Abstract
A better knowledge of the molecular mechanisms that govern leukocyte trafficking is of major relevance for the clinics. Both normal and pathologic extravasation of lymphocytes are a fine-tuned spatio-temporal event of migratory path-finding, likely regulated by molecular guidance cues underlying cell movements in other systems. We have recently reported that members of the Eph family of receptor tyrosine kinases, namely EphA2 and one of its ligands, ephrin-A4 (EFNA4) can mediate in the traffic of chronic lymphocytic leukemia (CLL) cells and presumably of normal B cells between the blood and the tissues. The importance of EphA2-EFNA4 interactions at the endothelium-lymphocyte interface during TEM could rely on their attractive/repulsive properties. In the present work, we expand on those results by including additional insights and new suggestions for future studies that discuss the relevance of these molecules in overall cell adhesion dynamic events.
Key words: Eph, ephrin, migration pathfinding, trafficking, leukemia, endothelium, lymphocyte, CLL, extravasation, transendothelial migration
Introduction
Lymphocyte trafficking between blood and the tissues is an essential process to assure lymphoid homeostasis.1–4 Lymphocytes migrate into (ingress) and out of (egress) lymphoid tissues at the level of specialized vascular structures named high endothelial venules (HEV). They harbor the molecular machinery for accommodating, upon cross-talk with putative transmigrating lymphocytes, transendothelial migration processes.3 In many pathological conditions, selective leukocyte recruitment takes place at the level of newly specialized endothelial venules as a consequence of context dependent signals including pro-inflammatory cytokines.3,5 A better knowledge of the molecular mechanisms orchestrating these processes is beneficial for the clinics including the development of new therapies for many human diseases.
Within the context of extravasation, lymphocytes and endothelial cells exchange bidirectional signals leading to mutually dependent alterations in their respective behaviors.6–8 A multi-step cell adhesion cascade is currently recognized to be involved in the extravasation process that includes successive conditional lymphocyte-endothelial cell contacts: (1) tethering and “rolling,” (2) firm adhesion, (3) crawling and (4) transmigration followed by tissue invasion.3,4,9,10 The first steps, rolling and tethering, are the most relevant to decision-making, while the following steps are more concerned with the transmigration itself. The whole process is determined by the balance of extracellular signals derived from the involved tissue, mostly in the form of soluble molecules like chemoattractants and/or repellents (chemokines and others) and other factors like shear flow.3,11 As such, the intra/extravasation of lymphocytes is a complex event where migratory path-finding is regulated by numerous trafficking signals that selectively control the movement of distinct subsets of immune cells into and out of specific tissues.
Substantial progress has been made in dissecting the molecular mechanisms that orchestrate the directed movement of leukocytes between the vasculature and the tissues7,11 and within the later ones,1,2 emphasizing the importance of existing attractive/repulsive signals underlying leukocyte migration.12 Molecular mechanisms that act in a context-dependent manner, such as bidirectional guiding cues during development or in adult plastic systems, can be relevant to leukocyte migration as well, like netrins, semaphorins, slit ligands and Eph/ephrins.
Eph/Ephrins
The Eph family of receptor tyrosine kinases and their ligands, ephrins (EFN), delivers attractive/repulsive signals that guide cell movements in a wide spectrum of processes during development including the axon growth cone during pathfinding, the boundary formation or the cell positioning during morphogenesis through restricting cell intermingling.13–16 An increasing body of knowledge is showing that Eph signaling also controls the architecture and homeostasis of different adult tissues under normal and pathological conditions,17–19 suggesting that common mechanisms of cell behavior can be modulated by them. They therefore represent putative new tools in cancer diagnosis and as potentially relevant molecular therapeutic targets.
