Abstract
The formation of complex tissues during embryonic development is often accompanied by directed cellular migration towards a target tissue. Specific mutual recognition between the migrating cell and its target tissue leads to the arrest of the cell migratory behavior and subsequent contact formation between the two interacting cell types. Recent studies implicated a novel family of surface proteins containing a trans-membrane domain and single leucine-rich repeat (LRR) domain in inter-cellular recognition and the arrest of cell migration. Here, we describe the involvement of a novel LRR surface protein, LRT, in targeting migrating muscles towards their corresponding tendon cells in the Drosophila embryo. LRT is specifically expressed by the target tendon cells and is essential for arresting the migratory behavior of the muscle cells. Additional studies in Drosophila S2 cultured cells suggest that LRT forms a protein complex with the Roundabout (Robo) receptor, essential for guiding muscles towards their tendon partners. Genetic analysis supports a model in which LRT performs its activity non-autonomously through its interaction with the Robo receptors expressed on the muscle surfaces. These results suggest a novel mechanism of intercellular recognition through interactions between LRR family members and Robo receptors.
Key words: muscle, tendon, migration, Drosophila, leucine rich repeat, Robo, Slit, LRRTM
Directed cellular migration is often guided by gradients of attractive and repellant cues that signal to the migrating cell and guide its movement toward a specific target tissue. Target recognition is associated with the arrest of cellular migration and is often followed by the formation of specific junctions between the two interacting cell types. Whereas several signaling mechanisms have been described that control directed cellular migration,1,2 little is known regarding the mechanisms that signal the arrest of migration following target recognition.
In the Drosophila embryo, somatic muscles develop from the mesoderm layer. The individual myoblasts then fuse to founder cells to form syncitial multinucleated myotubes, which extend towards their attachment sites in the ectoderm germ layer.3 Muscle extension towards the attachment sites occurs in a similar fashion to that of axon guidance, where the leading edge elongates towards the target tissue, while the cell body remains stationary.4 The muscle attachment cells are a specialized subset of ectodermal cells whose pattern is determined during ectoderm patterning by the segment polarity gene products.5,6 The muscle attachment cells, also called tendon cells, express the egr-like transcription factor Stripe, which is required for their differentiation to tendon-like cells.7,8 Following muscle contraction, the tendon cells acquire a typical moon-like morphology and an intricate array of polarized microtubules is formed, stretching from the basal to the apical aspects of the cell. The apical aspects of the tendon cell secrete the cuticle, which is part of the exoskeleton and, with the microtubule network within the cell, provide the tendon with resistance and elasticity following muscle contraction.9 Muscle connection to the tendon cells is mediated by an intricate network of integrin-mediated hemi-adherens junctions formed between these two cell types and referred to as the myo-tendinous junction (MTJ).10
A major signaling pathway mediating the attraction of the muscles to their corresponding tendon cells is the Slit/Roundabout (Robo) pathway.4,11 In contrast to the function of this signaling pathway in the nervous system where it provides repulsive cues leading to axonal repulsion, muscles appear to respond to Slit in two alternative fashions; the ventral muscles are repelled by Slit secreted from the ventral midline and are attracted to tendon cells via Slit/Robo signaling. Slit is expressed in tendon cells under the regulation of Stripe, and the secreted protein forms a gradient at the vicinity of the tendon cell.11,12 Muscles expressing Robo receptors respond to Slit by extending their leading edge towards the tendon cell (Fig. 1). Once the muscle cell has reached its destined tendon cell, it arrests its migratory behavior and initiates the formation of the MTJ. How the muscle cell “knows” that it has reached the target and the nature of the molecules inducing arrest of the muscle's migratory behavior are still open questions.
Figure 1.
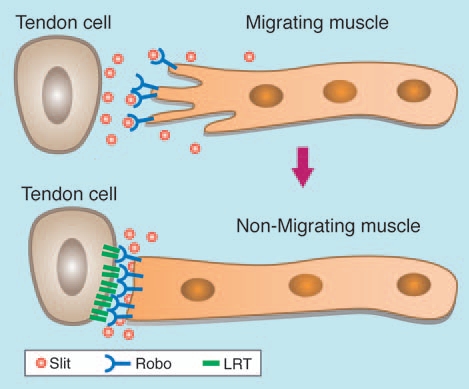
Slit/Robo dependent migration of muscle to its corresponding tendon cell. Migrating embryonic muscle expressing Robo receptors (blue) is attracted by Slit (orange) secreted from the tendon cell. Once the muscle has reached its target tendon cell, the Robo receptors accumulate at the muscle tendon adhesion site corresponding to the site of LRT (green) accumulation.
