Abstract
Wound closure and infection control are the primary goal of wound management. A variety of disinfectants and antimicrobial agents are widely available today and routinely achieve infection control. On the contrary, wound closure still remains a challenging goal. Cell adhesion, migration and contraction play significant roles in creating contractile force of patent wound margins and in contributing to wound closure. Modulations of these cellular behaviors have been investigated in the context of wound contraction; however, therapeutic strategy to achieve wound closure has not been established. Recently, we have reported that a previously unknown cytoskeleton molecule, wound inducible transcript-3.0 (wit3.0) also known as fibroblast growth factor receptor 1 oncogene partner 2 (FGFR1OP2), can significantly modulate fibroblast-driven wound closure in vitro and in vivo. The dynamic role of cytoskeleton in different experimental models may provide a novel platform for designing the therapeutic target of wound management.
Key words: wound closure, cell migration, cytoskeleton, FGFR1OP2/wit3.0, therapeutic target
Postoperative wound dehiscence and surgical site infections are common complications in surgical treatments. The severity of such complications may manifest from mild cases that require local wound care and antibiotics treatment to serious cases with repeated surgical interventions and a high mortality rate. When Eliason and McLaughlin (1934) published the classic review on postoperative wound complications, their focus was largely on surgical site infections.1 The effective use of prophylactic antibiotics and the unidirectional ventilation system for surgery and recovery suites are among the improvements developed in the past several decades, which have contributed to the reduction of surgical site infection risks.2,3 On the contrary, the challenge to prevent surgical wound dehiscence remains problematic and wounds that dehisce frequently are left to heal by secondary or tertiary intention, which could lead to a large scar formation. Incisional and excisional wounds in adult tissues do not spontaneously close and thus remain patent during the critical initial healing stages.4,5 Current approaches to achieve the initial wound closure employ sutures and adhesives that are essentially unchanged over a century.
Fetal Wound Closure
It has been reported that skin wounds created in early mammalian embryos exhibit spontaneous wound closure and wound tissue regeneration.6,7 In the adult wound, epithelial cells migrate into the wound center, but fetal wound epithelial cells remain blunt-faced and adherent to the underlying basal lamina. A thick cable of actin is formed in the basal epidermis at the leading edge of marginal cells surrounding the wound. The contraction of this actin cable is believed to provide the force required to draw the wound edges together in a “purse-string”-like manner.8,9 In addition to a filamentous actin cable, other components of the contractile machinery, including myosin heavy chain II, are also assembled in a coordinated manner, as are the cytoskeletal proteins that enable the intracellular cable to link with neighboring cells through adherent junctions.10 The purse-string wound closure appears to be unique in fetal wound healing and has not been found in wound healing of adult tissues.
Adult and fetal wound healing differs in cellular mediators, inflammatory cytokines, growth factors, and extracellular matrix (ECM) modulators.11,12 The intense characterization of fetal wounds revealed that wound induced inflammation was less extensive, and profiles of cytokines and growth factors were different than adult wounds.11,12 These observations provided the basis for the potential therapeutic targets such as neutralizing antibodies against TGFb1, TGFb2 and PDGF, as well as exogenous application of TGFb3.13,14 Considering the favorable fetal wound healing, control of the inflammatory response or modulation of selected inflammatory cytokines may be therapeutically feasible to reduce post-wound scarring. However, these growth factor-related therapies intending to generate “fetal-like” wound healing phenotype in adult wounds show only mixed results to date.
