Abstract
Mean pore size is an essential aspect of scaffolds for tissue-engineering. If pores are too small cells cannot migrate in towards the center of the construct limiting the diffusion of nutrients and removal of waste products. Conversely, if pores are too large there is a decrease in specific surface area available limiting cell attachment. However the relationship between scaffold pore size and cell activity is poorly understood and as a result there are conflicting reports within the literature on the optimal pore size required for successful tissue-engineering. Previous studies in bone tissue-engineering have indicated a range of mean pore sizes (96–150 µm) to facilitate optimal attachment. Other studies have shown a need for large pores (300–800 µm) for successful bone growth in scaffolds. These conflicting results indicate that a balance must be established between obtaining optimal cell attachment and facilitating bone growth. In this commentary we discuss our recent investigations into the effect of mean pore size in collagen-glycosaminoglycan (CG) scaffolds with pore sizes ranging from 85–325 µm and how it has provided an insight into the divergence within the literature.
Key words: bone tissue engineering, cell adhesion, collagen, extracellular matrix, pore size, scaffold
The goal of tissue engineering is to develop cell, construct and living system technologies to restore the structure and functional mechanical properties of damaged or degenerated tissue. While the field of tissue engineering may be relatively new, the idea of replacing tissue with another goes as far back as the 16th century when an Italian, Gasparo Tagliacozzi (1546–99), Professor of Surgery and Anatomy at the Bologna University, described a nose replacement that he had constructed from a forearm flap in his work “De Custorum Chirurigia per Insitionem” (The Surgery of Defects by Implantation) which was published in 1597. In modern times, the techniques of transplanting tissue from one site to another in the same patient (an autograft) or from one individual to another (transplant or allograft) have been revolutionary and lifesaving. However major problems exist with both techniques. Harvesting autografts is expensive, painful, constrained by anatomical limitations and associated with donor-site morbidity due to infection and hemorrhage. Transplants have serious constraints. The major problem is accessing enough tissue and organs for all of the patients who require them. Transplants are strongly associated with rejection by the patient's immune system and they are also limited by the potential risks of introducing infection or disease.
Tissue engineering was born from the belief that primary cells could be isolated from a patient, expanded in vitro and seeded onto a substrate that could be grafted back into the patient.1 It provides a biological alternative to transplantations and prosthesis. One of the first scaffolds pioneered for tissue regeneration was synthesized as a graft co-polymer of type I collagen and chondroitin 6-sulphate, a glycosaminoglycan. The development of these scaffolds, which are capable of supporting tissue synthesis when seeded with cells, marks the beginning of the field of tissue engineering.2,3 Since this early work, there have been rapid advances in bone tissue engineering with the development of porous, biocompatible, three-dimensional scaffolds. Regardless of the application, the scaffold should be biocompatible and imitate both the physical and biological function of the native extracellular matrix (ECM), as the ECM provides a substrate with specific ligands for cell adhesion as well as physical support for cells.4 When designing scaffolds for any tissue engineering application, a major consideration is the mean pore size. Scaffolds must be permeable with interconnecting pores to facilitate cell growth, migration and nutrient flow. A previous study demonstrated that permeability increases with increasing pore size due to a reduction in specific surface area.5 If pores are too small, cell migration is limited, resulting in the formation of a cellular capsule around the edges of the scaffold. This in turn can limit the distribution of nutrients and removal of waste products resulting in necrotic regions within the construct. Conversely if pores are too large there is a decrease in specific surface area.3 It has been proposed that a reduction in specific surface area reduces the ligand density available for cells to bind to.6 Cellular activity is influenced by specific integrin-ligand interactions between cells and surrounding ECM. Initial cell adhesion mediates all subsequent events such as proliferation, migration and differentiation within the scaffold. As a result the mean pore size within a scaffold affects cell adhesion and ensuing proliferation, migration and infiltration. Therefore maintaining a balance between the optimal pore size for cell migration and specific surface area for cell attachment is essential.4,7
In our laboratory we use a composite scaffold fabricated from collagen and a glycosaminoglycan (GAG) for bone tissue engineering applications produced by a lyophilisation (freeze-drying) fabrication process. The first generation of this collagen-GAG (CG) scaffold was originally developed for skin regeneration but has since been applied to a number of other tissue engineering applications, due to its high biological activity and resultant ability to promote cell growth and tissue development.2,8–12 Originally CG scaffolds were fabricated using a rapid uncontrolled quench process during lyophilisation which resulted in heterogeneous porous scaffolds with a large variation of pore size within certain areas of the scaffold.2 When these scaffolds were used in previous studies they were visually examined so that the areas of variation could be avoided resulting in subjective selection of scaffold samples for analysis.8 However, an improved lyophilisation technique was later developed which incorporated a constant cooling rate which controlled the formation and growth of ice-crystals thus resulting in CG scaffolds with homogenous pore structures.13 The traditional final temperature of freezing used to produce these scaffolds is −40°C; however, further modifications to the lyophilisation process demonstrated that by changing the final temperature of freezing, it is possible to tailor the mean pore size in the scaffolds. This study showed that by varying the temperature of freezing from −40 to −10°C it was possible to produce homogenous CG scaffolds with mean pore sizes ranging from 96–151 µm.6