The Eph family is classified into two sub-families, EphA (nine members in humans) and EphB (six members), depending on the similarity within each group of the extracellular domain sequences and on their affinity for the membrane bound ligands, ephrin (EFN), either of type A (five members), which are glycosylphosphatidylinositol (GPI) anchored proteins to the cell membrane, or of type B (three members), which have a single transmembrane domain. Receptor/ligand interactions are promiscuous within each subclass and certain inter-class interactions also exist which can contribute to the fine-tuning of cell processes. Most Eph receptors contain an active tyrosine kinase domain while ephrins lack intrinsic catalytic activity but associate with tyrosine kinases and bind to several intracellular targets.20 As such, an essential property of Eph-EFN interaction is that it can result in bidirectional signaling into both the Eph- (forward signaling) and the EFN-expressing cells (reverse signaling)21–23 and the occurrence of cell and non-cell autonomous roles.20
An Impaired Transendothelial Migration Potential of Chronic Lymphocytic Leukemia (CLL) Cells Can be Linked to Ephrin-A4 Expression
We have recently described a novel molecular interaction that could mediate in the trafficking of CLL cells and, presumably, normal B cells.24 Through comparing lymph node tissue biopsies and peripheral blood B-cell preparations from CLL patients and control subjects not suffering any kind of leukemia, as well as through several in vitro approaches, we showed that certain members of the Eph receptor family of tyrosine kinases, namely the EphA2 receptor and one of its ligands, the ephrin-A4 (EFNA4), could be involved in the B-cell trafficking through HEV. Overexpression of EFNA4 in CLL cells compared to normal B cells can partially explain the observed reduced extravasation capacity of the former ones through repulsive signals. Besides, CLL cells from patients presenting clinical lymphadenopathy expressed lower levels of EFNA4 in correlation with a better in vitro adhesion and TEM than those from patients who lack this clinical characteristic. Further experiments suggested that critical steps of the extravasation process, such as adhesion and/or transendothelial migration (TEM), can be modulated upon the interaction of EFNA4 expressed on lymphocyte cell surface with the EphA2 receptor found in the luminal side of CD31+ HEV from lymph nodes.
EphA2 Expression in the HEV of Human Lymph Nodes Could be Related to Specific Leukocyte Recruitment and/or Pathologic Conditions
Our study demonstrated strong expression of EphA2 in the HEV from human lymph nodes, supporting the hypothesis that EphA2-EFNA4 interaction could mediate in the leukocyte trafficking between the blood and the tissues, at least in CLL.24 EphA2 has been implicated in endothelial processes like angiogenesis and tumor neovascularization25,26 through orchestrating cell-cell contacts as well as in the paracellular permeability of some vascular vessels,27 suggesting that it could be implicated in the dynamics of endothelial junctions during leukocyte passage through HEV. Besides, EphA2 has been related with the adhesion of leukocytes to endothelia in a thrombin-mediated inflammation in vitro system28 and in other inflammatory conditions.29 In mice, class A Eph/ephrins has also been related with T-cells trafficking.30 We have recently realized that the high expression of EphA2 found in most of the HEV from CLL lymph nodes is limited to a “subset” of HEV within the control lymph nodes (Fig. 1), which we are currently characterizing. Thus, upregulated expression of EphA2 at certain HEV could be functionally related to specific leukocyte recruitment and/or pathologic conditions like in the CLL studied.
Figure 1.
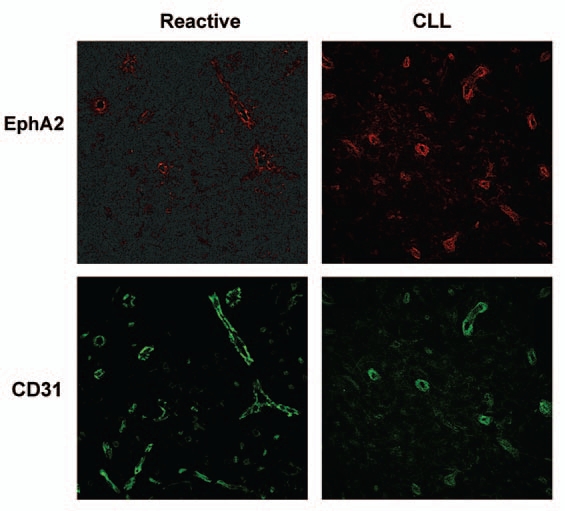
EphA2 is differentially expressed in the CD31+ vascular vessels of human lymph nodes. 8 µm thick cryosections from human lymph node biopsies of either control subjects (reactive lymph node) (left) or CLL patients (right) were immunostained with a primary polyclonal antibody for human EphA2 followed by AlexaFluor-546 conjugated specie-specific secondary antibody (shown in red) and an AlexaFluor-488 conjugated anti human CD31 (shown in green). representative experiments are shown. Fluorescence images were acquired with a confocal microscope (Leica TCS-SP2 AOBS; objective: 20x multi-immersion, 1.20 NA).
