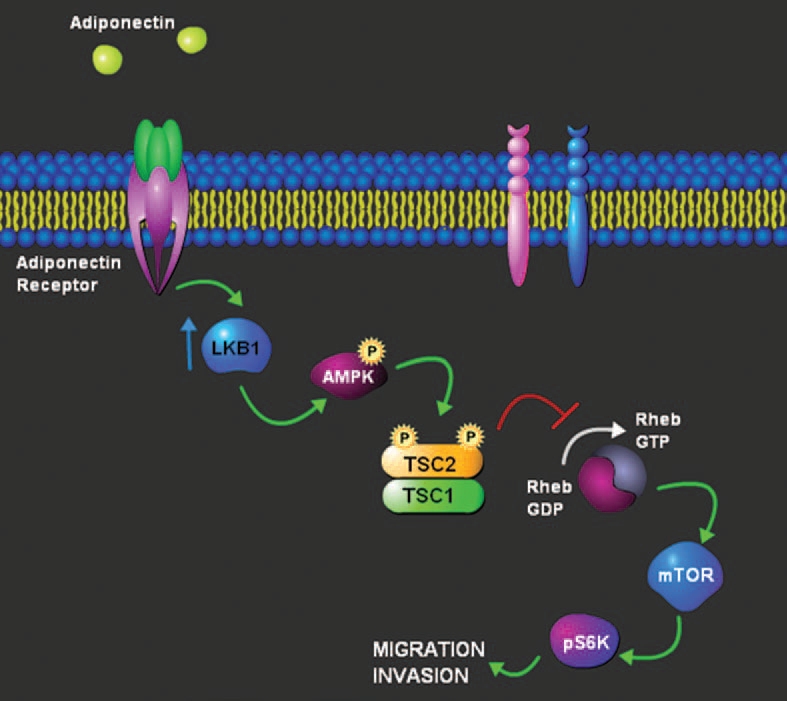
Model describing the underlying molecular mechanism involved in adiponectin mediated inhibition of migration and invasion of breast cancer cells. Adiponectin increases the expression of LKB1 resulting in increased phosphorylation of AMPK. AMPK activation results in modulation of TSC2/TSC1 leading to inhibition of S6K activation.
Taliaferro-Smith et al. demonstrate for the first time that adiponectin can increase LKB1 expression in human breast cancer cells, which play a critical role in mediating the inhibitory effects of adiponectin on mammary tumorigenesis.37 LKB1 kinase, a key determinant in Peutz-Jeghers syndrome, is known as a tumor suppressor gene that has an inherited propensity to gastrointestinal and other cancers including lung, pancreatic and breast cancer.38,39 Inactivation of LKB1 gene has been shown in a subset of sporadic lung and pancreatic cancer. A recent clinical study reporting extensive analysis of LKB1 expression in breast tumors using IHC show that while abrogation of LKB1 expression is not common in human breast carcinoma, it importantly correlates with high-grade DCIS and high-grade invasive ductal carcinoma.40 It is important to note that inhibition of LKB1 expression is only observed in the DCIS associated with invasion and not pure DCIS cases demonstrating that loss of LKB1 can potentially promote invasion. In agreement with these results, low LKB1 protein levels have been shown to correlate with poor prognosis in breast carcinoma.41 It has also been shown that adiponectin mediated increase in LKB1 expression diminishes invasion potential of breast cancer cells.37 LKB1 participates in regulating various cellular functions including controlling cell polarity, energy homeostasis, protein synthesis and cell cycle arrest. Studies using mutant LKB1 lacking the nuclear localization signal have reported that cytoplasmic pool of LKB1 plays an important role in mediating its tumor suppressor properties. Co-expression of LKB1 with STRAD and MO25 strikingly increase its localization in cytoplasm whereas mutant LKB1, unable to interact with STRAD and MO25 does not translocate to cytoplasm.42–44 It is speculated that adiponectin might also increase LKB1-STRAD-MO25 interaction in addition to overexpression of LKB1 thus increasing the functional pool of LKB1. Upstream kinase LKB1 itself gets phosphorylated at 8 residues (Ser31, Ser325, Thr366, Ser431 by upstream kinases and autophosphorylation at Thr185, Thr189, Thr336 and Ser404). The importance of these phosphorylation events is still questionable for LKB1 function as mutation of any of these sites of phosphorylation to either Ala to abolish phosphorylation or to Glu to mimic phosphorylation has thus far not been reported to45–47 significantly affect LKB1 catalytic activity in vitro or its cellular localization.43 Recent findings support the concept that LKB1 plays an integral role in breast cancer metastasis and higher expression of LKB1 in response to adiponectin treatment inhibits invasion and migration properties of breast cancer cells.