A cellular solid is one made up of an interconnecting porous network and cellular solids modeling techniques can be used to describe both mechanical and microstructural (i.e., specific surface area) properties of scaffolds. A cellular solids model utilizing a tetrakaidecahedral unit cell (a 14-sided polyhedron that packs to fill space) was used to determine the effect of mean pore size on specific surface area. Specific surface area can be related to the relative density of a scaffold and using a tetrakaidecahedral unit cell it was possible to model the geometry of the CG scaffolds.5,6,14 As a result the specific surface area (SA) per unit volume (V) available for cell adhesion in each of the scaffolds with different mean pore sizes (d) was estimated as:
| (1) |
This relationship demonstrates that the specific surface area is inversely proportional to the mean pore size. The authors then carried out a simple experiment and seeded the scaffold range with osteoblasts and monitored initial cell adhesion up to 48 h post-seeding. Cell adhesion is the binding of cells to their extracellular environment via specific ligand-integrin interactions. The results demonstrated that cell adhesion decreased with increasing pore size and that the highest levels of cell attachment were found on the scaffolds with the smallest pore size (96 µm). The rationale for this result, as suggested by the authors, was the effect of specific surface area on cell adhesion due to the scaffolds with larger pores having less available specific surface area and thus a lower ligand density for initial cell attachment.5,6
The results of this study conflicted with other studies within the literature which demonstrate a need for larger pores. The relationship between scaffold pore size and cell activity is not fully understood and as a result, over the years there have been conflicting reports on the optimal pore size required for bone tissue engineering. Pores ranging from 20–1,500 µm have been used in bone tissue engineering applications.15–18 Initial studies demonstrated that the minimum pore size for significant bone growth is 75–100 µm with an optimal range of 100–135 µm.15,19 Since this early work it has been reported that pores greater than ∼300 µm are essential for vascularisation of constructs and bone ingrowth, while pores smaller than ∼300 µm can encourage osteochondral ossification.20–22
In a very recent study in our laboratory, which utilized improved technical capability of our freeze-drying system and introduced a novel annealing step during lyophilisation, we have been able to further expand the range of mean pore sizes produced in the CG scaffolds from 96–151 µm up to 85–325 µm.23 We then investigated the effect of this new expanded range of scaffolds on initial cell attachment followed by migration and proliferation by monitoring cellular activity up to 7 days post-seeding (as opposed to 48 h in the earlier study6) to see whether the pattern of specific surface area affecting initial cell adhesion as seen in the previous studies would continue as cells proliferated.24
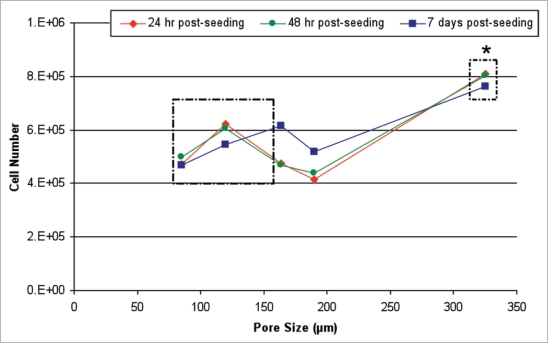
The results provide a possible insight into why there are conflicting reports in the literature on the optimal scaffold pore size for bone tissue engineering. A non-linear effect of pore size was seen on cell proliferation over the 7 day incubation period. Scaffolds with the largest pore size of 325 µm facilitated higher cell number at all time points in comparison to the other scaffold types. However, within the lower range of pore sizes there was a small peak in cell number at 24 h and 48 h post-seeding in scaffolds with a mean pore size of 120 µm. This peak disappeared by day 7 (Fig. 1). This peak is consistent with that seen in the earlier study6 and can therefore be explained by the effect of pore surface area on cell attachment. Collagen, a natural component of bone ECM, contains binding sites (ligands) that are recognized by specific cell surface receptors (integrins), the main collagen integrins being α1β1 and α2β1. Based on the interactions between integrins and their corresponding ligands, cells can detect subtle changes in ECM that can influence cell attachment and consequently determine cell proliferation, speed and migration. Our results reflected this within the smaller pore range (85–190 µm) when cell number was presented as a percentage of the cells seeded onto the scaffolds,24 indicating that high specific surface area in scaffolds is important for optimal cell attachment. However, when this range of pore sizes was expanded (85–325 µm) the linear relationship between mean pore size and specific surface area was no longer applicable (Fig. 1) and scaffolds with the largest pores showed the highest cell numbers even though the surface area is lower than that for the other scaffold variants. We propose that the effect of specific surface area is overcome in larger pores by the improved potential for cell migration and proliferation as was seen histologically in scaffolds with 325 µm.