Disclosing EphA2-EFNA4 Bidirectional Signaling Within the Molecular Context of Extravasation
As alluded to above, understanding how Eph/ephrin mediates their action is often complicated by the fact that both ligands and receptors are expressed in both cells involved in the interaction, which can lead to bi-directional signaling cascades. In order to evaluate in vitro the importance of each of the members in the performed assays,24 HUVECs or B cells were pre-incubated separately with soluble recombinant homodimers of the extracellular portion of human EFNA4 or EphA2, respectively, carrying a poly-His tag that allowed us further flow cytometry analysis of cell binding. In this way, we assured that EphA2-EFNA4 interactions were blocked in the co-cultures and that eventual signaling would only take place in the pre-incubated population. Both HUVEC and B cells can express other Eph/ephrin members that bind to the corresponding recombinant homodimer such as EphA4, in the case of TNF-a activated HUVECs,24 and several EFNA ligands, in the case of CLL or normal B cells.31 Binding assays demonstrated that HUVECs bound EFNA4-Fc mainly through EphA2, as determined by confocal microscopy, and that CLL cells bound EphA2-Fc mainly through EFNA4, as determined by flow cytometry.24
Co-expression of receptors and ligands within the same cell and/or cell subpopulation should be taken into consideration. Thus, the differential expression of some members within a given cell population, mainly B-cell subpopulations,31 could have side effects in the overall population response. Co-expression of different Eph/EFN members is commonly observed and some of them have been referred as a mechanism of regulation of Eph/EFNs signaling. These include receptor-ligand interactions in cis32–34 and the formation of Eph receptor heterocomplexes, including the participation of kinase-defective Eph receptors like EphB6 and/or splice variants,16 which modulates the magnitude and type of signaling and the cell outcome. Thus, it would be interesting to carry out adhesion and TEM assays by co-culturing mixtures of EphA2-Fc treated and non-treated B cells as well as different combinations of B-cell subpopulations according to other differentially co-expressed members.
The role played by EphA2-EFNA4 interactions in the TEM of CLL cells can also be related with chemokine-mediated chemotaxis.24 CCR7-mediated chemotaxis of CLL cells can be directly modulated, in a cell autonomous way, by EFNA4 signaling while CXCR4 or CXCR5 chemotaxis were only affected in the TEM assays. It suggests that cell-cell contact dependent bidirectional EphA2-EFNA4 signals between B cells and HUVECs differentially modulated chemokine-driven migration. Transmigrating lymphocytes might recognize endothelial-presented chemokines in a cell contact-dependent manner which is instrumental for integrin-mediated lymphocyte adhesion and transendothelial migration.35–37 In the case of CXCR4- or CXCR5-mediated TEM, the CLL cell outcome is the same, i.e., no or less migration whether or not HUVEC or CLL cells are pre-incubated with the corresponding recombinant molecule, suggesting that bidirectional Eph-EFN signals mediate the TEM induced by the chemokines. Another non-excluding possibility could be that the EphA2-EFNA4-mediated cell outcomes are also subjected to modulation by the endothelial-presented chemokines. Under shear flow conditions, leukocyte crawling onto endothelium is largely dictated by the endothelial-presented chemokines which coordinate spatio-temporal changes of the integrin mediated bonds,38 a process where EphA2-EFNA4 interactions are likely to contribute. In this respect, evaluation of the EphA2-EFNA4 signaling in lymphocyte TEM under shear flow conditions is mandatory.
The wide spectrum of biological cell processes and developmental stages orchestrated by Eph/EFN signaling relies on cross-communication with multiple signaling pathways.16,39–41 Thus, they can regulate and be regulated by several other molecules including cell adhesion molecules, chemokine receptors or extracellular matrix components. On this regard, it remains to be further investigated the possible cross-talk between EphA2-EFNA4 signaling and that generated by other molecules involved in lymphocyte TEM including ectoenzymes,11 cell adhesion molecules and the endothelial-presented chemokines but also from other Eph/EFN members.