To identify essential tendon-dependent gene products involved in muscle attraction and/or adhesion to tendon cells, we conducted a microarray screen in which we compared the expression profile of mRNAs that are upregulated in the ectoderm following ectoderm-specific overexpression of the key tendon-specific transcription factor, Stripe. Using the GAL4-UAS expression system, Stripe expression was driven in the entire ectoderm, converting the fate of all ectoderm cells into that of tendons. Analysis of the genes that were upregulated in the microarray screen revealed a number of novel tendon-specific genes.
One of these genes is a Leucine-Rich-Repeat (LRR) trans-membrane Tendon-specific protein (LRT).13 LRT belongs to a family of LRR proteins discovered recently in Drosophila.14,15 These proteins were found to be essential for correct recognition between muscles and their innervating motor axons. They are expressed by the target muscle tissue, while the identity of their interacting receptor on the axon surface has not yet been characterized. We found that the expression of LRT is restricted to tendon cell membranes, at a region that corresponds to the contact area between the muscle and the tendon cell. Importantly, embryos mutant for the entire lrt gene exhibit a phenotype of misguided muscle tracts. For example, the muscles at the ventral aspects of the embryo often do not fully extend towards their corresponding tendon cells.
To follow the migration path of a single muscle cell, its plasma membrane was labeled with the fluorescent protein CD8-GFP. We chose a muscle (called muscle 12) that originates in the vicinity of a more posterior tendon cell at the segmental border and extends towards a tendon cell located at a more anterior segmental border. At the end of migration, the two ends of muscle 12 are connected to two tendon cells, each located at consecutive segmental borders (Fig. 1). Strikingly, in lrt mutant embryos, the GFP labeled muscle 12 continues to send membrane filopodia in different directions, even after the muscle has established connections at both its ends to the tendon cells (Fig. 2). This behavior was interpreted to suggest that the muscle cell did not receive the appropriate signal from the tendon cell to arrest its migratory behavior, implicating LRT as part of a signaling pathway involved in the arrest of muscle filopodia formation.
Figure 2.
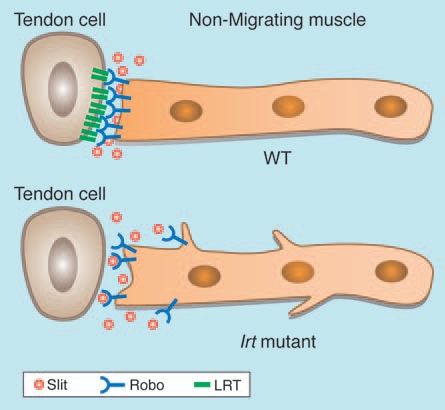
Muscle lacking lrt does not arrest its migratory behavior following its arrival to the tendon cell. Muscle in lrt mutant embryo exhibits extra-filopodia formation even after the muscle has reached its target tendon cell.
Both Slit and LRT share the LRR domains that were shown to be essential for binding to the Robo receptor in the Slit protein.16,17 Thus, we suggested that LRT expressed by the tendon cell might bind to Robo through its LRR domain and signal to the muscle cell through the Robo receptor to change its migratory behavior. To address possible interaction between Robo and LRT, S2 cells were transfected with HA-tagged Robo receptors and were subsequently exposed to conditioned medium containing a secreted form of LRT fused to GFP. The LRT-GFP secreted protein was produced by S2 cells transfected with a fusion construct of LRT-GFP lacking the trans-membrane domain. Only the S2 cells expressing Robo-HA exhibited specific association with the secreted GFP-LRT, indicating that the extracellular domain of LRT associates with Robo receptors. Co-immunoprecipitation experiments verified the formation of a protein complex between LRT and Robo.
The next question to be addressed was whether a functional link between Robo and LRT exists in vivo. When LRT was specifically overexpressed in tendon cells, muscle extension was inhibited (Fig. 3) and the muscle cells adhered to the nearest tendon cell overexpressing LRT. This interesting phenotype suggests that the levels of LRT protein must be tightly controlled. In the absence of LRT, muscle cells reach the tendon; however, they do not arrest their migratory behavior and continue to extend filopodia. In case LRT levels in the tendon cells are significantly elevated during the migration period, muscle migration towards the subsequent tendon cell is stalled. To address the relevance of Robo receptors to the stalled muscles, LRT was upregulated in tendon cells of embryos heterozygous for robo receptors, in which the expression level of Robo receptors was roughly half that in wild type embryos. In this case, the normal migratory behavior of the muscles was restored (Fig. 3). Together with the experiments performed in S2 cells indicating the interaction potential of LRT and Robo, these results support the direct involvement of LRT in Robodependent signaling during muscle migration. The exact role however remains to be elucidated.