LKB1 Modulates AMPK-mTOR Axis
Tumor suppressor function of LKB1 can be partly due to its ability to activate master metabolic regulator AMPK.39 Direct binding of AMP to the nucleotide- binding domain of AMPK induces a conformational change, exposing the activation loop of the catalytic kinase subunit. Upstream kinase LKB1 phosphorylates a critical threonine in this activation loop to activate AMPK.39 AMPK activation is dependent on LKB1 as genetic depletion of LKB1 in mouse embryonic fibroblasts (MEFs) results in a loss of AMPK activation even in the presence of energy stresses that raise AMP.48 Concurring to these findings, Taliaferro-Smith et al. show that silencing of LKB1 in breast cancer cells results in lower AMPK activation while overexpression of LKB1 increases AMPK activation which synergistically increases by concomitant adiponectin treatment.37 AMPK is an important downstream target of LKB1, which upon activation, regulates the activation of two other tumor suppressors, TSC1 and TSC2. Heterodimeric binding partners TSC2 and TSC1 regulate Rheb which is a critical regulator of mTOR protein kinase which in turn regulates a vast range of cellular activities, including transcription, translation, cell size, mRNA turnover, protein stability, ribosomal biogenesis and cytoskeletal organization.48 Analysis of these molecular cascades suggest that cells having reduced expression of LKB1 show hyperactivation of mTOR that may contribute to carcinogenesis.39,48 Indeed, it has been observed that LKB1 silencing results in higher mTOR activity whereas adiponectin-induced higher expression of LKB1 results in inhibition of mTOR activity in breast cancer cells. Based on these observations, it is hypothesized that development of adiponectin analogues may prove to be an excellent therapeutic option for carcinomas characterized by hyperactive mTOR signaling such as human familial cancer syndromes all of which are characterized by hamartoma-type tumors.
Many epidemiological studies have shown that higher serum levels of most of the adipocytokines are associated with poor prognosis for obesity-related diseases including carcinogenesis. In contrast, adiponectin has shown promising insulin-sensitizing, anti-inflammatory and antiatherogenic activities both in vitro and in vivo studies. Serum adiponectin levels are dramatically decreased in obesity and various obesity-related diseases. In vivo studies using animal models have shown that treatment with adiponectin can improve glucose/lipid homeostasis, increase insulin sensitivity and prevent atherosclerosis hence suggesting clinical relevance of adiponectin treatment.49–52 Recent studies show that adiponectin inhibits the metastatic properties of breast cancer cells via upregulation of LKB1 and modulation of LKB1AMPK-S6K axis thus providing mechanistic insights for the development of adiponectin analogues. Future studies may focus on the detailed mechanism of action of LKB1, the signaling pathways it uses and the possible interaction with other adipokines that might act in synergy. Is adiponectin the crucial, long-sought link between obesity and breast cancer? Although at this point adiponectin is unlikely to fully explain the relationship, there may be more interactions with other signaling pathways as well as other adipokines. These findings are important enough to lend credence to the idea that adiponectin or LKB1, its crucial downstream target, might represent exciting targets for the drug development. Thus, increasing adiponectin levels using adiponectin analogues or augmentation of its effectiveness by using TZDs Thiazolidinediones, can potentially become a future beneficial treatment for breast cancer patients for inhibiting invasion and migration properties of breast tumors and improving overall survival.
Acknowledgements
Grant Support: NIDDK NIH (K01DK077137 to N.K.S.), NCI NIH (R01CA131294 to D.S.), Wilbur and Hilda Glenn Foundation (grant to D.S.), CDMRP BCRP (grant BC030963 to D.S.), The Susan G. Komen for the Cure (grant BCTR0503526 to D.S.), Emory University Research Council (D.S.), BJ Foundation (D.S.) and Mary K. Ash Foundation (research grant to D.S.).