Alpha-Smooth Muscle Actin
Alpha-smooth muscle actin (α-SMA) is a 42 KDa cytoskeleton molecule, found as a predominant actin species in aortic smooth muscle cells.15 Gabbiani and Majno (1970) reported a group of fibroblasts in healing granulation tissue exhibiting ultrastructural features of smooth muscle cells such as microfilamant bundles.16 α-SMA was identified in these so-called “myofibroblasts,” and immediately postulated to play a central role in wound contraction. 17 Furthermore, various growth factors and inflammatory cytokines were found to influence the α-SMA expression,18 supporting the postulated involvement of α-SMA in wound contraction. Unlike cytoplasmic actin isoforms, which participate in the cytoskeletal appratus and microfilaments contributing to cell motility, α-SMA composes contractile sarcomeres and smooth muscle myofibrils in muscle tissues. In fact, α-SMA null mutant mice showed the decreased vascular contractility and lowered basal blood pressure.19 However, the role of α-SMA in myofibroblasts is less established. The induction of cell traction force in corneal fibroblasts did not require α-SMA;20 and the α-SMA knockout mutation did not significantly affect fibroblast cell motility.21 Cell traction force and cell migration play an important role in wound closure. These recent observations prompted us to search alternative therapeutic target molecules of wound management.
Dorsal Closure of Drosophila Embryo
The progressive closure of a dorsal gap during Drosophila embryogenesis has been considered another model of wound closure.22 The longitudinal epidermis margins on the either side of the dorsal opening close by approximation of the leading edges, which form seams at each canthus. It has been shown that dorsal closure is achieved by synchronized cellular forces generated by subcellular actin and nonmuscle myosin II in each “purse string” of epidermal margins.23 The dorsal opening is filled with specialized epithelial cells, amnioserosa, which undergo apoptosis during dorsal closure resulting in eliminating supernumerary cells.24 Recently, Solon et al. (2009) demonstrated that amnioserosa cells generated the pulsed force, which effectively displaced the epidermis margins.25 During this process, amnioserosa undergo extensive morphological changes from the initial squamous shapes to a narrow, tubular structure. Various molecules associated with the cytoskeleton and cell junctions have been postulated to regulate the dorsal closure. Further characterization of this model may contribute to the accelerated wound closure and wound management.
Oral Wound Closure
The healing sequence of oral wounds is similar to that in adult skin wounds; hemostatis, inflammation, granulation tissue formation and remodeling of the connective tissue matrix. However, clinical observations and experimental animal studies consistently indicate that the extent of granulation tissue and scar formation in oral mucosa is small, and oral wound healing demonstrates faster wound closure compared to the equivalent wound in skin.26 Both fetal and oral wounds are exposed to a moist environment, which has been postulated to be favorable for wound healing.27 Saliva also contains a group of cytokines and growth factors.28,29 Saliva of NOD mice contains decreased concentrations of epidermal growth factor, and this type I diabetes mouse model has been reported to exhibit the impaired oral wound healing.30 Certainly, the oral environment may assist better wound healing. However, because skin grafts transposed into the oral cavity maintain the skin wound healing phenotype,31 the contribution of constitutive oral cellular and molecular components to the rapid wound closure cannot be overlooked.
Oral mucosa and gingiva are composed of a thin keratinocyte layer with underlying highly-vascularized connective tissue.32 Epithelial cells of fetal wound and dorsal gap of Drosophila, which play the pivotal role in the “purse-string” closure, do not proliferate. In contrast, oral wound epithelium undergoes rapid proliferation. Oral keratinocytes constitutively express glucose transporter 1 (GLUT1) and contain fatty acids such as palmitate,33 suitable for cell proliferation, which requires the high level of energy metabolism. While oral keratinocytes may quickly re-establish the epithelial continuity, epithelial “purse-string” mechanism does not seem to occur in the oral wound. Therefore, the rapid oral wound approximation may be accomplished by a different mechanism.
FGFR1OP2/wit3.0
Collagen gel contraction by isolated oral fibroblasts occurs at a faster rate than with skin fibroblasts in vitro.34,35 Therefore, it has been postulated that oral fibroblasts possess distinctive characteristics promoting accelerated wound closure. We have isolated a previously unknown cDNA from the oral wound library encoding Wound Inducible Transcript-3.0 (wit3.0).36 The OMIM database identified the association of wit3.0 with 8p11 myeloproliferative syndrome, a case presented by Grand et al. (2004).37 An unusual (12; 8) (p11; p11p22) chromosomal insertion was found in a 75-year-old male who suffered from a T-cell lymphoblastic lymphoma that progressed rapidly to acute myelogenous leukemia (AML). The chromosomal abnormality facilitated the insertion of the wit3.0 N-terminal domain to the Fibroblast Growth Factor Receptor 1 (FGFR1) and resulted in the ligand-independent FGFR1 dimerization through coiled-coil structure derived from wit3.0. As such, the Genbank database resisters this molecule as FGFR1 Oncogene Partner-2 (FGFR1OP2).