Contextualizing EphA2-EFNA4 Interactions as Possible Guiding Cues During TEM
EphA2 accumulates at inter-endothelial cell junctions and is evenly distributed on the luminal surface of TNFα activated HUVEC monolayers.24 EphA2 aggregated on the surface of HUVECs upon incubation with EFNA4-Fc complexes and the EphA2-EFNA4Fc complexes were transcytosed toward a peri-nuclear basal location within 60 min.24 This was accompanied by ICAM-1 and VCAM-1 sequestration into endothelial vesicles24 reminiscent of the vesiculo-vacuolar organelles (VVO) or caveolae that have been related with the formation of a transcellular pore during leukocyte diapedesis through endothelium (trans-cellular route of migration).42–45
An increasing body of knowledge supports the notion that EphA2 expressed at endothelial and epithelial boundaries could contribute to the regulation of paracellular permeability at these tissue barriers through cross-talk with the junctional molecular machinery.27,46–48 In the context of paracellular TEM of leukocytes, repulsive signals delivered through EphA2-EFNA4 interactions could contribute to the weakening of endothelial cell adherens junctions in cooperation with the endothelial lateral border recycling compartment (LBRC)7 that surrounds migrating leukocytes. In support of this, our experiments showed that transmigrating lymphocytes, especially normal B cells, were surrounded by endothelial EphA2.
Thus, it is tentative to speculate that EphA2-EFNA4 interaction could participate in orchestrating the migratory pathfinding during TEM through regulating the dynamic podosome-like protrusions extended by crawling lymphocytes and/or in the regulated formation of endothelial trans-cellular pores used for transcellular migration and/or the paracellular route.
The CLL Context
We have proposed a model of EFNA4 regulated extravasation of lymphocytes which is based on the realization that the defective adhesion and TEM of CLL cells can be linked to overexpression of this molecule.24 Chronic lymphocytic leukemia (CLL) is a lymphoproliferative disorder characterized by peripheral lymphocytosis of CD5+ malignant B cells with varying degrees of lymphoid tissue dissemination.49–52 In accordance with our study,24 several lines of evidence support the notion that CLL cells have an impaired migration into tissues that can be linked to an adhesion deficiency to HEV.53–58
CLL cells form proliferation centers or “pseudofollicles” within the infiltrated lymphoid tissues that can contribute to their survival and/or expansion59,60 and also to the enlargement of lymph nodes or clinical lymphadenopathy observed in some patients.61 A defective egress of the CLL cell population leading to their retention within tissues has also been proposed but not firmly demonstrated.58 In such case, it is tentative to speculate that EFNA4 overexpression could be also implicated in a hypothetical defective egress of the CLL cell population that remains to be investigated.
Remarkably, signaling through EFNA4 further impairs CLL TEM in vitro,24 suggesting that management of this molecule could have a therapeutic potential through preventing their dissemination in the more severe cases. However, the challenge for the future is to assay its applicability through in vivo human-animal models and to clearly ascertain the specificity and/or side effects derived from EFNA4 signaling in CLL therapy.
Concluding Remarks
CLL could represent a “natural model” which has allowed the identification of EphA2-EFNA4 interaction as a putative new molecular mechanism which may govern lymphocyte trafficking in certain conditions. As indicated above, the differential expression of EphA2 in the HEV of non-CLL lymph nodes may be indicative of specific leukocyte recruitment and/or pathologic states which need further attention.
In conclusion, through orchestrating the cell adhesion dynamic events that take place during TEM, the EphA2-EFNA4 interaction could contribute to the migratory path-finding of lymphocytes through endothelial vessels.
Acknowledgements
This work has been supported by grants from the Spanish Ministry of Health (FIS-PI50571 and FIS PI080093) and the Spanish Ministry of Science (BFU 2004-03132). E.T. was supported by the Spanish Ministry of Health (FIS-PIO50571 and FIS RD06/0010/0003).