Figure 1.
Effect of mean pore size on cell number at each time point. Cell number increases to a small peak 24 h post seeding in scaffolds with a pore size of 120 µm. This peak declines at later time points. Cell number significantly peaks in scaffolds with a mean pore size of 325 µm. *p < 0.001 (reviewed in ref. 24).
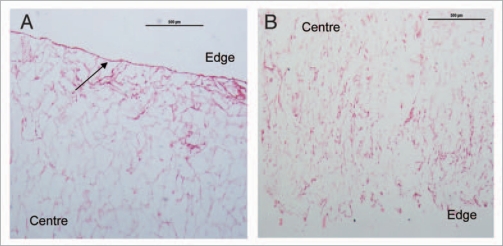
When seeding three-dimensional scaffolds it is desirable that the cells infiltrate and colonize the scaffold laying down their own ECM. The CG scaffolds are highly porous (∼99%)5 and it has previously been shown that cell migration behavior decreases with increasing pore size.26 However, similarly to other studies,6 these results were based on limited range of mean pore sizes incubated for less than 48 h. In this study, migration of cells was assessed histologically after 7 days incubation. Cells were observed lining the pores in all scaffolds. However, cell aggregations were seen along the edges of the scaffolds with smaller pore sizes of 85 µm–120 µm limiting the number of cells infiltrating the scaffold (Fig. 2A). Cell aggregations form a “skin” around the outer surface of the scaffold which restricts the diffusion of nutrients and removal of waste from the cells colonizing the center of the scaffold. As the mean pore size increased, cells migrated further away from the edges and in towards the center of the scaffold until cells were seen colonizing the center of the scaffolds with the largest mean pore size of 325 µm (Fig. 2B). An increase in cell number was seen in 120 µm pore size, but the aggregations seen on the surface of these scaffolds compound the hypothesis that this peak was related to initial cell adhesion and the advantages of this pore size were lost with subsequent cell proliferation and migration.
Figure 2.
Effect of mean pore size on cell infiltration and distribution CG scaffolds after 7 days. Scaffolds were stained with H&E: (A) 85 µm pore size at x40 magnification, (B) 325 µm pore size at ×40 magnification. Collagen scaffold is stained pink and cell nuclei a deep purple. The arrow indicates cell aggregations along the edges of the scaffold. Aggregations disappeared and cell migration increased with increasing pore size (reviewed in ref. 24).
The study24 had a number of limitations. It was not possible to determine the upper pore size limit for cell activity within a CG scaffold. If the pores become too large the mechanical properties of the scaffold will be compromised due to void volume7 and as pore size increases further, the specific surface area will eventually reduce to a level that will limit cell adhesion. Furthermore, this study has determined the optimal pore size for MC3T3-E1 pre-osteoblast activity. It has been hypothesised that the optimal pore size will vary with different cell types6 and another recent study from our laboratory has demonstrated that mesenchymal stem cells seeded on the smaller range of CG scaffolds and maintained in osteogenic culture for 3 weeks showed improved osteogenesis on the scaffolds with bigger pores25. For this reason it is important to repeat this study with different cell types. However, regardless of these limitations, this paper has demonstrated that mean pore size does affect cell behavior within a scaffold and that subtle changes in pore size can have a significant effect on cell behavior. We also provide an insight into why the literature reports conflicting results on the optimal pore size required for bone tissue engineering, whereby increased specific surface area provided by scaffolds with small pores has a benefi- cial effect on initial cell attachment, but this is overcome by the improved cellular infiltration provided by scaffolds with larger pores suggesting that these scaffolds might be optimal for longer term in vitro culture with the aim of facilitating bone tissue repair.
Acknowledgements
The author acknowledges Science Foundation Ireland, President of Ireland Young Researcher Award (04/Yl1/B531) for funding. Collagen materials were provided by Integra Life Sciences, Inc., through a Material Transfer Agreement.