The highly conserved FGFR1OP2/wit3.0 sequence has been identified in Homo sapiens, Rattus norvegicus, Mus musculus and Canis familiaris. FGFR1OP2/wit3.0 is located on human chromosome 12, at 12p11.23, spanning almost 27.5 kb; rat chromosome 4q44; mouse chromosome 6G3; and dog chromosome 27. Among these species, the FGFR1OP2/wit3.0 allele contains highly conserved seven exons, with a start and stop codons located in exon2 and 7, respectively. In addition, exon5 is alternatively spliced, resulting in two isoforms. The NCBI database revealed that the FGFR1OP2/wit3.0 peptides in humans, dogs, mice, rats, chicken, fruit flies, and mosquitoes contain a conserved domain, DUF837, which belongs to the protein family pfam05769.3. This domain is consistently located at the N-terminal in all species, and has appeared in several eukaryotic proteins, although the function is still not clear. BLAST search identified a match with myosin heavy chain II, in particular, a conserved domain in a myosin tail family (pfam01576.8). The FGFR1OP2/wit3.0 peptide also matched with the pfam COG4372, an uncharacterized protein conserved in bacteria with the myosin-like domain.
In oral wound healing, FGFR1OP2/wit3.0 was significantly upregulated and oral fibroblasts have been identified as the primary cellular source.38 Strikingly, oral fibroblasts expressing FGFR1OP2/wit3.0 were different from those expressing α-SMA, suggesting that FGFR1OP2/wit3.0 expressing oral wound fibroblasts were not myofibroblasts. In immunocytological analyses, FGFR1OP2/wit3.0 peptide was amorphously localized in cytoplasma, especially around the perinucleus area of both oral fibroblasts and skin fibroblasts. Skin fibroblasts derived from excisional wound exhibited the similar FGFR1OP2/wit3.0 distribution in cytoplasma (unpublished data, Fig. 1). On the contrary, oral fibroblasts derived from tooth extraction wound revealed the dramatically modulated cytological distribution. Immunostaining of FGFR1OP2/wit3.0 was peripherally spread to form a cytoskeletal filamentous structure. F-actin was also positive on the wit3.0-positive cytoskeletal filaments; however, these two molecules appeared to localize in a mutually exclusive fashion (Fig. 1).39
Figure 1.
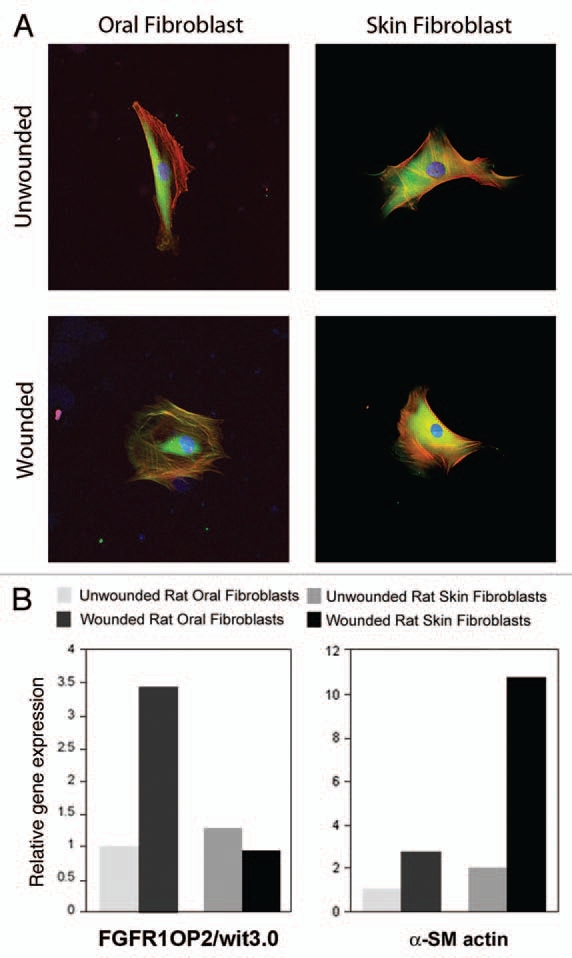
Expression of FGFR1OP2/wit3.0 in rat primary oral and skin fibroblasts. (A) Immunocytological staining identified FGFR1OP2/wit3.0 (green) amorphously in the cytoplasma surrounding nucleus (DAPI: blue) of oral and skin fibroblasts. In oral fibroblasts derived from gingiva of post-tooth extraction wounding, FGFR1OP2/wit3.0 localized in cytoskeletal network containing F-actin (red). In contrast, skin fibroblasts harvested after excisional wounding showed no change in FGFR1OP2/wit3.0 distribution. (Confocal laser scanning micrographs of oral fibroblasts are reproduced from Lin et al., Am J Pathol, 2010, 176:108–121, with permission.) (B) The steady state mRN A levels of FGFR1OP2/wit3.0 and alpha smooth muscle actin (α-SM actin) were characterized by real time reverse transcription polymerase chain reaction. Wounded oal and skin fibroblasts demonstrated the increase in a-SM actin expression, albeit at different magnitudes. In oral fibroblasts, wounding increased the expression of FGFR1OP2/wit3.0 expression; whereas wounding did not modulate its expression in skin fibroblasts.