Abbreviations
- CLL
chronic lymphocytic leukemia
- Eph
erythropoietin-producing hepatocellular carcinoma
- EFN
ephrin (eph receptor interacting protein)
- TEM
transendothelial migration
- HEV
high endothelial venule
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11586
References
- 1.Germain RN, Bajenoff M, Castellino F, Chieppa M, Egen JG, Huang AY, et al. Making friends in out-of-the-way places: how cells of the immune system get together and how they conduct their business as revealed by intravital imaging. Immunol Rev. 2008;221:42–45. doi: 10.1111/j.1600-065X.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 2.Bajenoff M, Egen JG, Qi H, Huang AY, Castellino F, Germain RN. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol. 2007;28:346–352. doi: 10.1016/j.it.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 4.Aurrand-Lions M, Johnson-Leger C, Imhof BA. The last molecular fortress in leukocyte trans-endothelial migration. Nat Immunol. 2002;3:116–118. doi: 10.1038/ni0202-116. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigo Mora J, Von Andrian UH. Specificity and plasticity of memory lymphocyte migration. Curr Top Microbiol Immunol. 2006;308:83–116. [PubMed] [Google Scholar]
- 6.Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–2545. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alon R, Luscinskas FW. Crawling and INTEGRating apical cues. Nat Immunol. 2004;5:351–353. doi: 10.1038/ni0404-351. [DOI] [PubMed] [Google Scholar]
- 9.Dunon D, Piali L, Imhof BA. To stick or not to stick: The new leukocyte homing paradigm. Curr Opin Cell Biol. 1996;8:714–723. doi: 10.1016/s0955-0674(96)80114-1. [DOI] [PubMed] [Google Scholar]
- 10.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 11.Salmi M, Jalkanen S. Cell-surface enzymes in control of leukocyte trafficking. Nat Rev Immunol. 2005;5:760–771. doi: 10.1038/nri1705. [DOI] [PubMed] [Google Scholar]
- 12.Huttenlocher A, Poznansky MC. Reverse leukocyte migration can be attractive or repulsive. Trends Cell Biol. 2008;18:298–306. doi: 10.1016/j.tcb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 14.Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, et al. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 15.Lackmann M, Boyd AW. Eph, a protein family coming of age: more confusion, insight or complexity? Sci Signal. 2008;1:2. doi: 10.1126/stke.115re2. [DOI] [PubMed] [Google Scholar]
- 16.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 17.Merlos-Suarez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol. 2008 doi: 10.1016/j.ceb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Janes PW, Adikari S, Lackmann M. Eph/ephrin signalling and function in oncogenesis: lessons from embryonic development. Curr Cancer Drug Targets. 2008;8:473–479. doi: 10.2174/156800908785699315. [DOI] [PubMed] [Google Scholar]
- 20.Davy A, Soriano P. Ephrin signaling in vivo: look both ways. Dev Dyn. 2005;232:1–10. doi: 10.1002/dvdy.20200. [DOI] [PubMed] [Google Scholar]
- 21.Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 22.Bruckner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- 23.Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, et al. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes & development. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinidad EM, Ballesteros M, Zuloaga J, Zapata A, Alonso-Colmenar LM. An impaired transendothelial migration potential of chronic lymphocytic leukemia (CLL) cells can be linked to ephrin-A4 expression. Blood. 2009;114:5081–5090. doi: 10.1182/blood-2009-03-210617. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 26.Brantley DM, Cheng N, Thompson EJ, Lin Q, Brekken RA, Thorpe PE, et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–7026. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- 27.Larson J, Schomberg S, Schroeder W, Carpenter TC. Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol. 2008;295:431–439. doi: 10.1152/ajplung.90256.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan B, Sukhatme VP. Receptor tyrosine kinase EphA2 mediates thrombin-induced upregulation of ICAM-1 in endothelial cells in vitro. Thromb Res. 2009;123:745–752. doi: 10.1016/j.thromres.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov AI, Romanovsky AA. Putative dual role of ephrin-Eph receptor interactions in inflammation. IUBMB Life. 2006;58:389–394. doi: 10.1080/15216540600756004. [DOI] [PubMed] [Google Scholar]