Figure 3.
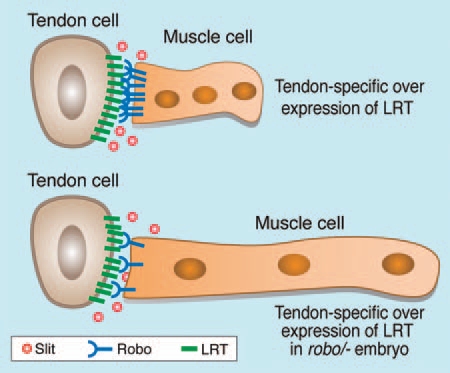
Overexpression of LRT arrest muscle extension. Muscles in embryos overexpressing LRT in tendon cells (using stripe-Gal4>UAS-lrt, upper picture) are often stalled and do not extend their end towards the next tendon cell. This phenotype is rescued by lowering the amount of Robo receptors on the muscle cells using embryos that are heterozygous for Robo and in addition overexpress LRT.
We therefore suggest that LRT is part of a mechanism involved in signaling to the muscle to arrest its migratory behavior. The high expression levels of LRT at the sites of muscle-tendon attachment, where Robo receptors accumulate, are consistent with a possible interaction between LRT and Robo receptors at these sites once the muscle cell has reached its corresponding tendon attachment site. The association between LRT and Robo might reduce the amount of Robo receptors available on the surfaces of the muscle cell to prevent further interaction with Slit. Alternatively the LRT/Robo interaction may directly signal into the cell to arrest its migratory behavior.
Leucine rich repeat proteins carrying a trans-membrane domain have been recently described in Drosophila to mediate correct olfactory axonal targeting in the CNS.15,18 In addition, a number of LRR proteins including Tartan, Capricious, Haf and others are expressed by distinct muscle types.15 These receptors contribute to the targeting specificity of peripheral axons to distinct types of muscles. Thus, membrane LRR proteins appear to be important for axonal targeting to muscles as well as for muscle targeting to tendons. It is not clear, however, which receptors on the axonal side transmit their activity. Based on our study, we suggest that these surface LRR proteins mediate their activity through receptors of the Robo family. The Drosophila genome contains genes encoding three Robo receptors. They are expressed by muscles as well as by different types of axons. Different combinations of Robo receptors together with different combinations of LRR surface proteins may direct the correct recognition between axons and their targeted axons or muscles. Similarly, a combination of Robo receptors may mediate the recognition between muscles and their corresponding tendon cells.
Support for this theory comes from two independent screens performed both in Zebrafish and mice, which have shown that LRR proteins that contain a trans-membrane domain (thus called LRRTM proteins) are important for trans-synaptic signaling and synapse formation.19,20 These include previously characterized proteins such as the Netrin-G-ligand (NGL) proteins NGL-2 and NGL-3, who have been shown to interact with Netrin-G and the LAR receptor protein phosphatase, respectively,21,22 and members of the Fibronectin Leucine Rich Transmembrane (FLRT) family of proteins. The screen performed in mice searched for synaptogenic proteins expressed in a co-culture of neurons and fibroblasts. This screen has identified the LRRTM1 protein as an important factor for synapse formation, as mutating it in mice caused altered distribution of the vesicular glutamate transporter VGLUT1 at the synapse.19 Strikingly, a recent screen performed in zebrafish aimed at identifying cell surface proteins involved in protein-protein interactions has discovered several LRRTM family members as interactors with Robo receptors, amongst which were Lrrc24, Elfn1 and LRRTM1.20 The high similarity between the domainstructure of LRT and LRRTM proteins expressed in the nervous systems of mice and zebrafish, coupled with the ability of some of the LRRTM proteins to bind Robo family members, implies that LRT's function in coordinating muscle/tendon adhesion through association with Robo might be an evolutionary conserved mechanism important for synapse formation.
In summary, the emerging family of LRRTM proteins identified in Drosophila appears to be involved in cellular recognition events between migrating cells and their target tissue. Our study suggests that these LRRTM proteins might mediate their activity through association with members of the Robo receptor family.
Acknowledgements
We thank E. Zelzer for fruitful discussions and S. Pietrokovski for precious help in the sequence comparisons between LRRTM proteins. S. Schwarzbaum helped in English corrections of the manuscript. This study was supported by a grant from the Israel Science Fund (ISF).