The three-dimensional collagen gel embedded with fibroblasts has been used as a reliable in vitro assay of wound contraction, in which volumetric changes of collagen gel substrate are thought to be generated by fibroblast migration and contraction force. Oral fibroblasts exhibit the greater rate of collagen gel contraction than skin fibroblasts. siRNA knockdown and overexpression of FGFR1OP2/wit3.0 resulted in the significant decrease and increase of oral fibroblast-embedded floating collagen gel contraction, respectively. The International Gene Trap Consortium (IGTC) represents all publicly available gene trap cell lines that are available on a non-collaborative basis, from which C57Bl/6 mouse ES cell lines carrying the gene trap mutagenesis in the FGFR1OP2/wit3.0 allele have been identified. The differentiated fibroblast-like cells from the mutant ES cells showed significantly decreased cell migration to the wound space in vitro.39 Furthermore, a proof-of-concept study in a mouse skin wound model revealed that the application of lentiviral vector carrying FGFR1OP2/wit3.0 generated accelerated wound closure similar to oral wounds and resulted in less scarring.39 Taken together, FGFR1OP2/wit3.0 may regulate the cell migration ability relevant to the fibroblast-driven wound closure; thus this newly characterized cytoskeleton molecule may present an alternative candidate for the new therapeutic target for wound management.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11917 DOI: 10.4161/cam.4.3.11917
References
- 1.Eliason EL, McLaughlin C. Post-operative wound complications. Ann Surg. 1934;100:1159–1176. doi: 10.1097/00000658-193412000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols RL. Preventing surgical site infections. Clin Med Res. 2004;2:115–118. doi: 10.3121/cmr.2.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smylie HG, Davidson AI, Macdonald A, Smith G. Ward design in relation to postoperative wound infection I. Br Med J. 1971;1:67–72. doi: 10.1136/bmj.1.5740.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin P. Wound healing-Aiming for perfrect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 5.Singer AJ, Clark RAF. Cutaneous wound healing. New Eng J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 6.Whitby DJ, Longaker MT, Harrison MR, Adzick NS, Ferguson MW. Rapid epithelialisation of fetal wounds is associated with the early deposition of tenascin. J Cell Sci. 1991;99:583–586. doi: 10.1242/jcs.99.3.583. [DOI] [PubMed] [Google Scholar]
- 7.Longaker MT, Whitby DJ, Jennings RW, Duncan BW, Ferguson MW, Harrison MR, Adzick NS. Fetal diaphragmatic wounds heal with scar formation. J Surg Res. 1991;50:375–385. doi: 10.1016/0022-4804(91)90206-2. [DOI] [PubMed] [Google Scholar]
- 8.McCluskey J, Martin P. Analysis of the tissue movements of embryonic wound healing—DiI studies in the limb bud stage mouse embryo. Dev Biol. 1995;170:102–114. doi: 10.1006/dbio.1995.1199. [DOI] [PubMed] [Google Scholar]