Abbreviations
- CG
collagen-glycosaminoglycan
- d
diameter of pore size
- ECM
extracellular matrix
- SA
surface area
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11747
References
- 1.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7:679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 2.Yannas IV. Tissue and organ regeneration in adults. New York: Springer; 2001. [Google Scholar]
- 3.Yannas IV. Tissue regeneration by use of collagen-glycosaminoglycan copolymers. Clin Mater. 1992;9:179–187. doi: 10.1016/0267-6605(92)90098-e. [DOI] [PubMed] [Google Scholar]
- 4.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien FJ, Harley BA, Waller MA, Yannas IV, Gibson LJ, Prendergast PJ. The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering. Technol Health Care. 2007;15:3–17. [PubMed] [Google Scholar]
- 6.O'Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–441. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 7.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Freyman TM, Yannas IV, Yokoo R, Gibson LJ. Fibroblast contraction of a collagen-GAG matrix. Biomaterials. 2001;22:2883–2891. doi: 10.1016/s0142-9612(01)00034-5. [DOI] [PubMed] [Google Scholar]
- 9.Farrell E, Byrne EM, Fischer J, O'Brien FJ, O'Connell BC, Prendergast PJ, et al. A comparison of the osteogenic potential of adult rat mesenchymal stem cells cultured in 2-D and on 3-D collagen glycosaminoglycan scaffolds. Technol Health Care. 2007;15:19–31. [PubMed] [Google Scholar]
- 10.Farrell E, O'Brien FJ, Doyle P, Fischer J, Yannas I, Harley BA, et al. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006;12:459–468. doi: 10.1089/ten.2006.12.459. [DOI] [PubMed] [Google Scholar]
- 11.Freyman TM, Yannas IV, Gibson LJ. Cellular materials as porous scaffolds for tissue engineering. Prog Mater Sci. 2001;46:273–282. [Google Scholar]
- 12.Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA. 1989;86:933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien FJ, Harley BA, Yannas IV, Gibson L. Influence of freezing rate on pore structure in freezedried collagen-GAG scaffolds. Biomaterials. 2004;25:1077–1086. doi: 10.1016/s0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 14.Harley BAC, Kim H-D, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction Interactions. Biophysical Journal. 2008;95:4013–4024. doi: 10.1529/biophysj.107.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulbert SF, Young FA, Mathews RS, Klawitter JJ, Talbert CD, Stelling FH. Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res. 1970;4:433–456. doi: 10.1002/jbm.820040309. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Lee IW, Lee YM, Lee HB, Khang G. Macroporous biodegradable natural/synthetic hybrid scaffolds as small intestine submucosa impregnated poly(D,L-lactide-co-glycolide) for tissue-engineered bone. J Biomater Sci Polym Ed. 2004;15:1003–1017. doi: 10.1163/1568562041526487. [DOI] [PubMed] [Google Scholar]
- 17.Nehrer S, Breinan HA, Ramappa A, Young G, Shortkroff S, Louie LK, et al. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18:769–776. doi: 10.1016/s0142-9612(97)00001-x. [DOI] [PubMed] [Google Scholar]
- 18.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 19.Klawitter JJ, Bagwell JG, Weinstein AM, Sauer BW. An evaluation of bone growth into porous high density polyethylene. J Biomed Mater Res. 1976;10:311–323. doi: 10.1002/jbm.820100212. [DOI] [PubMed] [Google Scholar]
- 20.Kuboki Y, Jin Q, Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg Am. 2001;83:105–115. [PubMed] [Google Scholar]
- 21.Roosa SM, Kemppainen JM, Moffitt EN, Krebsbach PH, Hollister SJ. The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J Biomed Mater Res A. 2010;92:359–368. doi: 10.1002/jbm.a.32381. [DOI] [PubMed] [Google Scholar]
- 22.Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem. 1997;121:317–324. doi: 10.1093/oxfordjournals.jbchem.a021589. [DOI] [PubMed] [Google Scholar]
- 23.Haugh MG, Murphy CM, O'Brien FJ. Novel freeze-drying methods to produce a range of collagen-GAG scaffolds with tailored mean pores sizes. Tissue Eng Part C Methods. 2009 doi: 10.1089/ten.TEC.2009.0422. in press. [DOI] [PubMed] [Google Scholar]
- 24.Murphy CM, Haugh MG, O'Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461–466. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 25.Byrne EM, Farrell E, McMahon LA, Haugh MG, O'Brien FJ, Campbell VA, et al. Gene expression by marrow stromal cells in a porous collagen-glycosaminoglycan scaffold is affected by pore size and mechanical stimulation. J Mater Sci Mater Med. 2008;19:3455–3463. doi: 10.1007/s10856-008-3506-2. [DOI] [PubMed] [Google Scholar]