- 30.Sharfe N, Nikolic M, Cimpeon L, Van De Kratts A, Freywald A, Roifman CM. EphA and ephrin-A proteins regulate integrin-mediated T lymphocyte interactions. Mol Immunol. 2008;45:1208–1220. doi: 10.1016/j.molimm.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Alonso CL, Trinidad EM, de Garcillan B, Ballesteros M, Castellanos M, Cotillo I, et al. Expression profile of Eph receptors and ephrin ligands in healthy human B lymphocytes and chronic lymphocytic leukemia B-cells. Leuk Res. 2009;33:395–406. doi: 10.1016/j.leukres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, et al. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–330. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- 33.Yin Y, Yamashita Y, Noda H, Okafuji T, Go MJ, Tanaka H. EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci Res. 2004;48:285–296. doi: 10.1016/j.neures.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, et al. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell. 2005;121:127–139. doi: 10.1016/j.cell.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Cinamon G, Grabovsky V, Winter E, Franitza S, Feigelson S, Shamri R, et al. Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow. J Leukoc Biol. 2001;69:860–866. [PubMed] [Google Scholar]
- 36.Grabovsky V, Feigelson S, Chen C, Bleijs DA, Peled A, Cinamon G, et al. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med. 2000;192:495–506. doi: 10.1084/jem.192.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piali L, Weber C, LaRosa G, Mackay CR, Springer TA, Clark-Lewis I, et al. The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur J Immunol. 1998;28:961–972. doi: 10.1002/(SICI)1521-4141(199803)28:03<961::AID-IMMU961>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol. 2007;19:534–542. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 42.De Bruyn PP, Cho Y, Michelson S. Endothelial attachment and plasmalemmal apposition in the transcellular movement of intravascular leukemic cells entering the myeloid parenchyma. Am J Anat. 1989;186:115–126. doi: 10.1002/aja.1001860202. [DOI] [PubMed] [Google Scholar]
- 43.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 45.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 46.Miura K, Nam JM, Kojima C, Mochizuki N, Sabe H. EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell-cell contacts. Mol Biol Cell. 2009;20:1949–1959. doi: 10.1091/mbc.E08-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor RE, Hendrix MJ. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther. 2006;5:228–233. doi: 10.4161/cbt.5.2.2510. [DOI] [PubMed] [Google Scholar]
- 48.Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–638. [PubMed] [Google Scholar]
- 49.Ghia P, Caligaris-Cappio F. The origin of B-cell chronic lymphocytic leukemia. Semin Oncol. 2006;33:150–156. doi: 10.1053/j.seminoncol.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Keating MJ, Chiorazzi N, Messmer B, Damle RN, Allen SL, Rai KR, et al. Biology and treatment of chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2003:153–175. doi: 10.1182/asheducation-2003.1.153. [DOI] [PubMed] [Google Scholar]
- 51.D'Arena G, Di Renzo N, Brugiatelli M, Vigliotti ML, Keating MJ. Biological and clinical heterogeneity of B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2003;44:223–228. doi: 10.1080/1042819021000035756. [DOI] [PubMed] [Google Scholar]
- 52.Hamblin T. Chronic lymphocytic leukaemia: One disease or two? Ann Hematol. 2002;81:299–303. doi: 10.1007/s00277-002-0476-1. [DOI] [PubMed] [Google Scholar]
- 53.Stauder R, Hamader S, Fasching B, Kemmler G, Thaler J, Huber H. Adhesion to high endothelial venules: a model for dissemination mechanisms in non-Hodgkin's lymphoma. Blood. 1993;82:262–267. [PubMed] [Google Scholar]
- 54.Chen JR, Gu BJ, Dao LP, Bradley CJ, Mulligan SP, Wiley JS. Transendothelial migration of lymphocytes in chronic lymphocytic leukaemia is impaired and involved downregulation of both L-selectin and CD23. Br J Haematol. 1999;105:181–189. [PubMed] [Google Scholar]
- 55.Gu B, Dao LP, Wiley J. Impaired transendothelial migration of B-CLL lymphocytes: A defect linked to low L-selectin expression. Leuk Lymphoma. 2001;42:5–12. doi: 10.3109/10428190109097671. [DOI] [PubMed] [Google Scholar]
- 56.Hartmann TN, Grabovsky V, Wang W, Desch P, Rubenzer G, Wollner S, et al. Circulating B-cell chronic lymphocytic leukemia cells display impaired migration to lymph nodes and bone marrow. Cancer Res. 2009;69:3121–3130. doi: 10.1158/0008-5472.CAN-08-4136. [DOI] [PubMed] [Google Scholar]
- 57.Bazerbashi MB, Reeve J, Chanarin I. Studies in chronic lymphocytic leukaemia. The kinetics of 51Cr-labelled lymphocytes. Scand J Haematol. 1978;20:37–51. [PubMed] [Google Scholar]
- 58.Dormer P, Theml H, Lau B. Chronic lymphocytic leukemia: A proliferative or accumulative disorder? Leuk Res. 1983;7:1–10. doi: 10.1016/0145-2126(83)90052-8. [DOI] [PubMed] [Google Scholar]
- 59.Munk Pedersen I, Reed J. Microenvironmental interactions and survival of CLL B-cells. Leuk Lymphoma. 2004;45:2365–2372. doi: 10.1080/10428190412331272703. [DOI] [PubMed] [Google Scholar]
- 60.Patten PE, Buggins AG, Richards J, Wotherspoon A, Salisbury J, Mufti GJ, et al. CD38 expression in chronic lymphocytic leukemia is regulated by the tumor microenvironment. Blood. 2008;111:5173–5181. doi: 10.1182/blood-2007-08-108605. [DOI] [PubMed] [Google Scholar]
- 61.Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115:755–764. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]