- 9.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 10.Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol. 1996;135:1097–1107. doi: 10.1083/jcb.135.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soo C, Hu FY, Zhang X, Wang Y, Beanes SR, Lorenz HP, et al. Differential expression of fibromodulin, a transforming growth factor-beta modulator, in fetal skin development and scarless repair. Am J Pathol. 2000;157:423–433. doi: 10.1016/s0002-9440(10)64555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colwell AS, Longaker MT, Lorenz HP. Fetal wound healing. Front Biosci. 2003;8:1240–1248. doi: 10.2741/1183. [DOI] [PubMed] [Google Scholar]
- 13.O'Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson MW, O'Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandekerckhove J, Weber K. Actin typing on total cellular extracts: a highly sensitive protein-chemical procedure able to distinguish different actins. Eur J Biochem. 1981;113:595–603. doi: 10.1111/j.1432-1033.1981.tb05104.x. [DOI] [PubMed] [Google Scholar]
- 16.Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR, Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972;135:719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabbiani G. Modulation of fibroblastic cytoskeletal features during wound healing and fibrosis. Pathol Res Pract. 1994;190:851–853. doi: 10.1016/S0344-0338(11)80988-X. [DOI] [PubMed] [Google Scholar]
- 18.Desmouliere A. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int. 1995;19:471–476. doi: 10.1006/cbir.1995.1090. [DOI] [PubMed] [Google Scholar]
- 19.Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle alpha-actin null mouse. Faseb J. 2000;14:2213–2220. doi: 10.1096/fj.99-0927com. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Li H, SundarRaj N, Wang JH. Alpha-smooth muscle actin expression enhances cell traction force. Cell Motil Cytoskeleton. 2007;64:248–257. doi: 10.1002/cm.20178. [DOI] [PubMed] [Google Scholar]
- 21.Takeji M, Moriyama T, Oseto S, Kawada N, Hori M, Imai E, Miwa T. Smooth muscle alpha-actin deficiency in myofibroblasts leads to enhanced renal tissue fibrosis. J Biol Chem. 2006;281:40193–40200. doi: 10.1074/jbc.M602182200. [DOI] [PubMed] [Google Scholar]
- 22.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 23.Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol. 2005;15:2208–2221. doi: 10.1016/j.cub.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 24.Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 2008;321:1683–1686. doi: 10.1126/science.1157052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–1342. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 26.Stephens P, Davies KJ, Occleston N, Pleass RD, Kon C, Daniels J, et al. Skin and oral fibroblasts exhibit phenotypic differences in extracellular matrix reorganization and matrix metalloproteinase activity. Br J Dermatol. 2001;144:229–237. doi: 10.1046/j.1365-2133.2001.04006.x. [DOI] [PubMed] [Google Scholar]
- 27.Okan D, Woo K, Ayello EA, Sibbald G. The role of moisture balance in wound healing. Adv Skin Wound Care. 2007;20:39–53. doi: 10.1097/00129334-200701000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Tyler LW, Donoff RB, Song B, Torio AJ, Gallagher GT, et al. Salivary EGF regulates eosinophil-derived TGFalpha expression in hamster oral wounds. Am J Physiol. 1996;270:191–202. doi: 10.1152/ajpgi.1996.270.1.G191. [DOI] [PubMed] [Google Scholar]