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11541
References
- 1.Gupta GP, Massague J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Schaffler A, Scholmerich J, Buechler C. Mechanisms of disease: adipokines and breast cancer—endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endocrinol Metab. 2007;3:345–354. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- 3.Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol. 2003;201:221–228. doi: 10.1002/path.1430. [DOI] [PubMed] [Google Scholar]
- 4.Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C, et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of antIapoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 5.Elliott BE, Tam SP, Dexter D, Chen ZQ. Capacity of adipose tissue to promote growth and metastasis of a murine mammary carcinoma: effect of estrogen and progesterone. Int J Cancer. 1992;51:416–424. doi: 10.1002/ijc.2910510314. [DOI] [PubMed] [Google Scholar]
- 6.Chamras H, Bagga D, Elstner E, Setoodeh K, Koeffler HP, Heber D. Preadipocytes stimulate breast cancer cell growth. Nutrition and Cancer. 1998;32:59–63. doi: 10.1080/01635589809514719. [DOI] [PubMed] [Google Scholar]
- 7.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 8.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocrine-Related Cancer. 2006;13:279–292. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 9.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:858–866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 10.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine and autocrine factors in breast cancer risk and progression. Endocrine-Related Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson GD, Rose DP. Breast cancer and obesity: an update. Nutrition Cancer. 2003;45:1–16. doi: 10.1207/S15327914NC4501_1. [DOI] [PubMed] [Google Scholar]
- 12.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 13.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 14.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 15.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 16.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 18.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 19.Fantuzzi G. Adipose tissue, adipokines and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 22.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 24.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen MB, McAinch AJ, Macaulay SL, Castelli LA, O'Brien PE, Dixon JB, et al. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab. 2005;90:3665–3672. doi: 10.1210/jc.2004-1980. [DOI] [PubMed] [Google Scholar]
- 26.Barb D, Pazaitou-Panayiotou K, Mantzoros CS. Adiponectin: a link between obesity and cancer. Expert Opin Investig Drugs. 2006;15:917–931. doi: 10.1517/13543784.15.8.917. [DOI] [PubMed] [Google Scholar]
- 27.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 28.Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- 30.Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2006;345:271–279. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, et al. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDAMB-231 cells in nude mice. Cancer Res. 2006;66:11462–11470. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- 32.Arditi JD, Venihaki M, Karalis KP, Chrousos GP. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: a potential hormonal link between obesity and cancer. Horm Metab Res. 2007;39:9–13. doi: 10.1055/s-2007-956518. [DOI] [PubMed] [Google Scholar]
- 33.Takahata C, Miyoshi Y, Irahara N, Taguchi T, Tamaki Y, Noguchi S. Demonstration of adiponectin receptors 1 and 2 mRNA expression in human breast cancer cells. Cancer Lett. 2007;250:229–236. doi: 10.1016/j.canlet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. 2008;20:971–977. [PubMed] [Google Scholar]
- 35.Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer. 2008;98:370–379. doi: 10.1038/sj.bjc.6604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Perrone D, Penault-Llorca F, et al. Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology. 2008;53:484–487. doi: 10.1111/j.1365-2559.2008.03121.x. [DOI] [PubMed] [Google Scholar]
- 37.Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene. 2009;28:2621–2633. doi: 10.1038/onc.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 39.Hardie DG. New roles for the LKB1—AMPK pathway. Curr Opin Cell Biol. 2005;17:167–173. doi: 10.1016/j.ceb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Fenton H, Carlile B, Montgomery EA, Carraway H, Herman J, Sahin F, et al. LKB1 protein expression in human breast cancer. Appl Immunohistochem Mol Morphol. 2006;14:146–153. doi: 10.1097/01.pai.0000176157.07908.20. [DOI] [PubMed] [Google Scholar]
- 41.Shen Z, Wen XF, Lan F, Shen ZZ, Shao ZM. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res. 2002;8:2085–2090. [PubMed] [Google Scholar]
- 42.Baas AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA, et al. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003;22:3062–3072. doi: 10.1093/emboj/cdg292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, et al. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boudeau J, Scott JW, Resta N, Deak M, Kieloch A, Komander D, et al. Analysis of the LKB1-STRAD-MO25 complex. J Cell Sci. 2004;117:6365–6375. doi: 10.1242/jcs.01571. [DOI] [PubMed] [Google Scholar]
- 45.Sapkota GP, Boudeau J, Deak M, Kieloch A, Morrice N, Alessi DR. Identification and characterization of four novel phosphorylation sites (Ser31, Ser325, Thr336 and Thr366) on LKB1/STK11, the protein kinase mutated in Peutz-Jeghers cancer syndrome. Biochem J. 2002;362:481–490. doi: 10.1042/0264-6021:3620481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, et al. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell growth. J Biol Chem. 2001;276:19469–19482. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- 47.Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27:6908–6919. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 48.Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 49.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 50.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]