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11606 DOI: 10.4161/cam.4.3.11606
References
- 1.O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larrivee B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circ Res. 2009;104:428–441. doi: 10.1161/CIRCRESAHA.108.188144. [DOI] [PubMed] [Google Scholar]
- 3.Beckett K, Baylies MK. The development of the Drosophila larval body wall muscles. Int Rev Neurobiol. 2006;75:55–70. doi: 10.1016/S0074-7742(06)75003-6. [DOI] [PubMed] [Google Scholar]
- 4.Schnorrer F, Dickson BJ. Muscle building; mechanisms of myotube guidance and attachment site selection. Dev Cell. 2004;7:9–20. doi: 10.1016/j.devcel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Volk T. Singling out Drosophila tendon cells: a dialogue between two distinct cell types. Trends Genet. 1999;15:448–453. doi: 10.1016/s0168-9525(99)01862-4. [DOI] [PubMed] [Google Scholar]
- 6.Hatini V, DiNardo S. Divide and conquer: Pattern formation in Drosophila embryonic epidermis. Trends Genet. 2001;17:574–579. doi: 10.1016/s0168-9525(01)02448-9. [DOI] [PubMed] [Google Scholar]
- 7.Frommer G, Vorbruggen G, Pasca G, Jackle H, Volk T. Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J. 1996;15:1642–1649. [PMC free article] [PubMed] [Google Scholar]
- 8.Becker S, Pasca G, Strumpf D, Min L, Volk T. Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development. 1997;124:2615–2622. doi: 10.1242/dev.124.13.2615. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian A, Prokop A, Yamamoto M, Sugimura K, Uemura T, Betschinger J, et al. Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle-tendon junction. Curr Biol. 2003;13:1086–1095. doi: 10.1016/s0960-9822(03)00416-0. [DOI] [PubMed] [Google Scholar]
- 10.Bokel C, Brown NH. Integrins in development: moving on responding to and sticking to the extracellular matrix. Dev Cell. 2002;3:311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- 11.Kramer SG, Kidd T, Simpson JH, Goodman CS. Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science. 2001;292:737–740. doi: 10.1126/science.1058766. [DOI] [PubMed] [Google Scholar]
- 12.Volohonsky G, Edenfeld G, Klambt C, Volk T. Muscle-dependent maturation of tendon cells is induced by post-transcriptional regulation of stripeA. Development. 2007;134:347–356. doi: 10.1242/dev.02735. [DOI] [PubMed] [Google Scholar]
- 13.Wayburn B, Volk T. LRT, a tendon-specific leucine-rich repeat protein, promotes muscle-tendon targeting through its interaction with Robo. Development. 2009;136:3607–3615. doi: 10.1242/dev.040329. [DOI] [PubMed] [Google Scholar]
- 14.Milan M, Weihe U, Perez L, Cohen SM. The LRR proteins capricious and Tartan mediate cell interactions during DV boundary formation in the Drosophila wing. Cell. 2001;106:785–794. doi: 10.1016/s0092-8674(01)00489-5. [DOI] [PubMed] [Google Scholar]
- 15.Kurusu M, Cording A, Taniguchi M, Menon K, Suzuki E, Zinn K. A screen of cell-surface molecules identifies leucine-rich repeat proteins as key mediators of synaptic target selection. Neuron. 2008;59:972–985. doi: 10.1016/j.neuron.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hohenester E. Structural insight into Slit-Robo signalling. Biochem Soc Trans. 2008;36:251–256. doi: 10.1042/BST0360251. [DOI] [PubMed] [Google Scholar]
- 17.Howitt JA, Clout NJ, Hohenester E. Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. EMBO J. 2004;23:4406–4412. doi: 10.1038/sj.emboj.7600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong W, Zhu H, Potter CJ, Barsh G, Kurusu M, Zinn K, et al. Leucine-rich repeat transmembrane proteins instruct discrete dendrite targeting in an olfactory map. Nat Neurosci. 2009;12:1542–1550. doi: 10.1038/nn.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, et al. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sollner C, Wright GJ. A cell surface interaction network of neural leucine-rich repeat receptors. Genome Biol. 2009;10:99. doi: 10.1186/gb-2009-10-9-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Burette A, Chung HS, Kwon SK, Woo J, Lee HW, et al. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294–1301. doi: 10.1038/nn1763. [DOI] [PubMed] [Google Scholar]
- 22.Woo J, Kwon SK, Choi S, Kim S, Lee JR, Dunah AW, et al. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12:428–437. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]