- 29.Ohshima M, Sato M, Ishikawa M, Maeno M, Otsuka K. Physiologic levels of epidermal growth factor in saliva stimulate cell migration of an oral epithelial cell line, HO-1-N-1. Eur J Oral Sci. 2002;110:130–136. doi: 10.1034/j.1600-0722.2002.11179.x. [DOI] [PubMed] [Google Scholar]
- 30.Nagy A, Nagashima H, Cha S, Oxford GE, Zelles T, Peck AB, Humphreys-Beher MG. Reduced oral wound healing in the NOD mouse model for type 1 autoimmune diabetes and its reversal by epidermal growth factor supplementation. Diabetes. 2001;50:2100–2104. doi: 10.2337/diabetes.50.9.2100. [DOI] [PubMed] [Google Scholar]
- 31.Bussi M, Valente G, Curato MP, Carlevato MT, Cortesina G. Is transposed skin transformed in major head and neck mucosal reconstruction? Acta Otolaryngol. 1995;115:348–351. doi: 10.3109/00016489509139327. [DOI] [PubMed] [Google Scholar]
- 32.Feliciani C, Gupta AK, Sauder DN. Keratinocytes and cytokine/growth factors. Crit Rev Oral Biol Med. 1996;7:300–318. doi: 10.1177/10454411960070040101. [DOI] [PubMed] [Google Scholar]
- 33.Kuroki S, Yokoo S, Terashi H, Hasegawa M, Komori T. Epithelialization in oral mucous wound healing in terms of energy metabolism. Kobe J Med Sci. 2009;55:5–15. [PubMed] [Google Scholar]
- 34.Chaussain Miller C, Septier D, Bonnefoix M, Lecolle S, Lebreton-Decoster C, Coulomb B, et al. Human dermal and gingival fibroblasts in a three-dimensional culture: a comparative study on matrix remodeling. Clin Oral Investig. 2002;6:39–50. doi: 10.1007/s00784-001-0143-2. [DOI] [PubMed] [Google Scholar]
- 35.Lorimier S, Hornebeck W, Godeau G, Pellat B, Gillery P, Maquart FX, Laurent-Maquin D. Morphometric studies of collagen and fibrin lattices contracted by human gingival fibroblasts; comparison with dermal fibroblasts. J Dent Res. 1998;77:1717–1729. doi: 10.1177/00220345980770090801. [DOI] [PubMed] [Google Scholar]
- 36.Sukotjo C, Abanmy AA, Ogawa T, Nishimura I. Molecular cloning of wound inducible transcript (wit 3.0) differentially expressed in edentulous oral mucosa undergoing tooth extraction wound-healing. J Dent Res. 2002;81:229–235. doi: 10.1177/154405910208100402. [DOI] [PubMed] [Google Scholar]
- 37.Grand EK, FH G, Chase AJ, Ross FM, Corcoran MM, Oscier DG, Cross NC. Identification of a novel gene, FGFR1OP2, fused to FGFR1 in 8p11 myeloproliferative syndrome. Genes Chromosomes Cancer. 2004;40:78–83. doi: 10.1002/gcc.20023. [DOI] [PubMed] [Google Scholar]
- 38.Sukotjo C, Lin A, Song K, Ogawa T, Wu B, Nishimura I. Oral fibroblast expression of woundinducible transcript 3.0 (wit3.0) accelerates the collagen gel contraction in vitro. J Biol Chem. 2003;278:51527–51534. doi: 10.1074/jbc.M309616200. [DOI] [PubMed] [Google Scholar]
- 39.Lin A, Hokugo A, Choi J, Nishimura I. Small cytoskeleton-associated molecule, fibroblast growth factor receptor 1 oncogene partner 2/wound inducible transcript-3.0 (FGFR1OP2/wit3.0), facilitates fibroblast-driven wound closure. Am J Pathol. 2010;176:108–121. doi: 10.2353/ajpath.2010.090256. [DOI] [PMC free article] [PubMed] [Google Scholar]


